Effects of Tuina static training on vascular endothelial cell dysfunction and adiponectin in obese rats
2024-02-27LITing李婷XIEZhouyu谢舟煜WEIJuan魏娟XIEYing谢颖WUYunchuan吴云川
LI Ting (李婷), XIE Zhouyu (谢舟煜), WEI Juan (魏娟), XIE Ying (谢颖), WU Yunchuan (吴云川)
School of Acupuncture-Moxibustion and Tuina of Nanjing University of Chinese Medicine, Nanjing 210023, China
Abstract Objective: To observe the effects and explore the mechanism of Tuina (Chinese therapeutic massage) static training on vascular endothelial cell dysfunction and adiponectin (APN) in obese rats.
Keywords: Tuina; Massage; Static Training; Static Qigong; Obesity; Vascular Endothelial Cells; Adiponectin; Rats
Obesity is a kind of endocrine and metabolic disorder with fat accumulation and body mass increase as the main manifestations, and it is also one of the main causes of cardiovascular diseases[1].Obesity usually destroys lipid metabolism and damages vascular endothelial cells, resulting in endothelial cell dysfunction[2].Vascular endothelial cell dysfunction leads to inflammatory reactions in blood vessels, thus aggravating atherosclerosis and causing cardiovascular diseases[2-3].Therefore, it is very important to reduce obesity and improve vascular endothelial cell dysfunction for the prevention and treatment of cardiovascular diseases.
Adipose tissue is not only an energy storage tissue but also an important endocrine organ.It can secrete many cytokines and adipose factors, thus regulating and balancing endothelial function.In addition, fat accumulation caused by obesity is easy to cause chronic inflammation and abnormal adipose factor secretion by adipose tissues[2].Adiponectin (APN), an anti-obesity factor secreted by adipocytes, regulates glucose metabolism and lipid metabolism, protects vascular endothelium, and reduces the incidence of cardiovascular diseases[4].
As a traditional exercise method of traditional Chinese medicine, Tuina (Chinese therapeutic massage)Gongfa plays an important role in the prevention,treatment, and rehabilitation of cardiovascular diseases.For example, Ba Duan Jin (Eight-section Exercise)consumes fat and promotes metabolism in obese patients[5].Wu Qin Xi (Five Mimic-animal Exercise)reduces blood pressure and prevents cardiovascular diseases without adverse reactions[6].Static training, the core movement of Tuina, is a training method that exerts still force without changing the position and shape of muscles.This training enhances the strength of core muscles and improves balance ability.Studies have shown that static training reduces obesity and the risk of atherosclerosis[7].However, the effect of static training on vascular endothelial cell dysfunction and APN is not clear.Therefore, we used static training to intervene in obese rats here for exploring the effect of Tuina static training on vascular endothelial cell dysfunction and APN in obese rats, as well as the mechanism, so as to provide basis for the prevention and treatment of cardiovascular diseases by Tuina.
1 Materials and Methods
1.1 Laboratory animals
Forty healthy male Sprague-Dawley (SD) rats, 7 weeks old, with a body mass of (200±20) g, were provided by Qinglongshan Animal Breeding Farm in Nanjing, and the animal license number was SCXK (Su)2017-0001.Rats were reared in a specific-pathogen-free barrier at the Laboratory Animal Center of Nanjing University of Chinese Medicine with the feeding environment: alternating light and shade of 12 h/12h,temperature of 20-22 ℃, and relative humidity of 45%-50%.The experimental scheme was approved by the Ethics Committee of Animal Experiment Center of Nanjing University of Chinese Medicine (Animal Ethics Approval No.202011A012).
1.2 Main reagents and instruments
Triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), and high-density lipoprotein (HDL)determination kits (Cat.No.S03027, No.S03042,No.S03029, No.S03025, Shenzhen Rayto Life and Analytical Sciences Co., Ltd., China); rat nitric oxide (NO)detection kit, rat endothelin-1 (ET-1), and APN enzymelinked immunosorbent assay (ELISA) kits (Cat.No.ZC-S0374, No.ZC-37024, No.ZC-37610, Shanghai Zhuocai Biotechnology Co., Ltd., China); rat soluble thrombomodulin (sTM) ELISA kit (Cat.No.BH-R101192,Shanghai Bohu Biotechnology Co., Ltd., China).
MICROCL21R centrifuge (Thermo Fisher Scientific Inc.,USA); Chemray 800 automatic biochemical analyzer(Shenzhen Rayto Life and Analytical Sciences Co., Ltd.,China); Epoch microplate reader (BioTeK, USA); RM2235 microtome and DM-1000 microscope (Leica, Germany).
1.3 Animal grouping and modeling
After 1 week of routine adaptive feeding, 8 of the 40 SD rats were randomly selected as a normal group, and the rest of the rats were made obesity models.The normal group was fed a common diet, while the model group was fed a high-fat diet, containing 45% fat, 35%carbohydrate, and 20% protein[8].All rats were weighed after 8 weeks of feeding.
Obesity modeling standard: The body mass of model rats exceeded that of normal rats by 20%[9].After successful modeling, obese SD rats were randomly divided into three groups: a model group, an aerobic exercise group, and a static training group, with 8 rats in each group.
1.4 Interventions
Normal group: Rats in the normal group were fed the common feed without other intervention during the entire 8-week experiment.
Model group: Rats in the model group were fed a high-fat diet without other intervention during the entire 8-week experiment.
Aerobic exercise group: Rats in the aerobic exercise group were fed a high-fat diet with aerobic exercise intervention during the entire 8-week experiment.
The animal experimental treadmill with a slope of 0°was used for weight-free running training according to Bedford’s animal exercise load standard[10]and the reported aerobic exercise scheme[9,11].Moderateintensity aerobic exercises were applied: 15 m/min for 60 min as a session, 6-day intervention with a 1-day rest in one week for 8 consecutive weeks.
Static training group: Rats in the static training group ate a high-fat diet throughout the entire experiment and carried out static training.A self-made static training device was made referring to the reported static training experimental scheme[12-13].Hanged the rats upside down by tying their hind feet to the crossbar.Then, the rats flexed their upper limbs and head forward to move closer to their hind limbs with their body being curled.At this time, the whole-body muscles of the rats were in a static force status.Tempted the rats with food to keep them in a static training status if they recovered upside down.The training lasted for 30 min each time, with 6-day interventions and a 1-day rest in one week for 8 consecutive weeks.
1.5 Sample collection
The rats fasted for 12 h after the last intervention.The 20% urethane was prepared for intraperitoneal injection anesthesia at a dosage of 7 mL/(kg·bw).After anesthesia, 5 mL of blood was collected from the aorta and centrifuged at 3 500 r/min for 15 min.The upper serum was harvested and stored in a refrigerator at-80 ℃.The epicardial adipose tissue, perirenal fat, and peri-epididymal fat were separated, weighed, and stored in the refrigerator at -80 ℃.The thoracic aorta was separated, and the connective tissue was stripped off in normal saline.After rinsing, a 3 mm segment containing the aortic arch was cut and fixed with 4%paraformaldehyde.
1.6 Observation items
1.6.1 General situation
Weighed and recorded the body mass, and measured the body length (the distance from nasal tip to anus) to calculate the Lee’s index of rats in each group every week[14-15].After sample collection, the perirenal fat and peri-epididymal fat were weighed, and the fat/body mass ratio was calculated[16].
Lee’s index = [Body mass (g)1/3× 1 000] ÷ Body length(cm).
Lipid/body mass ratio = [Peri-epididymal fat mass (g)+ Perirenal fat mass (g)] ÷ Body mass (g) × 100%.
1.6.2 Morphological observation of aorta
The aortic arch was taken out in paraformaldehyde fixative solution, and the aortic arch tissue of 3 mm was cut, repaired, dehydrated, paraffin-embedded,sectioned, baked, dewaxed, rehydrated, washed,stained with hematoxylin-eosin, dehydrated, treated with transparency, and sealed with neutral resin in turn.The morphological changes of aorta were observed under the microscope and photographed.
1.6.3 Lipid metabolism indicators
Serum from the -80 ℃ refrigerator was subject to TG,TC, LDL, and HDL determination with kits following the instructions under the Chemray 800 automatic biochemical analyzer.The data were derived and the rat serum levels of TG, TC, LDL, and HDL in each group were calculated according to the formula in the instruction manual.
1.6.4 Serum levels of NO, ET-1, sTM, and APN detected by ELISA
Serum from the -80 ℃ refrigerator was loaded,followed by enzyme administration, incubation, liquid preparation, washing, color development, and detection according to the instructions of NO, ET-1, sTM,and APN ELISA kits.The absorbance of each sample was determined by the microplate reader in sequence.The standard curve was drawn with the concentration of the standard substance as the ordinate and the optical density value as the abscissa, and the corresponding levels of NO, ET-1, sTM, and APN were calculated.
1.7 Statistical analysis
All data were in accordance with normal distribution and expressed as mean ± standard deviation (±s).Pairedt-test was used in intra-group comparisons before and after intervention.One-way analysis of variance was used to compare the data of multiple groups.Bonferroni method was used to correct the difference between groups when the variance was homogeneous, and Tamhane’s method was used to correct the difference when the variance was uneven.Pearson correlation was used to analyze the correlation between indexes.P<0.05 indicated that the difference was statistically significant.
2 Results
2.1 Morphological observation of rat aorta in each group
As shown in Figure 1, the aorta structure was intact,and the endothelial cells were not damaged in the normal group; the aortic wall was loose, the endothelial cells were damaged and shed in the model group; the aorta shape was basically similar, and the endothelial cell injury was not obvious in the aerobic exercise group and the static training group, but the static training group was slightly better than the aerobic exercise group.
2.2 Comparison of body mass, body length, and Lee’s index of rats among groups
The body mass of the model group increased linearly and was higher than that of the normal group, while the body mass of the aerobic exercise group and the static training group changed less significantly than that of the normal group and the model group during the intervention (Figure 2-Figure 4).The body length of the model group increased gradually and was longer than that of the normal group, while the body length growth range of the aerobic exercise group and the static training group was smaller than that of the model group but larger than that of the normal group.The Lee’s index in the normal group and the model group first decreased, then increased, and then decreased, while the Lee’s index in the aerobic exercise group and the static training group showed a downward trend.

Figure 3 Changes in rat body length in each group during intervention (n=8)
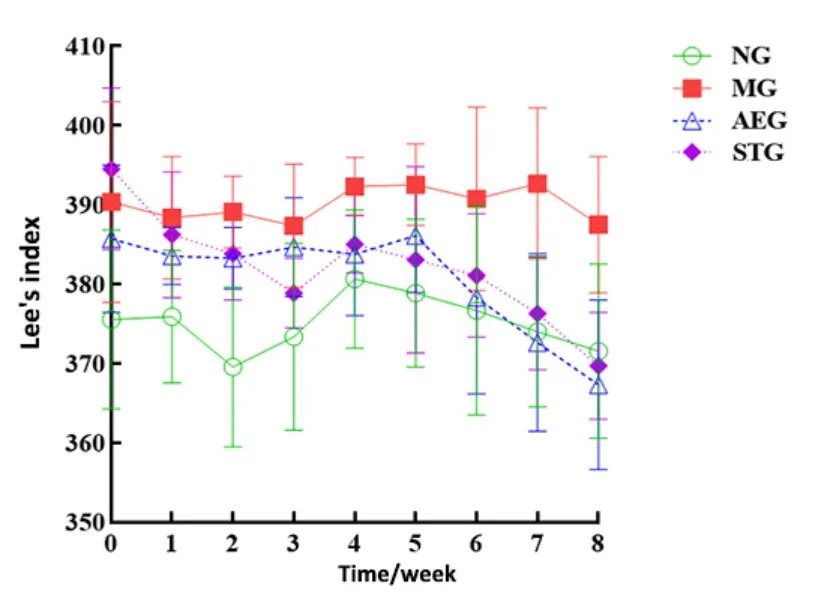
Figure 4 Changes in Lee’s index in each group during intervention (n=8)
After intervention, compared with the normal group,the body mass, body length, and Lee’s index of the model group increased significantly (P<0.01 orP<0.05);compared with the model group, the body mass and Lee’s index of the aerobic exercise group and the static training group decreased significantly (P<0.01), but there was no significant difference in the Lee’s index and body length between the aerobic exercise group and the static training group (P>0.05).Compared with before intervention, the body mass and body length increased significantly (P<0.01), while the Lee’s index decreased without statistical significance in the normal group and the model group (P>0.05); there was no statistical significance in the body mass change(P>0.05), but the body length increased significantly(P<0.01), and the Lee’s index decreased significantly(P<0.01) in the aerobic exercise group and the static training group.See Table 1.
采用LogMAR视力表检查裸眼视力(UCVA)、屈光状态及BCVA(1%阿托品睫状肌麻痹后矫正再进行视力检查);裂隙灯显微镜及眼底照相检查排除眼部器质性疾病;遮盖-去遮盖检查、交替遮盖检查及眼球运动检查排除显斜视及眼球运动障碍疾病;同视机(L-2510B,日本Inami公司)检查远立体视;随机点立体视图谱(RDS图谱)检查近立体视。为便于分析,参考文献[5],将立体视进行赋值评分量化,见表1-2。
2.3 Comparison of rat perirenal fat mass,peri-epididymal fat mass, and fat/body mass ratio among groups
Compared with the normal group, the perirenal fat mass and fat/body mass ratio of the model group, the aerobic exercise group, and the static training group increased obviously (P<0.01), and the peri-epididymal fat mass of the model group and the aerobic exercise group increased obviously (P<0.01), while the peri-epididymal fat mass of the static training group was slightly larger without significant difference.Compared with the model group, the perirenal fat mass and fat/body mass ratio of the aerobic exercise group and the static training group were significantly smaller(P<0.01); the peri-epididymal fat mass of the aerobic exercise group (P<0.05) and the static training group(P<0.01) was significantly smaller.See Table 2.
Table 1 Comparison of the body mass, body length, and Lee’s index before and after intervention ( ±s)
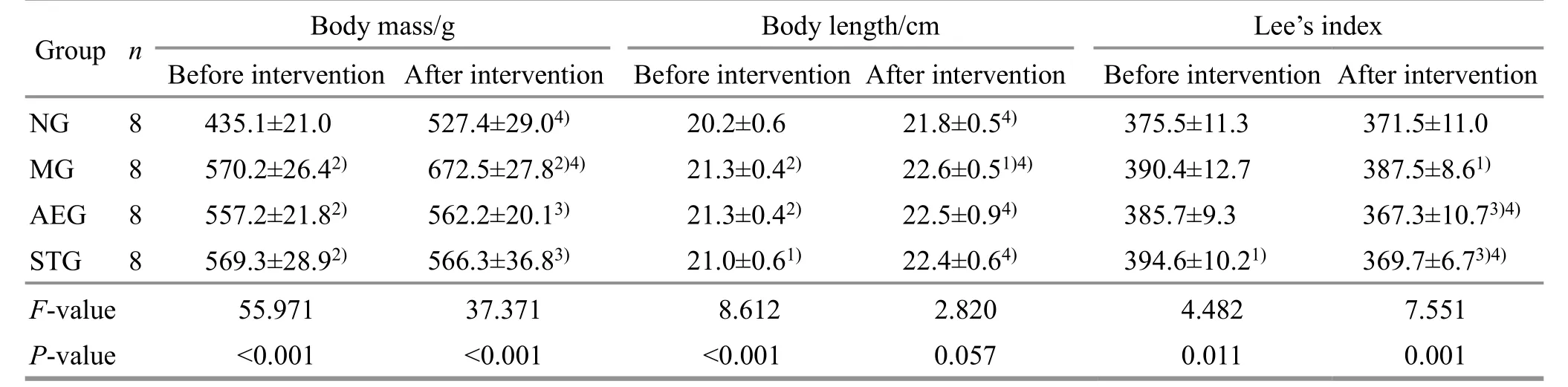
Table 1 Comparison of the body mass, body length, and Lee’s index before and after intervention ( ±s)
Note: NG=Normal group; MG=Model group; AEG=Aerobic exercise group; STG=Static training group; compared with the normal group, 1) P<0.05, 2) P<0.01; compared with the model group, 3) P<0.01; compared with the same group before intervention, 4) P<0.01.
Group n Body mass/g Body length/cm Lee’s index Before intervention After intervention Before intervention After intervention Before intervention After intervention NG 8 435.1±21.0 527.4±29.04) 20.2±0.6 21.8±0.54) 375.5±11.3 371.5±11.0 MG 8 570.2±26.42) 672.5±27.82)4) 21.3±0.42) 22.6±0.51)4) 390.4±12.7 387.5±8.61)AEG 8 557.2±21.82) 562.2±20.13) 21.3±0.42) 22.5±0.94) 385.7±9.3 367.3±10.73)4)STG 8 569.3±28.92) 566.3±36.83) 21.0±0.61) 22.4±0.64) 394.6±10.21) 369.7±6.73)4)F-value 55.971 37.371 8.612 2.820 4.482 7.551 P-value <0.001 <0.001 <0.001 0.057 0.011 0.001
Table 2 Comparison of the perirenal fat mass, peri-epididymal fat mass and fat/body mass ratio of rats among groups ( ±s)
Note: NG=Normal group; MG=Model group; AEG=Aerobic exercise group; STG=Static training group; compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.05, 3) P<0.01.
Group n Perirenal fat mass/g Peri-epididymal fat mass/g Fat/body mass ratio/%NG 8 3.59±1.22 3.52±0.59 1.35±0.30 MG 8 15.20±1.961) 8.75±1.991) 3.56±0.381)AEG 8 8.84±1.181)3) 6.46±1.761)2) 2.71±0.371)3)STG 8 8.49±1.461)3) 5.49±1.213) 2.46±0.351)3)F-value 81.650 16.993 53.753 P-value <0.001 <0.001 <0.001
2.4 Blood lipid comparison
Compared with the normal group, the levels of TC and LDL were obviously reduced (P<0.01), the HDL level was obviously decreased (P<0.01), and the TG level was obviously increased (P<0.05) in the model group; the LDL levels were increased significantly (P<0.01), the HDL levels were significantly reduced (P<0.01), while the TG and TC levels were increased slightly without statistical significance in the aerobic exercise group and the static training group (P>0.05).See Table 3.
Compared with the model group, the levels of TC and LDL were significantly reduced (P<0.01), while the HDL levels were significantly increased in the aerobic exercise group and the static training group (P<0.01);the TG level in the aerobic exercise group was slightly lower without statistical significance (P>0.05), and it was significantly lower in the static training group(P<0.05).
2.5 Comparison of serum NO, ET-1, sTM, and APN among groups
Compared with the normal group, the levels of NO and APN in the model group were significantly reduced(P<0.01), while the levels of ET-1 and sTM in the model group and aerobic exercise group increased significantly(P<0.01); the levels of NO and APN in the aerobic exercise group were slightly lower without statistical significance (P>0.05); the level of ET-1 was significantly increased (P<0.05), while the levels of NO and sTM were slightly higher, and APN was slightly lower without statistical significance (P>0.05) in the static training group.Compared with the model group, the NO level increased significantly (P<0.05), and the ET-1 level decreased significantly (P<0.05) in the aerobic exercise group; the sTM level decreased significantly (P<0.01),the APN level increased significantly (P<0.01) in the aerobic exercise group and the static training group; the NO level increased significantly (P<0.01) and the ET-1 level decreased significantly (P<0.01) in the static training group.The NO level in the static training group was significantly higher than that in the aerobic exercise group (P<0.05).See Table 4.
2.6 Correlation between the serum APN and NO, ET-1,or sTM in obese rats
There was a significant positive correlation between APN and NO (r=0.55) and a significant negative correlation between APN and ET-1 (r=-0.58) or sTM(r=-0.73) in obese rats (P<0.01).See Figure 5-Figure 7.
Table 3 Comparison of blood lipid indicators of rats among groups ( ±s) Unit: mmol/L
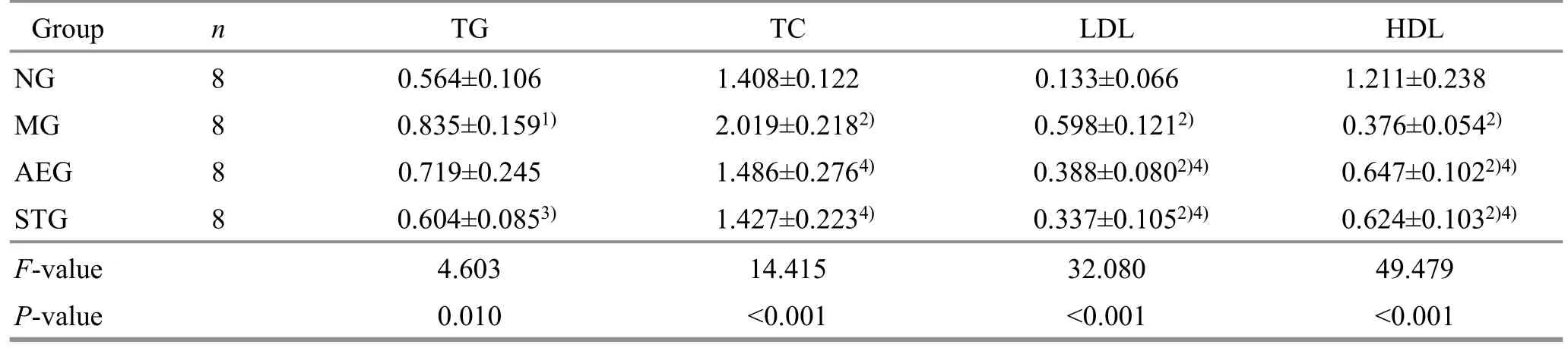
Table 3 Comparison of blood lipid indicators of rats among groups ( ±s) Unit: mmol/L
Note: NG=Normal group; MG=Model group; AEG=Aerobic exercise group; STG=Static training group; TG=Triglyceride; TC=Total cholesterol; LDL=Low-density lipoprotein; HDL=High-density lipoprotein; compared with the normal group, 1) P<0.05, 2) P<0.01;compared with the model group, 3) P<0.05, 4) P<0.01.
Group n TG TC LDL HDL NG 8 0.564±0.106 1.408±0.122 0.133±0.066 1.211±0.238 MG 8 0.835±0.1591) 2.019±0.2182) 0.598±0.1212) 0.376±0.0542)AEG 8 0.719±0.245 1.486±0.2764) 0.388±0.0802)4) 0.647±0.1022)4)STG 8 0.604±0.0853) 1.427±0.2234) 0.337±0.1052)4) 0.624±0.1032)4)F-value 4.603 14.415 32.080 49.479 P-value 0.010 <0.001 <0.001 <0.001
Table 4 Comparison of the serum NO, ET-1, sTM, and APN levels among groups ( ±s)
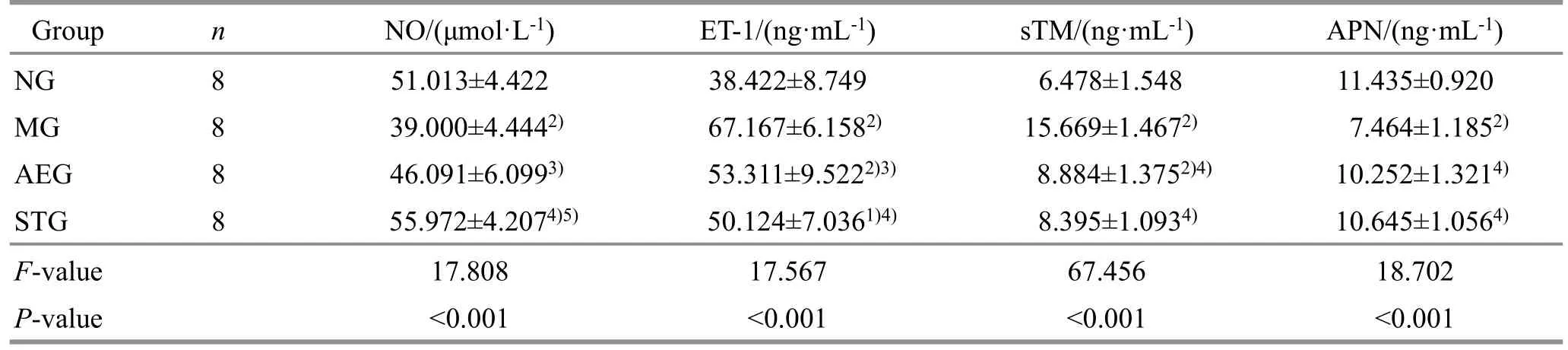
Table 4 Comparison of the serum NO, ET-1, sTM, and APN levels among groups ( ±s)
Note: NG=Normal group; MG=Model group; AEG=Aerobic exercise group; STG=Static training group; NO=Nitric oxide;ET-1=Endothelin-1; sTM=Soluble thrombomodulin; APN=Adiponectin; compared with the normal group, 1) P<0.05, 2) P<0.01;compared with the model group, 3) P<0.05, 4) P<0.01; compared with aerobic exercise group, 5) P<0.05.
Group n NO/(μmol·L-1) ET-1/(ng·mL-1) sTM/(ng·mL-1) APN/(ng·mL-1)NG 8 51.013±4.422 38.422±8.749 6.478±1.548 11.435±0.920 MG 8 39.000±4.4442) 67.167±6.1582) 15.669±1.4672) 7.464±1.1852)AEG 8 46.091±6.0993) 53.311±9.5222)3) 8.884±1.3752)4) 10.252±1.3214)STG 8 55.972±4.2074)5) 50.124±7.0361)4) 8.395±1.0934) 10.645±1.0564)F-value 17.808 17.567 67.456 18.702 P-value <0.001 <0.001 <0.001 <0.001
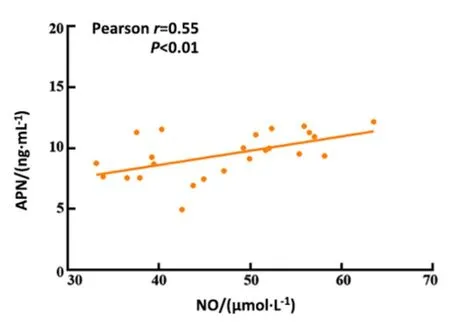
Figure 5 Correlation between APN and NO in obese rats
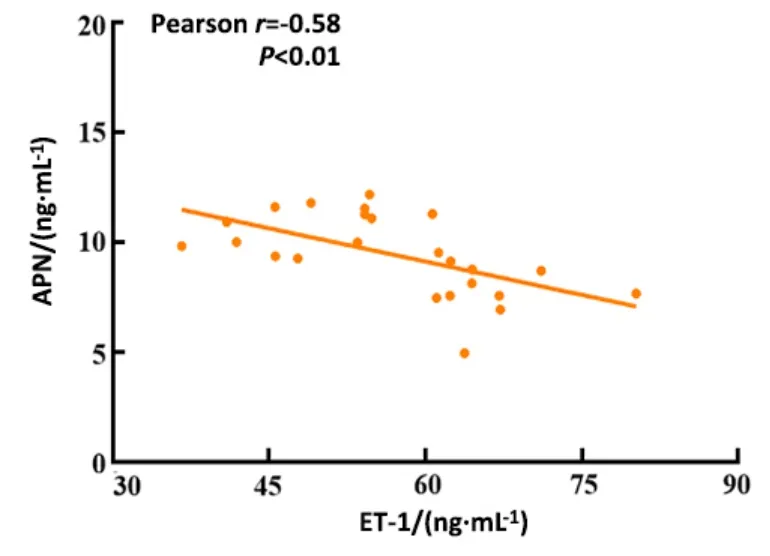
Figure 6 Correlation between APN and ET-1 in obese rats

Figure 7 Correlation between APN and sTM in obese rats
3 Discussion
Obesity usually causes dysfunction of glucose metabolism and lipid metabolism, inflammation, and oxidative stress, leading to vascular endothelial cell dysfunction[2].Vascular endothelial cell dysfunction caused by obesity is also the pathological basis of atherosclerosis, which leads to the occurrence of cardiovascular diseases[3].Tuina improves obesity,reduces body fat, regulates blood pressure, and has a certain therapeutic effect on vascular diseases[17].Therefore, whether the Tuina static training improves vascular endothelial cell dysfunction caused by obesity is of great significance to the prevention and treatment of cardiovascular diseases.In this study, the obese rats were given Tuina static training for 8 weeks to observe the effects on body mass increase, visceral fat mass increase, lipid metabolism dysfunction, vascular endothelial cell dysfunction-related factors, aortic morphological changes, and APN, so as to explore the therapeutic effect and mechanism of Tuina static training on obesity, and provide theoretical basis for clinical prevention and treatment of cardiovascular diseases with Tuina.
Excessive fat accumulation caused by obesity will hinder lipid metabolism and cause damage to vascular endothelial cells[3].Studies have shown that the lipid metabolism dysfunction caused by obesity is characterized by increased TG, TC, and LDL and decreased HDL, which can lead to vascular endothelial cell dysfunction[3,8].NO and ET-1, a pair of vasodilation and contraction regulators produced by endothelial cells, evaluate vasodilation and contraction function and indirectly reflect the injury degree of vascular endothelial cells.Obesity reduces NO utilization by endothelial cells, enhances the ET-1 expression,damages vasomotor function, and induces vascular endothelial cell dysfunction[2,21].In addition,thrombomodulin (TM) is an anticoagulant factor on the surface of vascular endothelial cells.When endothelial cells are injured and stimulated, TM will fall off and hydrolyze into sTM to enter the blood[22].Therefore,sTM is considered an important marker for the diagnosis and prognosis of vascular endothelial cell dysfunction[23].Besides, high-content sTM is closely related to atherosclerosis[24].Therefore, the progress of vascular endothelial cell dysfunction is directly reflected by the serum sTM level.Here we found that the body mass, body length, Lee’s index, perirenal fat mass, peri-epididymal fat mass, lipid/body mass ratio,ET-1, and sTM levels of obese rats were significantly higher, the NO level was significantly lower, and the aortic endothelial cells were scattered and damaged seriously in the model group than those in the normal group, which just explained that obesity would lead to lipid metabolism disorder, thus causing vascular endothelial cell dysfunction.
Obesity often leads to abnormal secretion of adipokines and induces vascular endothelial cell dysfunction.APN is an anti-inflammatory factor secreted by adipocytes.It is closely related to vascular endothelial cell dysfunction and plays a central role in the occurrence and development of obesity-related diseases such as type 2 diabetes, hyperlipidemia, and atherosclerosis[25-26].It has been found that APN reduces pro-inflammatory cytokines released by activating the cAMP/PKA signaling pathway to show an anti-inflammatory effect and regulate coronary microcirculation, thus alleviating vascular endothelial cell dysfunction[27-28].In addition, studies have shown that APN increases endothelial nitric oxide synthase(eNOS) phosphorylation, promotes NO production, and reduces ET-1 level through AMPK/eNOS signaling pathway, thus protecting vascular endothelium[29-32].The results of this study showed that the serum APN level in obese rats was significantly reduced, and the aortic endothelial cells were seriously damaged with shedding, which indicated that the lipid metabolism disorder caused by obesity would reduce the release of APN, resulting in the injury of aortic endothelial cells and the dysfunction of vascular endothelial cells.This suggests that APN is related to the occurrence and development of cardiovascular diseases.
Studies have found that exercise reduces fat accumulation, corrects lipid metabolism disorder,regulates endothelial systolic and diastolic function,protects vascular endothelial cells, improves obesity,and reduces the incidence of cardiovascular diseases[10,33-35].WANG X W,et al[36]found that aerobic exercise reduced the buttock subcutaneous and abdominal fat levels, and increased the serum APN level,indicating that APN secretion is correlated with fat mass decrease.Another study found that 8 weeks of aerobic exercise improved the serum APN level and reduced obesity[37].In addition, some studies have found that Tuina reduces the accumulation of glucose and lipids in rats and hinders cell dysfunction[38].GOKER A,et al[39]found that Tuina on the back increased breast milk production and the serum APN level in postpartum women.LEE K J,et al[40]have confirmed that abdominal Tuina regulates dyslipidemia, reduces fat levels, and prevents cardiovascular diseases in obese patients.
Our results showed that the body mass, Lee’s index,perirenal fat mass, peri-epididymal fat mass, lipid/body mass ratio, TG, TC, LDL, ET-1, and sTM levels were significantly reduced, while the levels of HDL, NO, and APN were significantly increased in the aerobic exercise group and the static training group compared with the model group; but the difference in the TG level was more obvious, and the NO level was significantly higher in the static training group than that in the aerobic exercise group.In addition, aortic endothelial cells were arranged relatively neatly with some injured endothelial cells in the aerobic exercise group, and the cells were arranged relatively neatly without obvious endothelial cell defects in the static training group, indicating that the injury degree of aortic endothelial cells in the static training group was lighter than that in the aerobic exercise group.At the same time, APN was negatively correlated with ET-1 and sTM and positively correlated with NO in obese rats, indicating that the function of aortic endothelial cells is closely related to APN.Increased APN secretion upregulated the NO level,reduced the levels of ET-1 and sTM, protected vascular endothelial cells, and thus hindered the occurrence of vascular endothelial cell dysfunction.
In conclusion, the changes in blood lipids and APN indirectly reflect the functional state of vascular endothelium.In this study, by monitoring the changes in blood lipids, APN, and other indicators in obese rats,we have found that static training can regulate lipid metabolism, balance fat factor secretion, improve vasomotor function, protect endothelial cells, and thus inhibit the process of endothelial dysfunction.Moreover,static training may protect vascular endothelial cells by correcting lipid metabolism disorder, promoting APN secretion, and regulating vasomotor function, and the effect of static training in improving lipid metabolism and vasomotor function and protecting vascular endothelial cells was more significant than aerobic exercise.
This study observed the effects of Tuina static training on the fat level, lipid metabolism, and aortic morphology in obese rats.It was found that Tuina static training improved vascular endothelium injury of aorta in obese rats, which may be related to regulating lipid metabolism and promoting APN secretion.Our results provide not only ideas and a basis for in-depth studies on the mechanism of Tuina intervention in cardiovascular diseases but also a theoretical basis for clinical prevention and treatment of cardiovascular diseases with Tuina.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Project of 2021 Jiangsu Graduate Research Innovation Plan (2021年江苏省研究生科研创新计划, No.KYCX21_1692).
Statement of Human and Animal Rights
The treatment of animals in this experiment conformed to the ethical criteria.
Received: 1 February 2023/Accepted: 28 August 2023
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Study on the mechanism of herb cake-partitioned moxibustion inhibiting tumor growth in colitis-associated colorectal cancer based on KDM4D receptor
- Effects of electroacupuncture on gut microbiota and related inflammatory factors in rats with Crohn disease
- Clinical study of electroacupuncture combined with exercise therapy in improving the balance function of patients with knee osteoarthritis
- Effects of warming triple needling plus Chinese medication on inflammatory responses and daily functioning ability in knee osteoarthritis patients
- Clinical observation of kidney-tonifying and mindcalming acupuncture therapy in the treatment of perimenopausal insomnia
- Clinical study of electroacupuncture improving sleep electroencephalogram and event-related potential in patients with somatoform disorders
