lnsight into small eyes: a practical description from phenotypes presentations to the management
2024-02-23SanaNiaziSorchaDhubhghaillFaridehDoroodgarZisisGatzioufasMohammadHosseinDehghan
Sana Niazi, Sorcha N Dhubhghaill, Farideh Doroodgar,, Zisis Gatzioufas, Mohammad Hossein Dehghan
1Translational Ophthalmology Research Center, Tehran University of Medical Sciences, Tehran 1416753955, Iran
2Antwerp University Hospital, Edegem 2650, Belgium
3Faculty of Health Sciences, Antwerp University, Antwerpen 2000, Edegem, Belgium
4Negah Aref Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran 16666, Iran
5University Eye Hospital Basel, Basel 4031, Switzerland
Abstract
● KEYWORDS: microphthalmos; nanophthalmos; lenses;intraocular; anterior chamber; axial length; eye; cataract microcornea syndrome
INTRODUCTION
The complex process of eye development begins at approximately the third week of pregnancy, starting with the formation of the optic vesicle.This vesicle then transforms into a two-layered optic cup through invagination.The outer layer contributes to creating the cornea, sclera, and anterior chamber, while the inner layer develops into the retina and pigmented epithelium.The lens forms from the lens placode,positioned between the front and back chambers of the eye.The presence of smaller eyes results from a mix of genetic and developmental factors.Genetic mutations that affect eye growth and development are often responsible for structural abnormalities, such as a crowded front segment, reduced ability to adjust focus, and a higher risk of complications like angle-closure glaucoma and serous retinal detachments[1-5].
Microphthalmos, in addition to being a rare ocular disorder(0.046%-0.11% of ophthalmology patients)[6], is quite challenging to appropriately manage due to its complexity.When indicated, cataract surgery requires more intraocular manipulation and extended surgical duration, and may have increased risk of intraoperative and postoperative complications[7-9].As such, these eyes should be recognized during routine cataract inspections and referred to expert care,where possible.The term microphthalmos refers to eyes with axial lengths (ALs) smaller than the age-matched mean for the population of at least 2 standard deviations (SDs)[10-11], in which lack of development include both anterior and posterior segment of eye[12].Recent study by Zhanget al[13]showed that microphthalmos affect orbital height and depth maximally and minimally, respectively.Posterior orbital retardation also occurred at 3 years old, suggesting that intervention in the affected eye should be performed before the age of four.Eyes shorter than 18.0 mm have also been referred to as extreme microphthalmos[14].Nanophthalmos[11]refers to a clinical variant of simple microphthalmos accompanied by thickened sclera, without any systemic or major ocular anomalies[12].These AL-based definitions encompass a variety of “smalleye” phenotypes[15], all of which arising from abnormalities in the evolution of the primary optic vesicle[6,14,16](Figure 1).
For optimal decision-making, better visual outcomes, and lower complication rate[17-19], we need to recognize and apply the criteria in the classification[14], particularly given the broad spectrum concerning microphthalmos in nomenclature (Figure 1).

Figure 1 Overview of small eye terminology in the literature Nanophthalmos: short axial length caused by shortening of the anterior and posterior segments, accompanied by thickened sclera; Relative anterior microphthalmos: short axial length caused by shortening of the anterior segment, with a normal-sized posterior segment and without scleral thickening, and Posterior microphthalmos refers to short axial length in which cause by shortening of the posterior segment, with a normal-sized anterior segment and thickened sclera.Posterior segment changes are inescapable both in posterior microphthalmos and nanophthalmos.Complications like angle closure glaucoma and exudative retinal detachment are likely to occur in eyes with nanophthalmos but not with posterior microphthalmos.Detailed multimodal image analysis found that papillomacular folds was partially a neural retinal issue, suggesting that redundancy of retinal issues involved only inner retinal layers.*In some references, the term posterior microphthalmos overlaps with nanophthalmos.It is due to retinal changes, from clinical findings of advanced anterior segment optical coherence tomography (AS-OCT), fluorescein angiography (FA), or indocyanine green (ICG) angiography findings, that definition boundaries are intertrial[19]; AL: Axial length; HCD: Horizontal corneal diameter; ACD: Anterior chamber depth; RAM:Relative anterior microphthalmos (measurements in millimeters).
Study SelectionThe first comprehensive search was done on the main online databases (including CENTRAL, PubMed,Scopus, Cochrane, ISI Web of Science and Embase) on 23 November 2022, and did not include any restrictions on language or type of study.Several supplementary searches have been conducted in the following months and the suitable papers have been included in the search algorithm.We used the following MeSH and Non-MeSH keywords and search model for this systematic review:
#1 Cataract (Topic)
#2 “microphthalm*”OR “small eye*”OR “hyeropia*” OR“non ophthal*” (Topic)
#3 “surger*” OR “preoperat*” OR “intraoperat*” OR“postoperat*” OR “manag*” OR “lens calculat*” OR“lens design*” OR “formula*” OR “iol calculat*” OR“phenotyp*”(Topic)#1 AND #2 AND #3 The search results in each database were imported to the Endnote software, and duplicate articles were removed.Eligible articles were selected by screening the titles and abstracts.For potentially eligible articles, full-text was then reviewed.In our initial search, we found 1162 articles from the online databases were included in the full-text assessment,of them, 330 papers were duplicate and therefore were excluded.Of the remaining 832 papers, 699 publications were considered irrelevant after looking at their titles and abstracts.Among the remaining 133 studies, 40 studies were excluded due to exclusion criteria.Finally, 93 articles were included in the full-text assessment (Figure 2).The study is registered in the PROSPERO International Prospective Register of systematic reviews, and it was assigned the approval ID CRD42023478311.The institutional review board of Negah Eye Hospital, Shahid Beheshti University of Medical Science approved it.
Part A: The Disease Category
Microphthalmos
The diagnostic criteria, clinical findings and preoperative examinationIt is described as high hyperopia, with anterior chamber depth (ACD) ≤2.2 mm[20]and AL≤20.0-21.0 mm[20-23].It is also referred to as simple microphthalmos (sometimes pure microphthalmos or nanophthalmos[24]), if not associated with other congenital ocular anomalies.When associated with other anomalies, the condition is called complex microphthalmos,which can present itself with pathologies such as persistent hyperplastic primary vitreous, chorioretinal colobomas (a rare congenital defect of the posterior segment brought on by the embryonic fissure's inadequate closing during fetal development), and retinal dysplasia[11,14,22,25].Coloboma has also been reported as the most common posterior segment abnormality in the contralateral eye of congenital unilateral blind microphthalmic or anophthalmic patients[26].
Important pre- and post-operative considerationsDespite the advances in phaco surgery, small eyes have anatomical issues that can cause problems during cataract surgery[10,27].These eyes can be difficult to approach as they sit deeper in the orbit.Since the horizontal width of cornea is greater than its vertical diameter, a temporal approach can make instrument positioning and manipulation easier and avoid any issues of a prominent brow[10,28].Paracenteses and the primary corneal wound should also be modified for thicker corneal pachymetry to avoid excessive tunnel length, which would hinder lens access.That being said, these eyes are more prone to iris prolapse so positioning the main incision slightly more anteriorly can be helpful in preventing it.When putting the IOL in the capsular container filled with cohesive ophthalmic viscosurgical devices (OVDs), caution must be used about zonular weakening in these eyes.Indeed, for subsequent piggyback IOL, its precise positioning is crucial[14,28-29].
Nanophthalmos
The diagnostic criteria, clinical findings and preoperative examinationIt is characterized by a short AL (≤21.0 mm), a shallow anterior chamber (≤2.2 mm), thickened choroid and sclera (>1.7 mm), but no other structural deformities[10,28,30].
As the lens thickness increases with age, it tends to protrude preferentially into the anterior chamber making these eyes more prone to angle-closure glaucoma over time[31].Even in the absence of exudative lesions, uveal effusions may be seen preoperatively or postoperatively, due to the resistance of the thicker sclera to venous outflow from the vortex veins[10,28,32].Scleral thickness can be measuredviaultrasonography,anterior segment optical coherence tomography (AS-OCT),or ultrasound biomicroscopy (UBM), and may be helpful in predicting a postoperative effusion.In addition, UBM should be used to rule out preoperative mild uveal effusions that might worsen during or after cataract surgery.
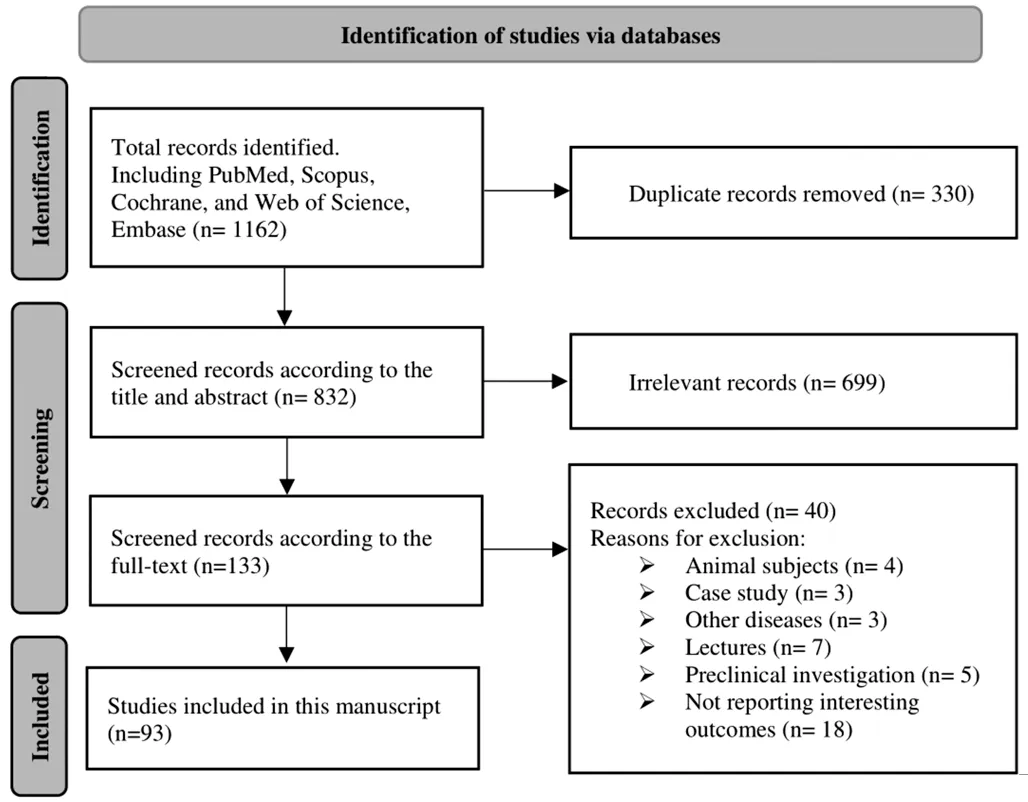
Figure 2 Study selection.
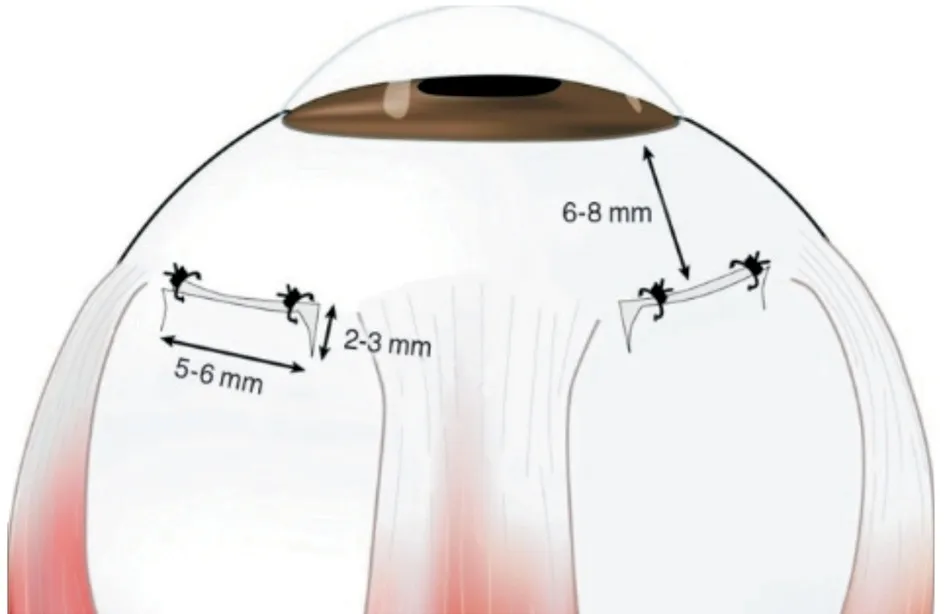
Figure 3 Creating a scleral window prevents uveal effusions A square bracket shaped full-thickness scleral incision was made in the quadrant between the rectus muscles[34].[This figure is distributed under the terms of the Creative Commons Attribution-NonCommercial 3.0 Unported (https://creativecommons.org/licenses/by-nc/3.0/), which permits use, duplication, adaptation,distribution, and reproduction in any noncommercial medium or format].
Important pre- and post-operative considerationsCycloplegics, steroids, or even the creation of scleral windows should be considered to treat these effusions weeks before cataract surgery, as per the surgeon’s opinion[10,33].Creating scleral windows[34](Figure 3) at the start of surgery can prevent uveal effusions in nanophthalmos eyes[30,35].Intraoperative intraocular pressure (IOP) fluctuations should be controlled to prevent uveal effusion or suprachoroidal bleeding[10,20,32].If the surgery is complicated by a sharp rise in IOP and sudden shallowing of the anterior chamber, an intraoperative effusion should be suspected.If needed, the surgeon can perform inferior sclerectomies to lower IOP at the conclusion of the procedure if uveal effusions develop during phacoemulsification.Alternatively, the surgeon should close all wounds and finish the operation, deferring further surgery until the effusion has cleared up[10].
Relative anterior microphthalmos
The diagnostic criteria, clinical findings and preoperative examinationIn the case of relative anterior microphthalmos,which has a prevalence of 6%[8,14], despite an AL greater than 20 mm, eyes have an ACD≤2.2 mm and corneal diameter(CD) ≤11 mm with normal scleral thickness.While the AL may be considered normal, the relatively small side of the anterior segment means that angle-closure glaucoma,pseudoexfoliation, and corneal guttata have similar incidence to nanophthalmos[8,10,14,28,30].
In the presence of a shallow anterior chamber, it is important to document IOP, ACD, peripheral anterior synechiae, posterior synechiae, or anterior segment dysgenesis in the preoperative evaluation.When this occurs, careful slit lamp biomicroscopy should be accompanied by the use of other diagnostic equipment, such as dynamic gonioscopy, AS-OCT, or UBM.If the angle is constricted, IOP should be controlled using topical treatment, peripheral iridotomy, IOP measurement devices (e.g.MEHDI-IOP measurement model[36]) and/or laser iridoplasty before surgery, particularly in the case of elevated IOP and glaucomatous neuropathy.Greater baseline of lens thickness,and lens vault values can be associated with more increase in anterior segment biometric parameters after laser peripheral iridotomy[37].
Important pre- and post-operative considerationsPreoperative laser iridotomy/iridoplasty may increase the angle and eventually elevate the ACD which could simplify surgical movement during cataract surgery.Evaluation of endothelial cell counts and central corneal pachymetry is also recommended for patients with relative anterior microphthalmos, since these eyes are more prone to phacoemulsification damage and Fuchs endothelial dystrophy[10,28,30,38].
In small eyes with a shallow anterior chamber, posterior pressure can make surgery challenging and raise the risk for complications.Preoperatively, intravenous mannitol dehydrates the vitreous, reducing its volume and the likelihood of significant posterior pressure[10,35].
Iris hooks are preferable to other iris extension devices for small eyes with inadequate dilation, small pupillary aperture,or iris coloboma, especially when a shallow anterior chamber is present[10,28].
Posterior microphthalmos
The diagnostic criteria, clinical findings and preoperative examinationIn these eyes, the high axial hyperopia and the standard anterior segment are associated with retinal findings[19].This is thought to be due to an inhibition in the growth of the outer layer of the eye [such as sclera, choroid,and retinal pigment epithelium (RPE)], while the growth in the inner layer of the sensory retina continues.As a result,posterior microphthalmos is associated with retinal findings such as sclerochoroidal thickening, papillomacular folds,macular hole, uveal effusion, retinoschisis, foveoschisis,pigmentary retinopathy, serous retinal detachment, crowded optic discs, pseudo papilledema, and avascular zone that may be seen in the extreme periphery of the retina[11,16,39].The allelic presentation of both posterior microphthalmos and nanophthalmos is likewise speculated[40-41].It is possible that the anterior segment is also affected in posterior microphthalmos, though to a lesser extent than the posterior segment, as seen by the subnormal dimensions of ACD and angle in posterior microphthalmos cases (Figure 4).On the other hand, previous reports illustrated complete vascularization of the fovea and prominent depletion of the foveal avascular zone (FAZ), macular hypoplasia in timedomain optical coherence tomography (TD-OCT) and spectraldomain optical coherence tomography (SD-OCT), and cystic nonleaking structural changes in microphthalmos (Figure 5)[19].
Important pre and postoperative considerationsOptical coherence tomography angiography (OCTA) equipment, such as the AngioVue XR Avanti (RTVue XR; Optovue Inc) can be used to precisely demonstrate the macular and periphery retinal perfusion status, detect retinal and/or disc neovascularization and inflammatory or non-inflammatory lesions[42], and finally demonstrate both shallow and deep capillary plexus in microphthalmos, indicating the lack of FAZ[11].
Axial high hyperopia
Diagnostic criteria, clinical findings and preoperative examinationAxial high hyperopia is characterized by a normal ACD with a short AL and high hyperopia[14,17,21-22].Fortunately, most cases of small eyes seen in a typical ophthalmology practice will be cases of simple microphthalmos,in which the anterior segment is normal but the AL is short.
Important pre- and post-operative considerationsThe majority of these procedures may be expected to go offwithout a problem, both during and after the operation[28,43].Even so, in spite of advances in IOL calculation[44-49], and the effective lens position may affect the long-term refractive outcome[50].There is no global consensus on the optimum IOL and the formula for calculating power in small eyes[6,8,14,24,51-54].However, these patients typically require lenses of very high dioptric correction which often require custom lens orders or the combination of two lenses, known as piggyback IOLs.Although eyes with equivalent preoperative biometric values usually have the same effective lens position (ELP), the postoperative refraction from the first eye can refine the second eye's IOL calculation[10,55-56].Given these uncertainties (Figure 1), a thorough pre-, intraand postoperative care (Figure 6) is recommended to identify other causes of impaired vision beyond high hyperopic amblyopia[10].Preoperative optical refractive assessments are the most important concern that will be discussed in the part B.Part B: Optical Refractive AssessmentsPreoperatively, the patients should be thoroughly counseled regarding the risks and difficulties associated with microphthalmia.These patients have a higher incidence of difficulties in achieving emmetropia in their postoperative refractive outcomes.Some causes for subpar results in these cases include the presence of ametropic amblyopia[51], residual hyperopia[51-52], refractive surprise[24],and errors in the calculation[52].Moreover, surgeries are more difficult[57]and often require different techniques (e.g., two[52]or three[24]IOLs in the bag), secondary iris-claw lenses[51]or a combinations of lenses in the sulcus, bag or anterior chamber[14,58].

Figure 4 Overcrowding of the angle in a spectrum of narrow angle glaucoma, uveal effusion and posterior microphthalmos ATA: Angle to angle; STS: Sulcus to sulcus; ACD: Anterior chamber depth; TEMPO: Temporal; INFER: Inferior; INFRATEM TRANS: Inferior trabecular meshwork transversely.

Figure 5 Optical coherence tomography of the macula of both eyes illustrates subretinal fluid, a small pigment epithelial detachment,intraretinal cysts and congenital absence of a foveal pit ILM: Internal limiting membrane.
In the small eyes, the ultrasound axiometers calibrated with average velocities for normal eyes are not perfect, less consistent and more prone to error[52].Thereby, and even with the presumption of the perfect IOL calculations[53-54], achieving emmetropia is elusive[24,52].Despite the use of various formulae for IOL calculations, and even with an overcorrection of 10%,eyes have a high tendency to remain hyperopic after surgery(range 2.25 to 12.25 D)[52].

Figure 6 Associated perioperative ocular disease and complications relative to microphthalmos PCR: Posterior capsule rupture; IOP: Intraocular pressure; IOL: Intra-ocular lens; PCO: Posterior capsule opacity; AC: Anterior chamber; Nd-YAG: Neodymium-doped yttrium aluminum garnet; PPV: Pars plana vitrectomy; LPI: Laser peripheral iridotomy.
The laser interferometries by IOL master, immersion, and applanation ultrasound, have not showed significant differences for measurements of AL and ACD values in cases of otherwise“normal” cataract eyes[59-60].Lens-Star also uses a swept-source laser that has a better signal-to-noise ratio[54].Ultrasound biometry with indentation of the cornea reflects the light at the internal limiting membrane (ILM) rather than the RPE,which makes a difference of about 130 µm.This difference between ultrasound and optical biometry is key, showing that measurements by the ultrasound biometry may be underestimated when compared to optical biometry.On the other hand, about 9%-11.1% of the patients cannot fixate to the target due to nystagmus, tremor, respiratory distress, ocular surface and lid problems, extreme opacity of media, membrane formation or retinal detachment, making laser interferometry difficult to perform, and in some cases impossible[54].In these cases, immersion ultrasound biometry is the gold standard.
IOL Calculations and FormulaeThere have been many studies on IOL calculation over the years as technology has improved.These include the primitive-generation formulae such as SRK/II[8], SRK/T[6]and the Hoffer Q, with the latter having been regularly recommended for moderately short eyes[6]and nanophthalmos[24].Fourth-generation formulae include the Holladay II technique, which has been recommended for extremely short eyes (i.e., 18 mm )[6], relative anterior microphthalmos and posterior microphthalmos[24].The Haigis formula has also been recommended for short eyes[8,54].Finally, fifth-generation formulae such as Barrett Universal II and Olsen may also prove to perform highly acceptable in these cases also.
In a large study on 8108 eyes with varying ALs, the Hoffer Q formula showed the best accuracy for eyes with an AL≤21.50 mm (134 eyes) when compared the Holladay 1 and SRK/T formulae[61].However, there is limited data in small eyes (18.80≤AL≤22.00 mm), because there were no statistically significant differences with the latest seven generation formulae (Barrett Universal II, Haigis, Hill-RBF 2.0, Hoffer Q, Holladay 1, Holladay 2, and Olsen)[53].Small myopic refractive prediction errors were found in Hoffer Q and Holladay 2 (about -0.22 and -0.23 D, respectively), which may not be too detrimental for the postoperative visual outcome as residual myopic is better tolerated than residual hyperopia[53].Furthermore, a 2018 Meta-analysis concluded that the Haigis formula is better to the Hoffer Q, Holladay 1, Holladay 2,SRK/T, and SRK II formulae[62].Along with Hoffer Q, the fourth-generation formulae Haigis and Holladay 2 appear suitable for short AL eyes since they take into account factors such as age, preoperative refraction, lens thickness, ACD, and white-to-white (WTW) measurement[10].
The Kane formula is a newer formula that is a combination of theoretical optics, regression and artificial intelligence (AI)technology to improve results in the extremes of biometry[63].In recent studies which included long and short eye subgroups,the Kane formula was the most precise[64].In a study of 270 eyes using an SA60AT IOL with a spherical equivalent power of 30 D, a number of IOL calculation methods were used to examine the results in cases with an AL mean of 20.82±0.63 mm[65].The improved lens constants were used to compute predicted refraction, which was then checked with the actual refractive result to provide prediction errors.Barrett Universal II, Haigis,Hill-RBF 2.0, Holladay 1 and 2-AL adjusted, Hoffer Q, Olsen,and SRK/T were all less accurate than Kane.When compared to the Barrett or Hoffer Q formulae, 20% more patients use the Kane approach to get a result within 0.50 D[65].Although the new Kane method looks promising for short AL eyes[28,65],more studies should evaluate the impact of IOL optimization particularly in short eye[66].In fact, deformation and progress in the flattening[52]of the IOLs’ curvatures within the contact zone is partly responsible for the postoperative residual hyperopia in short eyes.Fundamentally, we still lack methods to quantify and perfectly predict the ELP where the implant lens will sit postoperatively[51].
IOL predictions and a shallow anterior chamberA shallow anterior chamber is a frequent feature of microphthalmos.Recently, there has been considerable interest in optimizing the IOL formula selected for these patients[67-69].It has been shown that the lens vault (LV) and the accuracy of the IOL formulae are related, and a summary of the formulae that best fit each scenario is provided in Figure 7[70-71].A study by Yanet al[67]on the accuracy of the Barrett Universal Ⅱ, Haigis, Hoffer Q,Hoffer QST, Holladay 1, Kane, and SRK/T formulae in eyes with shallow anterior chambers, showed that the Barrett and Kane formulae were better than others in generating the least median absolute error both short and normal ALs.This is likely because of the integrations of parameters such as ACD, WTW,central corneal thickness (CCT), lens thickness and gender, as well as the conventionally used ALs and corneal power.These have been shown to be the most accurate methods throughout a broad range of ALs[64,72-76].
A large rise in LV is typically seen in shallow ACD brought on by lens-related causes.In certain circumstances, lens-related variables that contribute to shallow ACD can be eliminated by cataract surgery[77].The LV subgrouping has no impact on the precision of the Barrett and Kane calculation, which considers preoperative ACD (Figure 7).This may be owed to the fact that these two formulations include lens thickness as additional variables, which may help reduce the interference caused by lens-related factors in the calculation of IOL power, improving the accuracy and decreasing the sensitivity to fluctuations of the refractive findings[67].
The postoperative refractive errors of the remaining formulae,with the exception of the Barrett and Kane, exhibited a strong correlation with LV, thereby indicating a trend towards hyperopia as the LV increases[67,70-71].Patients with large LV also tend to have zonular laxity[78], which might exacerbate postoperative ACD, and increase the chance of postoperative hyperopia.In formulae like Haigis and Hoffer QST in particular,which incorporate ACD but not lens thickness as a variable,large preoperative LV may lead to hyperopic refractive outcomes.Altogether, the Barrett and Kane method is more commonly advised in patients with shallow anterior chambers,and LV may indeed be important to take into account[67].
Intraocular lens designWhen operating these smaller eyes,not only it is essential to know the lens options and prepare for in the bag placement, but also be prepared to change the surgical plan and consider sulcus placement, bag-in-the-lens technique, piggyback placement, iris fixation, anterior chamber placement, and scleral fixation[79].
Advantages of bag-in-the-lens technique such as the surgeon-controlled IOL centration along the patient’s line of sight[80], the low amount of surgical induced astigmatism,the IOL stability[81], the long term posterior capsule opacity(PCO) prevention[82-84].have been reported.However the surgeons should consider all precautions about all surgical considerations preoperatively[85-86].Moreover, in spite of optimizing in biocompatibility of IOL and visual performance, iris claws and piggyback implantation, are less reliable in terms of achieving emmetropia and are prone to complications such as early and late lens displacement, erosion and transillumination of the iris, uveitis, endothelial cell loss,iris ovalization, and atrophy[51,87].

Figure 7 Relationship between LV and the precision of the IOL formulae in short and normal AL AL: Axial length; N/A LV: Not applicable (no considering) lens vault; MAE: Mean absolute error.
One High Dioptric Primary IOL ImplantationWhere possible, a single lens should be placed into the capsular bag.Many companies routinely deliver lenses up to +30 D and may be requested to make lenses of an even higher power as a custom order.It is important to remember that the higher the diopter,the thicker the lens and this should be considered as they can be difficult to inject through very small incision sizes.If there is a hyperopic refractive surprise postoperatively, a secondary lens implanted into the ciliary sulcus (also known as a piggyback lens) can be used to correct the result.In the postoperative period, it is possible to more precisely calculate the height of the piggyback IOL and determine whether or not there is enough space for a sulcus-placed IOL.Refractive vergence can be determined with the help of the nomogram of Nichamin[88]or it can be calculated with the Holladay IOL Consultant or vergence formulae provided by the lens manufacturers.
Two Lenses Primary IOL ImplantationsIn some cases where the full dioptric power cannot be achieved with one lens, two implants used together may be the best means of correcting the refractive error.Originally, these lenses were both implanted in the capsular bag but this approach is no longer recommended as direct contact between the two lenses can cause multiple issues.These issues included situations where the posterior lens was displaced, damage and opacification at the interface of the two lenses, deformation decreasing total IOL power, optical distortion, aberration and deterioration of vision[6,24].Longer term complications,such as Elschnig pearls, pigment dispersion, interlenticular opacification, and glaucoma, can also result in a reduction of visual acuity[89-90].As a result, we should reiterate that placing both lenses in the capsular bag is not advised.In a case of severe nanophthalmos, Caoet al[24]performed implantation of a 30 D acrylic IOL and a 9 D silicone IOL in the capsular bag,followed by a 30 D silicone IOL in the ciliary sulcus for a total of three IOLs in one eye.The one year follow up showed that the lenses were still well tolerated.For silicon IOLs, in order to decrease the contact zone between the lenses and minimize the interlenticular opacification (ILO), the acrylic IOL used was thinner, which produced satisfying outcomes.
Nowadays, when the piggyback approach is used, one lens is placed in the bag and one is placed in the ciliary sulcus.In most cases, an acrylic lens is inserted into the capsular bag, and a three-piece IOL (commonly silicone) inserted into the ciliary sulcus.This piggyback IOL can be placed during cataract surgery or at a later time (may be the better option) as a followup procedure, as mentioned above[14].This technique reduces the development of interlenticular membranes, opacifications,and late hyperopic shifts that have been associated with two lenses in the capsular bag[91].

Figure 8 Intra- and post-operative precautions OVD: Ophthalmic viscoelastic devices; PPVitx: Pars plana vitrectomy; CS: Cortico-steroid; IOP:Intraocular pressure; Nd-YAG: Neodymium-doped yttrium aluminum garnet; CPC: Cyclophotocoagulation.
The piggyback IOLs have many advantages, such as the ability to achieve the required lens power and less spherical aberration(enhanced image quality).On the other hand, ILO[24,52],Newton ring formation[52], and deterioration of the optical quality secondary to the aberration of the distorted soft lens material[52], residual hyperopia, technical difficulties, extended surgical duration and consequent secondary complications are counted as their disadvantages[6,52].Finally, with a piggyback lens implant, red rock syndrome or ILO can occur, in which Elschnig pearls form at the lens interface.In the worse-case scenario, the sulcus lenses can be explanted with good results,even years after surgery, which was not so simple for the inthe-bag placed lenses[92].
Care of potential complication after cataract surgeryMany studies have linked preoperative risk factors to complications during surgery, and of those, an AL shorter than 20 mm and raised IOP confer higher risks of complications (5.76%-27.9%)[8,21-22,28-29](Figures 6 and 8).Common complications include posterior capsule rupture, zonular dehiscence, iris prolapse,trauma to the corneal endothelial or Descemet membrane, transient severe corneal edema, cystoid macular edema, anterior uveitis,uveal effusion, angle-closure glaucoma, retinal detachment,and aqueous misdirection[10,22,28-29,32].Adequate experience and preparation can help mitigate these risks.
Finally, once the surgery is completed, the patient should be carefully followed up.Refractive surprises, suboptimal visual outcomes, IOP and choroidal effusions are more common in these cases, and early detection can greatly assist in addressing them[93].
CONCLUSION
Small eye cataract surgery involves several obstacles, and is more prone to intraoperative and postoperative problems.Anatomic classification and a thorough preoperative examination assist with customizing each case and allows the surgeon to anticipate surgical hurdles.Therefore, in addition to anatomic classification, exact preoperative biometric evaluations and IOL calculations, attention to surgical method and postoperative treatment, as well as rigorous preoperative planning, are essential for providing high quality visual results.
ACKNOWLEDGEMENTS
Conflicts of Interest:Niazi S,None;Dhubhghaill SN,None;Doroodgar F,None;Gatzioufas Z,None;Dehghan MH,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Using choroidal thickness to detect myopic macular degeneration
- lmpact of multifocal gas-permeable lens designs on short-term choroidal response, axial length, and retinal defocus profile
- Baerveldt glaucoma implant with Supramid© ripcord stent in neovascular glaucoma: a case series
- Efficacy and safety of Usights UC100 illuminated microcatheter in microcatheter-assisted trabeculotomy
- Quantifying peripapillary vessel density and retinal nerve fibre layer in type 1 diabetic children without clinically detectable retinopathy using OCTA
- Nomogram to predict severe retinopathy of prematurity in Southeast China
