The photosynthetic oxygen evolution does not exclude the important role and contribution of bicarbonate photolysis
2024-03-06YanyouWuShaogangGuo
Yanyou Wu • Shaogang Guo,2
Abstract Photosynthesis is the most important biochemical reaction on Earth.It has co-evolved and developed with the Earth,driving the biogeochemical cycle of all elements on the planet and serving as the only chemical process in nature that can convert light energy into chemical energy.Some heavy oxygen isotopic (18O) labeling experiments have‘‘conclusively’’ demonstrated that the oxygen released by photosynthesis comes only from water and are written into textbooks.However,it is not difficult to find that bicarbonate has never been excluded from the direct substrate of photosynthesis from beginning to end during the history of photosynthesis research.No convincing mechanism can be used to explain photosynthetic oxygen evolution solely from water photolysis.The bicarbonate effect,the Dole effect,the thermodynamic convenience of bicarbonate photolysis,the crystal structure characteristics of photosystem II,and the reinterpretation of heavy oxygen isotopic labeling (18O)experiments all indicate that the photosynthetic oxygen evolution does not exclude the important role and contribution of bicarbonate photolysis.The recently proposed view that bicarbonate photolysis is the premise of water photolysis,bicarbonate photolysis and water photolysis work together with a 1:1 (mol/mol) stoichiometric relationship,and the stoichiometric relationship between oxygen and carbon dioxide released during photosynthetic oxygen evolution is also 1:1,has excellent applicability and objectivity,which can logically and reasonably explain the precise coordination between light and dark reactions during photosynthesis,the bicarbonate effect,the Dole effect,the Kok cycle and the neutrality of water and carbon in nature.This is of great significance for constructing the bionic artificial photosynthetic reactors and scientifically answering the question of the source of elemental stoichiometric relationships in nature.
Keywords Bicarbonate effect ∙Dole effect ∙Kok cycle ∙Heavy oxygen isotope ∙Artificial photosynthetic reactor
1 Introduction
Photosynthesis is the only chemical process on Earth that converts light energy into chemical energy.Along with the evolution and development of the Earth,it drives the biogeochemical cycle of all the elements on the planet.The widely accepted view is that the photosynthetic oxygen evolution comes from water,not from inorganic carbon.However,from the photosynthesisdiscovery,photosynthetic oxygen evolution experiments and the structural characteristics of the photosystem II in the oxygen-evolving center,it can be seen that the photosynthetic oxygen evolution can never exclude the important role and contribution of bicarbonate photolysis.From the thermodynamic convenience of bicarbonate photolysis,the overall equation of photosynthesis,the earth’s own water/carbon balance and the Dole effect,bicarbonate photolysis dominates the photosynthetic oxygen evolution,and bicarbonate photolysis and water photolysis play a role in photosynthetic oxygen evolution with a 1:1(mol/mol)stoichiometric relationship(Wu 2023).
2 Photosynthetic inorganic carbon assimilation does not preclude bicarbonate assimilation
Although it is widely accepted that the oxygen evolution during photosynthesis comes from water rather than inorganic carbon (Shevela et al.2023),However,from the history of photosynthesis research,it is not difficult to find that people have never excluded bicarbonate from the direct substrate of photosynthesis.In Van Helmont’s experiment,not only rain or distilled water itself could nourish the willow tree,but also the bicarbonate dissolved in it.In the experiments of Ingen-Housz and Senebier,leaves submerged in solutions could absorb not only carbon dioxide but also but also bicarbonates dissolved in water.Similarly,in de Saussure’s experiments,the additional weight gained by plants could come from water or bicarbonate dissolved therein(Govindjee et al.2006;Wu and Rao 2023).Not only aquatic plants such as algae can directly utilize bicarbonate(Yamano et al.2015;Xie and Wu 2017),but also terrestrial plants have numerous evidences that they can directly use root-derived bicarbonate (Gao and Zhou 2001;Wu and Xing 2012;Rao and Wu 2017).
3 Photosynthetic oxygen evolution does not exclude bicarbonate photolysis
Although the experiment using heavy oxygen isotopic(18O) labeling by Ruben et al.(1941) ‘‘conclusively’’proved that the oxygen release during photosynthesis came solely from water (Ruben et al.1941)and was written into textbooks.However,there is still a lack of convincing explanation for the precise mechanism of solely water photolysis to account for the oxygen evolution during photosystem II.Especially,‘‘whether the photosynthetic oxygen-evolving center in the S3→S4→S0transition state of the Kok cycle is bound to water or not’’ lacks theoretical(large energy barrier problem)and experimental basis (S4state is not directly observed) (Guo et al.2023;Song and Wang 2023;Song et al.2023).Moreover,more and more experimental evidence also proves that the Hill reaction increases the amount of oxygen release several times or even more than ten times under the stimulation of bicarbonate (bicarbonate effect).The standard free energy of bicarbonate photolysis (H++HCO3-→1/2O2-+2e-+2H++CO2) (24.8 kcal/mol) significantly less than that of water photolysis (H2O →1/2O2+2e--+2H+) (37.3 kcal/mol) (Dismukes et al.2001).These have raised serious doubts about that the photosynthetic oxygen evolution comes solely from water (Warburg and Krippahl 1958;Stemler and Radmer 1975;Govindjee et al.2006;Wu 2021a).
Photosynthetic oxygen evolution is the physiological response of plants under specific physiological conditions.These experiments of heavy oxygen isotopic(18O)labeling to conclusively prove oxygen released solely from water(Ruben et al.1941;Stemler and Radmer 1975;Radmer and Ollinger 1980;Clausen et al.2005;Hillier et al.2006),the hydroxylamine treatment experiments(Shevela et al.2008;Ulas et al.2008),the analysis of Fourier transform infrared difference spectroscopy (Aoyama et al.2008),and the resolution of crystal structures in photosystem II(Loll et al.2005;Umena et al.2011;Bhowmick et al 2023)have been conducted under non-actual physiological conditions,their results cannot rule out the possibility of oxygen released from bicarbonate photolysis under actual physiological conditions.
Chloroplasts possess diverse carbonic anhydrases isoenzymes,thylakoid carbonic anhydrase has the characteristics of photosystem II,photosystem II exhibits carbonic anhydrase activity,and dehydration and hydration of thylakoid carbonic anhydrase depends on pH.The above characteristics of photosystem II and thylakoid carbonic anhydrase make oxygen of OH,COOH,O–O,C=O and other groups exchange rapidly with that of water in the photosynthetic oxygen-evolving system.The oxygen of labeled bicarbonate from heavy oxygen isotopic (18O)labeling experiments conducted by Ruben et al.(1941)and Stemler and Radmer (1975) is almost completely exchanged with that of other groups,so the isotope composition of oxygen released are consistent with that of water (Wu 2021a).Even so,it is remain uncertain whether it came solely from water or from bicarbonate because18O of bicarbonate had be exchanged almost entirely with16O of water in these experiments.
The degree to which bicarbonate binds to the photosynthetic oxygen-evolving center depends on physiological conditions (Bearden and Malkin 1973;Stemler and Murphy 1983;Bowden et al.1991;Moubarak-Milad and Stemler 1994;Lu and Stemler 2007;Kozlov et al.2010;Tikhonov et al.2018;Shevela et al.2020).The18O-labeled experiments of saturated flash illuminating dark-adapted chloroplasts demonstrated that the released oxygen was not related to exogenous added bicarbonates (Radmer and Ollinger 1980;Clausen et al.2005;Hillier et al.2006),but it cannot be ruled out that the oxygen in these experiments came from bicarbonates,because exogenous added bicarbonates are difficult to reach the photosystem II oxygenevolving center under saturated flash illumination (Wu 2023).Therefore,the oxygen release of photosystem II is independent of exogenous bicarbonate,but the bound-bicarbonate photolysis of photosystem II is not excluded,which can be supported by the fact that the photosynthetic oxygen evolution of photosystem II under a short-time saturated flash is less than that under the continuous illumination by a factor of 10(Radmer and Ollinger 1980),and the binding of bicarbonate to photosystem II is controlled by pH and redox potential (Stemler and Murphy 1983;Kozlov et al.2010).
Fourier transform infrared difference spectroscopy analysis shows the similarity between the H12CO3–-minus-H13CO3–double difference spectra of photosystem II oxygen-evolving center and that of photosystem II non-heme iron under first flash illumination.However,it cannot be ruled out that bicarbonate of the photosystem II oxygenevolving center,which treated by a lower pH(pH 6.0)prior to infrared spectral determination,may have been dissociated by weak acids(Aoyama et al.2008).The absence of CO2release from the photosystem II oxygen-evolving center by hydroxylamine destruction does not exclude the binding of bicarbonate to the photosystem II oxygenevolving center under physiological conditions,as the binding of bicarbonate to the photosystem II oxygenevolving center may have been liberated by a weak acid environment (pH 6.0) prior to hydroxylamine treatment in their experiments (Shevela et al.2008;Ulas et al.2008).
Similarly,Loll et al.(2005),Umena et al.(2011) and Bhowmick et al.(2023) did not observe the binding of bicarbonate to the oxygen-evolving center of photosystem II crystals grown at pH lower than 7.0,which does not rule out the binding of bicarbonate to the oxygen-evolving center of photosystem II under physiological conditions.The binding probability of bicarbonate to the oxygenevolving center of photosystem II crystals grown at lower pH is significantly lower than that grown at pH 7.5 (Ferreira et al.2004).The binding of bicarbonate to the oxygen-evolving center is most likely not observed in photosystem II crystals grown at pH below 7.0,while this binding can be observed in the crystal structure of photosystem II grown at pH 7.5 with a high probability(Ferreira et al.2004).The binding of the oxygen-evolving center of photosystem II and bicarbonate may depend on the physiological environment of crystal growth such as pH and redox potential,and plant physiological environment may determine the photosynthetic function.
If water photolysis of plants is the sole source of atmospheric O2,and the concentration of O2in the atmosphere remains essentially unchanged on geological scales,and seawater constitutes the vast majority of Earth’s water,then the content of18O in atmospheric O2should be consistent with that in seawater.However,paradoxically,the18O content of O2in the atmosphere is significantly higher than that of water in ocean and lower than that of CO2dissolved in seawater.The enrichment of18O in atmospheric O2is nearly 24‰ higher than that in seawater(currently 23.5‰),and this enrichment of18O is known as the Dole effect (Dole 1935;Dole and Jenks 1944).
Many scientists have attempted to analyze the causes of the Dole effect by studying the fractionation of oxygen isotopes in the nature,but ultimately failed to identify the main contributor to the Dole effect other than photosynthetic oxygen evolution.The transportation of photosynthetic oxygen released by plants hardly undergoes isotopic fractionation(Guy et al.1993).The global18O enrichment of water in leaves relative to seawater is only 4.4‰ (Farquhar et al.1993).Even considering global canopy transpiration,the reduction of the Dole effect induced by terrestrial vegetation is very small (0.3‰–0.4‰) (Bender et al.1994);the ratio of global terrestrial and oceanic primary production remains between 1.8 and 1.0,which does not affect the Dole effect in the mid-Holocene(Beerling 1999).The isotopic fractionation caused by respiration and diffusion in soil is also small,with the δ18O content of soil O2ranging from -1.6‰ to 0.06‰.Even considering global soil respiration,it would only reduce the Dole effect by 1–1.5 ‰ (Angert and Luz 2001;Angert et al.2001,2003).The photochemical reaction between stratospheric CO2and O2causes isotopic exchange,reducing the δ18O of atmospheric O2by only 0.4 ‰(Bender et al.1994).Overall,when scientists consider all factors (chemical,physical,and biological) on a global scale,the18O enrichment of atmospheric O2relative to that of seawater (Dole effect) remains at 21–24 ‰ (Bender et al.1994;Hoffmann et al.2004;Mader et al.2017).Therefore,we can’t help but question whether the photosynthetic oxygen released by plants only comes from water(Metzner 1975;Wu 2023).
4 Bicarbonate photolysis and water photolysis has a 1:1 (mol/mol) stoichiometric relationship
Any evidence that water is the sole substrate for photosynthetic oxygen evolution is becoming increasingly indefensible in the face of new knowledge and discoveries.Since it cannot be ruled out that bicarbonate photolysis occurs in photosynthetic oxygen evolution,does it mean that bicarbonate photolysis and water photolysis coexist in the process of photosynthetic oxygen evolution? What is the stoichiometric relationship between bicarbonate photolysis and water photolysis?
Although the fact that photosynthetic oxygen release is several times higher under stimulation by bicarbonate indicates that bicarbonate photolysis dominates and plays an absolute superior role in photosynthetic oxygen evolution (Warburg and Krippahl 1958;Stemler and Radmer 1975).However,the fact that a very small amount of oxygen in HC18O3-labeling experiments comes from labeled HC18O3-seems to indicate that bicarbonate photolysis accounts for a small share,and the role ofbicarbonate photolysis in photosynthetic oxygen release is minimal or even negligible (Hillier et al.2006).To correctly understand the process and mechanism of photosynthetic oxygen evolution,we need to discover the natural laws from these two contradictory ‘‘appearances’’ and eliminate the false while preserving the true,and understand them from the surface to the essence.
The standard free energy of bicarbonate photolysis to release oxygen (24.8 kcal/mol) is significantly lower than that of water photolysis to release oxygen (37.3 kcal/mol),indicating that bicarbonate photolysis has a thermodynamic advantage (Dismukes et al.2001).In the overall equation of photosynthesis,CO2+2H2O (H2O+H++HCO3-)+light chlorophyll→C(H2O)+O2,it can be seen that if bicarbonate photolysis produces CO2,which then enters the dark reaction (carbon dioxide concentrating mechanisms (CCMs) in photosystem II) (Wu 2021b),maintaining the balance of the chemical reactions requires a stoichiometric relationship of 1:1 mol/mol between bicarbonate photolysis and water photolysis.Additionally,from the perspective of the Earth’s own water and carbon balance,the stoichiometric relationship between bicarbonate photolysis and water photolysis is also 1:1 mol/mol(Wu and Wu 2022;Wu 2023).
In addition,the chemical stoichiometry of bicarbonate photolysis and water photolysis can be obtained from the oxygen isotopic composition from the atmospheric O2,water and bicarbonate dissolved in seawater.Table 1 shows the δ18O and18O content of different components in the atmosphere and seawater (data from Metzner 1975).If the oxygen in the atmosphere comes only from water,the δ18O and18O content of water in the ocean should be close to those in the atmosphere.However,in fact,the difference between δ18O of water in the ocean and that in the atmosphere is 21‰ to 24‰,which is called the Dole effect(Mader et al.2017).If the oxygen in the atmosphere comes from both water and bicarbonate dissolved in water,we can calculate the proportion of bicarbonate photolysis to total photosynthetic oxygen evolution with different degrees of oxygen isotope exchange between carbon dioxide and bicarbonate ion (maximum exchange,medium exchange,and 0 exchange)according to the data in Table 1,which are 42.59%,48.42% and 55.42%,respectively.When the degree of oxygen isotope exchange between carbon dioxide and bicarbonate reaches 42%,the stoichiometric relationship between bicarbonate photolysis and water photolysis is 1:1 (mol/mol).
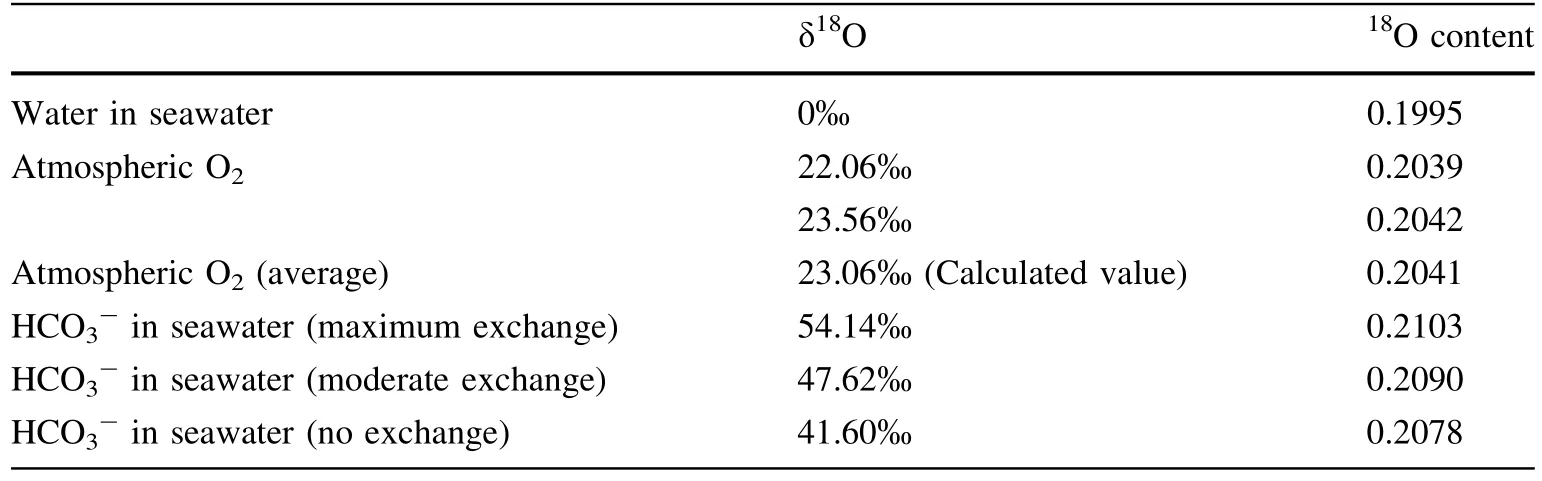
Table 1 δ18O and 18O content of different components in the atmosphere and seawater (data from Metzner 1975) (maximum oxygen exchange coefficient between carbon dioxide and bicarbonate is 1.012,data from Reid and Urey (1943))
If we calculate the enrichment of18O in atmospheric O2based on the assumption that bicarbonate photolysis and water photolysis account for half of the photosynthetic oxygen production,the results are 0.2036 (no exchange),0.2043 (moderate exchange),and 0.2049 (maximum exchange),respectively.When the degree of oxygen isotope exchange between CO2and HCO3-is moderate,the calculated value (0.2043) is very close to the observed average value (0.2041).
5 Conclusion and outlook
From the history of photosynthesis discovery,the mechanism of known photosynthetic oxygen evolution,the thermodynamic characteristics of bicarbonate photolysis and water photolysis,the general equation of photosynthesis,the water and carbon balance in nature,and the verification calculation based on the oxygen isotopic composition of O2in the atmosphere,water in seawater,and bicarbonate dissolved in seawater,it can be concluded that plants can assimilate bicarbonate,bicarbonate photolysis is the prerequisite for water photolysis,and bicarbonate photolysis and water photolysis work together in a 1:1 (mol/mol)stoichiometric relationship.The stoichiometric relationship between oxygen and carbon dioxide released during photosynthetic oxygen evolution is also 1:1.
In the future,we can use the dual-element bidirectional isotope tracer (culture) technology to obtain evidence and stoichiometric relationship between bicarbonate photolysis and water photolysis,confirm the occurrence of bicarbonate photolysis events by investigating the crystal structure of photosystem II in a simulated physiological dynamic environment,and further verify the stoichiometric relationship between bicarbonate photolysis and water photolysis in photosynthetic oxygen release by measuring the tri-oxygen isotopic composition of water in ocean,bicarbonate dissolved in seawater and atmospheric oxygen in the biosphere,challenging the traditional view of oxygen evolved only from water splitting.The regulation of photosynthesis by inorganic carbon is extended to the photoreaction stage,which provides a theoretical basis for revising the chapter on photosynthesis in textbooks and helping people reconsider the mechanism of photosynthesis.
AcknowledgementsWe thank the Support Plan Projects of Science and Technology Department of Guizhou Province [No.(2021)YB453].
Declarations
Conflict of interestThe author declares no conflict of interest.
杂志排行
Acta Geochimica的其它文章
- Geochemical modeling to aid experimental design for multiple isotope tracer studies of coupled dissolution and precipitation reaction kinetics
- Mafic and felsic magmatism in the Wadi Kalalat area,South Eastern Desert,Egypt:mineralogy,geochemistry and geodynamic evolution during the Neoproterozoic in the Nubian Shield
- The co-transport of Cd(II) and nZnO in saturated soil packed column: effects of ionic strength and pH
- Facies development and sedimentology of the Middle Miocene carbonates of the Raghama Formation,northeastern Saudi Arabia
- Petrogenesis and tectonic implications of the Silurian adakitic granitoids in the eastern segment of the Qilian Orogenic Belt,Northwest China
- Distribution,health and ecological risk assessments of trace elements in Nigerian oil sands
