Structure of The BLUF Protein TePixD Y8F Mutant*
2024-02-26HURuiXingZHOUYaLinLINLinDINGBeiLUQing
HU Rui-Xing, ZHOU Ya-Lin, LIN Lin, DING Bei**, LU Qing**
(1)Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders (Ministry of Education), Bio-X Institutes,Shanghai Jiao Tong University, Shanghai 200230, China;2)Center for Ultrafast Science and Technology, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China)
Abstract Objective TePixD (Tll0078) is a blue light-using flavin (BLUF) photoreceptor protein from Thermosynechococcus elongatus BP-1. TePixD protein has a conserved Tyr8-Gln50-Met93 triad around the FAD pocket to mediate the proton-coupled electron transfer (PCET) process. But the detailed light response mechanism needs further study. We aimed to elucidate the structure and biochemical properties of TePixD mutants at key light response sites to analyze the light response process of TePixD.Methods We employed X-ray crystallography to resolve the crystal structure of the TePixD Y8F mutant. The side chain of Tyr8 is involved in PCET while Phe8 in mutation loses the function due to the loss of its hydroxyl group. We compared the structure of TePixD Y8F mutation to TePixD wild type (WT) and its homology protein SyPixD Y8F. Using multi-angle light scattering (MALS),we analyzed the oligomerization of multiple TePixD mutations (Y8F, Q50L, W91F, Y8F/W91F, and Q50L/W91F), focusing specifically on mutational sites that are critical residues for the protein’s photo response to dark and light conditions. Results We resolved the crystal structure of TePixD Y8F mutant at a resolution of 2.54 Å and found that it shares a similar overall structure with the TePixD WT but exhibits significant differences from the SyPixD Y8F structure. Biochemical analysis revealed differences in molecular mass and elution profiles between the TePixD mutants and the WT under dark and light conditions, indicating the perturbation on the light-induced conformational change by the mutants. Conclusion Our structure determination and biochemical analyses will add information to reveal the light response mechanism of BLUF proteins.
Key words TePixD, photoreceptor, oligomerization, light response
Photoreceptor proteins contain several protein families that respond to specific light absorption at limited wavelengths and intensities, then transduce the light signal downstream by conformational transitions[1]. Following the identification of a new kind of photosensory domain in the protein AppA(activation of photopigment andpucexpression) in 1995[2], a family of photoreceptors termed sensors of blue light using flavin (BLUF) was characterized[3].BLUF domain is distributed in a wide range of the proteobacteria and the cyanobacteria. Multiple members of BLUF family have been identified through the use of Basic Local Alignment Search Tool(BLAST) searches in protein and genome databases[4].BLUF is one of the four families of blue light photoreceptors, with light oxygen voltage (LOV),cryptochrome (CRY), and photoactive yellow protein(PYP)[5-8]. CRY, LOV and BLUF all use flavins as chromophores, while their photocycles are various: in the photolyase homology region (PHR) of CRY, stable flavin adenine dinucleotide (FAD) radical states are generated; in LOV domain, flavin mononucleotide(FMN) forms a covalent bond with a cysteine residue;and in BLUF domain, the hydrogen bond network around FAD is rearranged[9-11].
The BLUF domain is a conserved protein structure approximately 100 residues long,characterized by a βαββαββ fold, which revealed by several high-resolution X-ray crystallographic and NMR data sets[12-13]. BLUF domain proteins contain two categories: “Group I” proteins (or “long”proteins), containing multi-domains and appearing to be homodimers; “Group II” proteins (or “short”proteins), composed of a BLUF domain plus 30-70 additional residues. Among the conserved residues,those near the FAD pocket are particularly important,with Tyr8, Gln50, and Met93 being crucial for the light-driven PCET process[14-15]. Although various models have been proposed to explain the dynamic light-induced processes, the details need further studies. The crystal structure represents a static snapshot, and the packing of crystal or molecular heterogeneity in solution can be challenging to capture the dynamic light-response procedures accurately[16]. To reveal the dynamic mechanisms and chemical properties, computational molecular physics(CMP) is blooming, comprehensive applying quantum mechanics (QM) and molecular mechanics (MM) to describe molecular dynamics (MD) details and explain experimental data[17]. However, the accuracy and convergence of the calculated properties depend on the accuracy of the input structural data[18]. In this case, experimental structural data remains crucial for providing the basis for mechanism modeling and validating the computed models.
TePixD is a “short” BLUF protein derived fromThermosynechococcus elongatusBP-1, consisting of 143 residues[19]. The crystal structure of TePixD was determined as a decamer assembly, where five monomers array in a ring to form a pentamer, and two pentamers are positioned face-to-face to form a decamer[12]. In our study, we crystallized several mutations of TePixD, and solved the high-resolution crystal structure of TePixD Y8F. The structure of a similar mutation, Y8F in SyPixD (Synechocystispositive phototaxis factor, 43% sequence identity with TePixD) was reported to be locked at a pseudo-light state[20]. Through the comparison of the crystal structures of TePixD Y8F with TePixD WT and SyPixD Y8F, along with biochemical characterization of TePixD mutations under dark and light conditions,we were able to capture the light-induced conformational change of BLUF domain involving Tyr8. Our research provides new insights into the molecular dynamics of the light response of BLUF proteins.
1 Methods
1.1 Constructs and protein expression
The coding sequence ofTePixD(WP_011055933.1, residues 1-143) was PCR-amplified fromThermosynechococcus elongatusPixD and cloned into the pET28a vector. Protein was expressed inEscherichia coliBL21(DE3) cells. The TePixD constructs were transformed intoEscherichia coliBL21(DE3) cells and cultured in LB medium at 37℃.Isopropyl β-D-thiogalactoside (IPTG) (0.2 mmol/L)was added to induce protein expression whenA600of the culture was 0.6, then cells were cultured at 16℃for 16 h. The His-tagged proteins were purified using a Ni2+-nitrilotriacetic acid agarose column and subsequent size-exclusion chromatography. Proteins were shaken with 20 mg FMN per 1 L cells for 1 h before loaded to Ni2+column. All point mutations of TePixD used in the current study were created with the standard PCR-based mutagenesis method and conformed by DNA sequencing. TePixD WT and mutations used in this research had His-tag and thrombin site at N-terminal.
1.2 Crystallography
Crystals of TePixD Y8F in complex with FMN(in 50 mmol/L Tris pH 8.0, 100 mmol/L NaCl,1 mmol/L EDTA, and 1 mmol/L tris (2-carboxyethyl)phosphine (TCEP)) were obtained by hanging-drop vapor-diffusion methods at 16°C. The crystals were grown in buffer containing 30% (v/v) 2-methyl-2,4-pentanediol (MPD), 100 mmol/L sodium acetate/hydrochloric acid pH 4.6, 20 mmol/L calcium chloride. Crystals grown to proper size in 4 weeks.Crystals were soaked in crystallization solution containing 30% glycerin for cryoprotection. Data sets were collected at the Shanghai Synchrotron Radiation Facility (SSRF) at 100 K. Wavelength used for this experiment was 0.919 73 Å. Data were processed and scaled with HKL3000 (http://www.hkl-xray.com/).
Using the structure of the TePixD (PDB ID:1X0P) as the search model, the initial structural model was solved using the molecular replacement method in PHASER (https://www.phenix-online.org/).The model was then refined by phenix.refinement(https://www.phenix-online.org/). Coot (http://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/) was used for peptide modeling and model adjustments. The final structure was validated by the phenix.model_vs_data validation tools (https://www.phenix-online.org/).
1.3 FPLC coupled with static light scattering
Protein samples (100 μl at a concentration of 1 g/L, preequilibrated with corresponding column buffer) was injected into an AKTA FPLC system with a Superose 12 10/300 GL column (GE Healthcare)with the column buffer of 50 mmol/L Tris-HCl pH 8.0, 100 mmol/L NaCl, 1 mmol/L EDTA, and 1 mmol/L TCEP. In experiments in dark, the column was covered by tin foil paper. In experiments in light,the column was lighted by 25 W blue light lamp(wave length 450-460 nm). The chromatography system was coupled to a static light-scattering detector (miniDawn, Wyatt) and differential refractive index detector (Optilab, Wyatt). Data were analyzed with ASTRA 7 (Wyatt).
2 Results
2.1 Crystal structure of the ground state of TePixD Y8F
The structure of TePixD with the resolution of 2.00 Å was elucidated in 2005, which is the first solved structure of the BLUF family[12]. While the dynamic process of TePixD response to the blue light has been extensively studied, with multiple proposed models in several papers[14,17,21-22], the detailed electron transfer chain is still up in the air. We aligned the BLUF domains from various BLUF proteins(Figure 1a). These BLUF domains have mutual secondary structures, and the residues involved in electron transfer and flavin-binding (Tyr8, Gln50,Arg65, Trp91 and Met93) are conserved. To gain further insight, we set out to characterize the involvement of these key residues of BLUF proteins by biochemical analysis and structural determination with crystallography.
Here we designed several mutations of TePixD at critical points of electron transport and aimed to get the crystal structure of mutations. We expressed the mutated TePixD proteins (Y8F, Q50A, Q50L, W91F,Y8F/W91F and Q50L/W91F) inEscherichia coliBL21(DE3) strain and tagged them with a His-tag,followed by purification using Ni-chelating affinity chromatography and size exclusion chromatography(SEC). The crystal of mutations grew under dark conditions at 24℃. After several rounds of optimization, we finally obtained ideal crystals of TePixD Y8F. We solved the structure of TePixD Y8F at 2.54 Å resolution through X-ray diffraction (Figure 1b, Table 1).
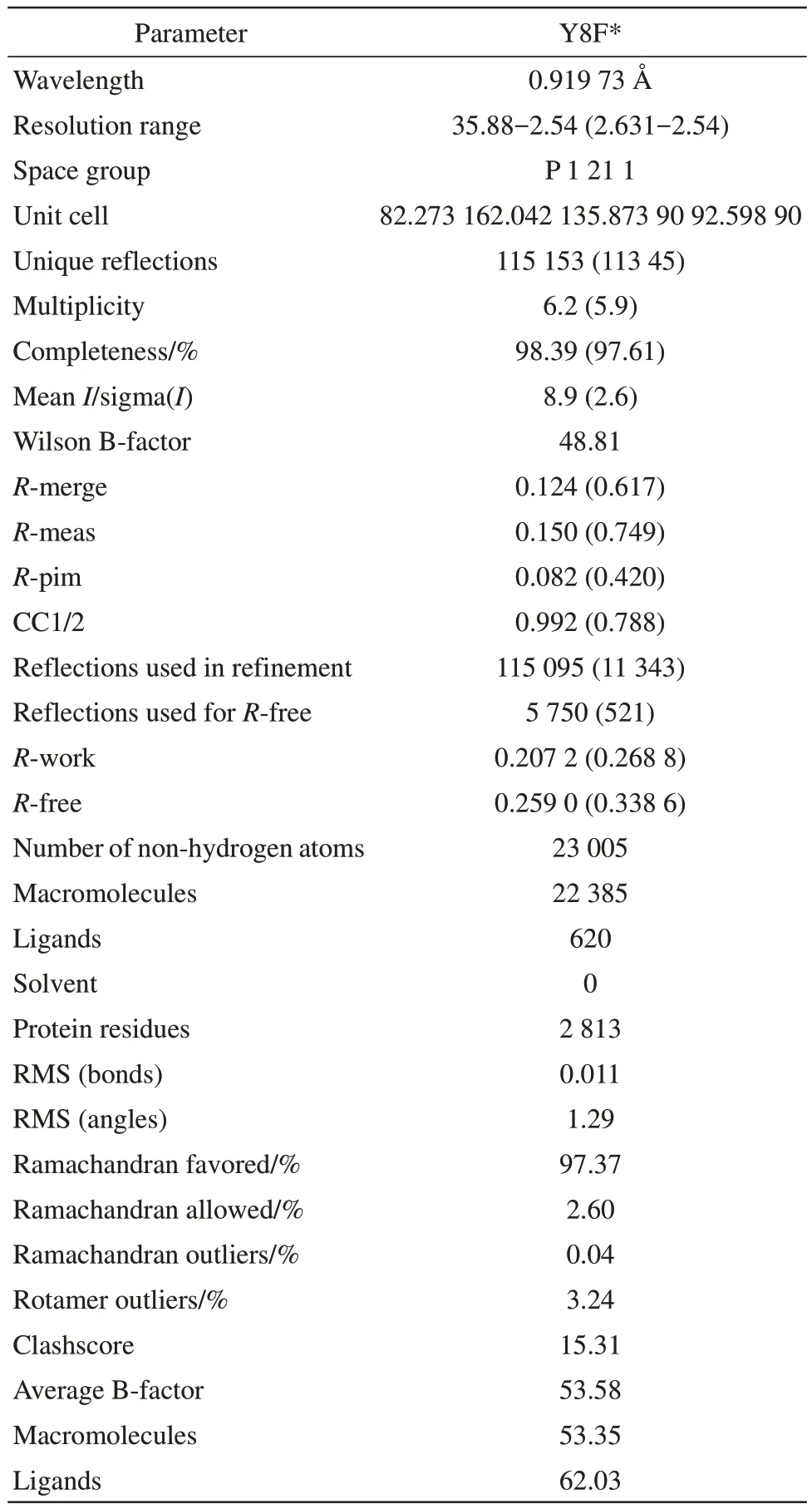
Table 1 Data collection and refinement statistics(PDB ID:8I98)
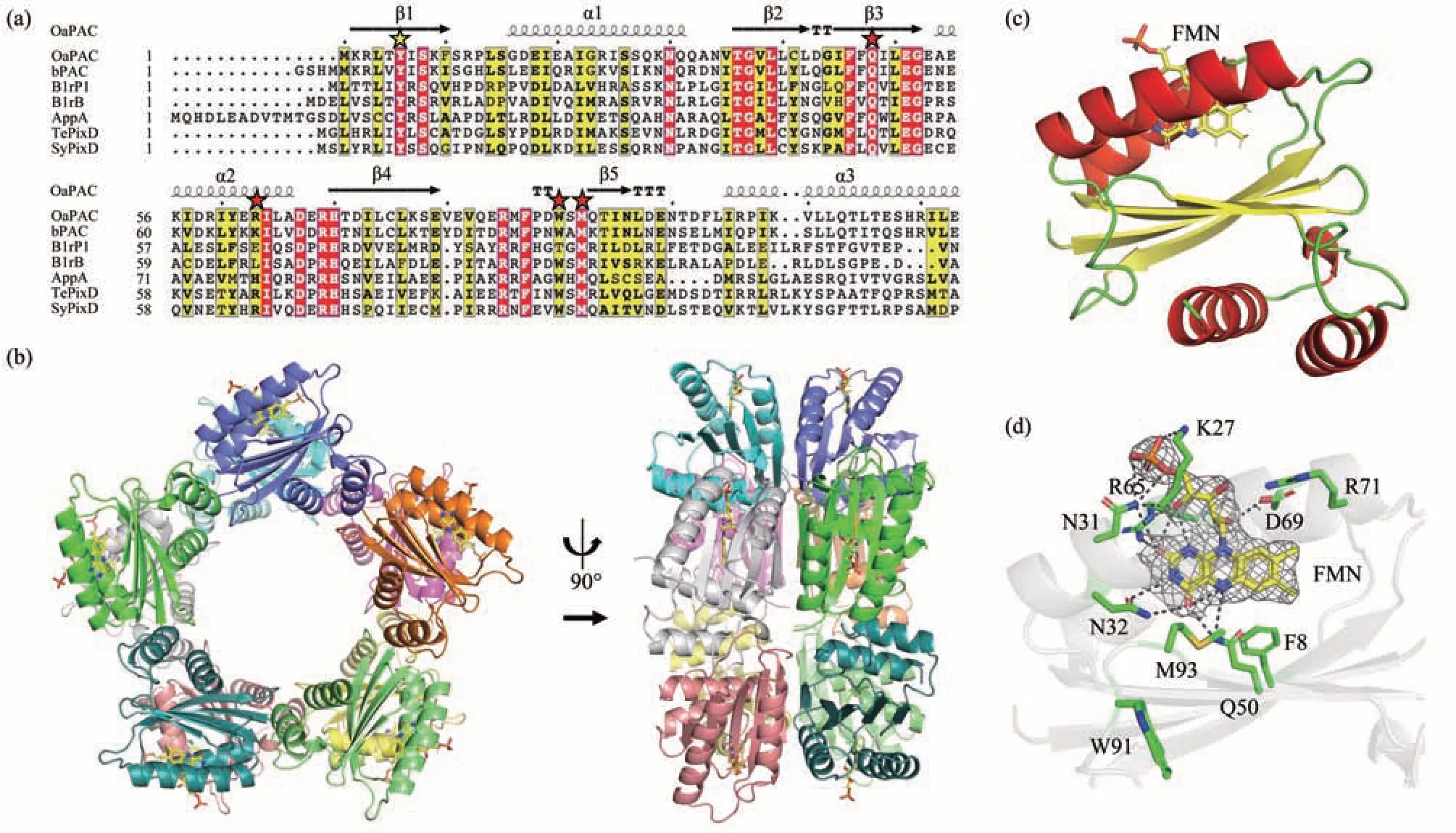
Fig.1 Overall structure of TePixD Y8F
TePixD Y8F also forms a decamer assembly similar to TePixD WT (PDB ID: 1X0P). Each TePixD Y8F monomer consists of a BLUF domain (β1α1β2β 3α2β4β5) and a short C-terminal region (α3α4)(Figure 1c). Five monomers form a pentamer ring with a 5-fold axis, and two pentamer rings are positioned face-to-face to form a decamer.
The hydrogen bond network associated with FMN in Y8F is similar to WT, with the exception of Phe8, which does not form a hydrogen bond with Gln50 due to the lack of hydroxyl group compared to WT (Figure 1d). In the previously reported crystal structures of BLUF proteins, residues near flavinbinding pocket have two different conformations:Trpin(e.g., PDB ID: 1YRX) and Metin(Trpout) (e.g.,PDB ID: 2IYG)[17]. The crystal structure of TePixD Y8F that we obtained corresponds to the Metinconformation, which is the same as that of TePixD WT. Met93 is located in the FMN binding pocket and Trp91 is located far from the pocket.
2.2 Structural comparison of TePixD WT and Y8F
After obtaining the high-resolution crystal structure of TePixD Y8F, we compared it with TePixD WT (PDB ID: 1X0P, resolution: 2.00 Å) to identify any potential structural shifts (Figure 2a). The structural comparison showed WT and Y8F have similar overall structures. Then we analyzed the detailed structure of residues involved in the electron transfer network. Specifically, we focused on the Tyr8-Gln50-Met93 triad in TePixD WT and the corresponding residues in Y8F (Figure 2b). In comparison to WT, Y8F has no hydrogen bond between Phe8 and Gln50 due to Tyr to Phe mutation,resulting in the partial flexibility of the acylamino of Gln50. The distances between Gln50 (Nε/Oε) and Met93 (Sγ) in individual Y8F chains indicate distance range from 3.3 to 4.5 Å, which is longer than the range of 3.1 to 3.5 Å observed in TePixD WT. This increase in distance suggests that the Tyr to Phe mutation impairs the hydrogen bond between Gln50 and Met93.
Next, we aligned the Trp91 residues in WT and Y8F, which is a potential key residue in signal transduction (Figure 2b). Recent research has shown that both Trpinand Metinstructures are stable candidates for the dark-adapted state or light-induced state, but the dark-to-light differences are more likely related to keto-enol tautomerization of Gln instead of Trpinand Metindifferences[17]. In SyPixD and TePixD,Trp91 are only found in the “out” conformation by X-ray crystallography, spectrum and macroscopic phototactic response assay[23]. According to structural alignment, Trp91 of TePixD Y8F is also on the “out”conformation, although partly flexible, in accordance with TePixD WT. We also analyzed Arg65 residues in Y8F (Figure 2c). In WT, Arg65 is in close proximity to FMN and forms a hydrogen bond between Arg65(Nζ) and FMN (Oβ). In most monomers of a Y8F decamer, Arg65 are in hydrogen bond distance with FMN, except two Arg65 side chains in a decamer have longer distances with FMN (3.4 Å and >7 Å)unable to form hydrogen bonds.
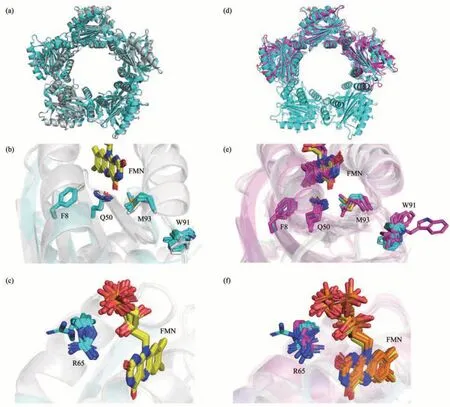
Fig.2 Structural comparison of TePixD WT, TePixD Y8F and SyPixD Y8F
2.3 Structural comparison of TePixD Y8F and SyPixD Y8F
Then we compared TePixD Y8F to SyPixD Y8F(PDB ID: 3MZI, resolution: 2.30 Å) in order to identify the differences between these two molecules.Previous research has shown that both TePixD WT and SyPixD WT form the decamer assembly, but the crystal unit of SyPixD Y8F is the hexamer with subunits arranged in a double half circle. The structural comparison reveals some difference between SyPixD Y8F and TePixD Y8F (Figure 2d),especially in the C-terminal region of the BLUF domain, including β5, α3, α4 and the loops between them. We analyzed the detailed structure of residues related to electron transfer network, as described above (Figure 2e, f). Although the positions of Phe8,Gln50, Met93, Trp91 and Arg65 are similar, these residues in SyPixD Y8F are more flexible than those in TePixD Y8F. The loops located Trp91 of SyPixD Y8F are more flexible. The distances between Gln50(Nε/Oε) and Met93 (Sγ) in individual SyPixD Y8F chains range from 2.7 to 4.4 Å, which is similar to TePixD Y8F (3.3 to 4.5 Å). In SyPixD Y8F, the distances between Arg65 side chains and FMN range from 2.6 Å to 4.2 Å, with three out of the six monomers in a hexamer have longer distances (3.5 Å,3.8 Å and 4.2 Å) that are unable to form hydrogen bonds. In comparation, two out of the ten monomers in TePixD Y8F have longer distances (3.5 Å and >7 Å) hard to form hydrogen bonds.
2.4 Oligomerization of mutations in dark and light conditions
To investigate the dynamic behavior of TePixD protein oligomerization in solution, we conducted multi-angle lightscattering (MALS) assays to analyze the protein conformation and determine the molecular mass (m) of WT and mutant proteins in both dark and light conditions. Although the crystal structures provide static representations of the protein, studying the oligomerization process requires experiments that capture the dynamic changes in the kinetic cycle.
We observed that most of the proteins, including the WT and mutants, formed decamers in both dark and light conditions, but exhibit different lightinduced conformational changes (Figure 3a-d). Size exclusion chromatography (SEC) was used to separate different states of protein samples and then used MALS to detect the specific molecular mass of each part. The molecular masses of TePixD WT are~188.0 ku in dark and ~194.6 ku in light (an increase of 6.6 ku induced by light), and the elution curves show a right-toward shift induced by light (Figure 3a),indicating the conformational change induced by light. The molecular masses of TePixD Y8F are~190.3 ku in dark and ~194.9 ku in light (an increase of 4.6 ku), with elution peaks at approximate positions(Figure 3b), indicating the perturbation on the lightinduced conformational change by Y8F.

Fig.3 Oligomerization of mutations in dark and light conditions
We also measured the molecular mass of several key residue mutants, including Q50L and W91F,which are known to disrupt the hydrogen network in the flavin pocket (Figure 3c, in dark; Figure 3d, in light). The MALS measurements reveal that these mutants also form decamers with only slight shifts in the elution peak (Table S1). Interestingly, Q50L and Q50L/W91F showed a decrease in molecular mass in light than in dark, whereas the molecular masses of WT, Y8F, W91F, and Y8F/W91F increased slightly in light. Based on the structure of TePixD flavin pocket(Figure 1d), two hydrogen bonds are formed between Gln50 and FMN. Therefore, the mutation Q50L may impair the binding of FMN to the apo protein. We speculate that the Q50L mutation may affect the conformational change of TePixD decamers upon exposure to light.
3 Discussion
Here we report the crystal structure of TePixD Y8F, a key residue mutation in a BLUF protein. As a kind of photoreceptor, the BLUF protein family has been known for over twenty years[4], and has been shown to be involved in various metabolic pathways in response to light. TePixD is a short BLUF protein and was the first in BLUF protein to have its crystal structure solved in 2005[12]. In TePixD, Tyr8 is a key residue that provides electrons and protons for flavin(FAD or FMN) during proton-coupled electron transfer, and the Y8F mutation loses the function[24-25].
Our overall structure of TePixD Y8F is similar to that of TePixD WT, forming the decamer assembly with only slight differences. Interestingly, previous research reported SyPixD is also the decamer assembly, but the SyPixD Y8F molecule is a hexamer that consists of two trimers in the semi-circle[20].Specifically, the Gln50 side chain become partly flexible due to the loss of the hydroxyl group in the Y8F mutation, and the hydrogen bond between Gln50 and Trp93 is impaired due to the increased distance between their side chains. Meanwhile, the hydrogen bond between Arg65 and FMN shows slight variation.
In the molecular masst tests of the mutations in both dark and light conditions, we observed slight shifts in the elution peaks, indicating the perturbation on the light-induced conformational change, and the protein oligomerization was not significantly affected by the mutations. Previous studies have also reported that TePixD and SyPixD form decamers both in dark and light conditions. However, under high pressure(above 60 MPa) in light, they dissociate into dimers[26-27]. Oligomerization of the proteins is also known to be dependent on the concentration of protein in the solution. At high concentrations, such as those in crystal formation solutions, decamers are the dominant species, while at low concentrations, they can form pentamers[28].
4 Conclusion
We conducted several experiments to investigate the effects of mutations of key residues in photocycle on the structure and function of a BLUF protein,TePixD.
By crystallizing and determining the crystal structure of TePixD Y8F at a resolution of 2.54 Å, we were able to capture the intermediate state of the photocycle. Detailed analysis indicated that the assemble mode of TePixD Y8F mutant was much different to that of SyPixD Y8F mutant. We also clarified the subtle differences of several key residues related to light-induced PCET, including Phe8, Gln50,Trp91, Met93 and Arg65.
To further understand the impact of these mutations on the conformational changes induced by light, we performed MALS measurements on TePixD mutations under both dark and light conditions. We found the perturbation on the light-induced conformational change by the Y8F mutation, along with other mutations (Q50L, W91F, Y8F/W91F and Q50L/W91F). Interestingly, we observed differences in the changes of molecular masses among these mutations, suggesting that the Q50L mutation may specifically influence the conformational response of TePixD decamers to light.
By combining our structural determination and biochemical analyses, we have contributed valuable information towards elucidating the light response mechanism of BLUF proteins.
AcknowledgementsThe authors thank the staff in Shanghai Synchrotron Radiation Facility(SSRF)BL18U1 for X-ray beam time.
SupplementaryAvailable online (http://www.pibb.ac.cn or http://www.cnki.net):PIBB_20230095_Figure_S1.pdf
PIBB_20230095_Table_S1.pdf
Data availabilityThe atomic coordinates of the TePixD Y8F decamer is deposited to the Protein Data Bank under the accession code 8I98.