lmpact of multifocal gas-permeable lens designs on short-term choroidal response, axial length, and retinal defocus profile
2024-02-23MutebAlanaziPatrickCarolineAmaneAlshamraniMariaLiu
Muteb Alanazi, Patrick Caroline, Amane Alshamrani, Maria Liu
1Optometry Department, College of Applied Medical Sciences,King Saud University, Riyadh 14813, Saudi Arabia
2College of Optometry, Pacific University, Forest Grove,Oregon 97116-1756, United States
3Optometry School, University of California at Berkeley,California 94720, United States
Abstract
● KEYWORDS: choroid; gas-permeable contact lens;retinal defocus; axial length; myopia
INTRODUCTION
Myopia is reaching epidemic levels worldwide[1].High myopia is one of the leading causes of vision loss in the world due to the increased risk of serious ocular pathologies[2].Clinical myopia control options have been explored during the recent years, many of which including novel spectacle and contact lens designs showed clinically significant efficacy[3].
Retinal image quality and defocus profile during visual development plays an essential role in regulating ocular growth.Evidence from a wide range of animal studies in refractive development suggests that ocular growth is a visually guided process that is mediated by local retinal mechanisms.These local mechanisms operate over both central and peripheral retina in spatially specific manners[4-5].Studies also indicated that myopic defocus imposed to a larger retinal area tends to have a greater myopia control effect[6].Converging evidence also suggests that peripheral vision plays a critical role in the overall regulation of ocular growth and central refractive development with or without cues from central vision[7-8].
In clinical trials investigating the efficacy of anti-myopia interventions, changes in axial length (AL) and refractive error are usually considered as the primary outcome variables, both of which take a long time to detect a clinically meaningful differences, rendering the clinical studies time consuming,costly, and suffer great potential for high dropout rates.On the other hand, more recent studies in both animal and human demonstrated shorter-term ocular changes that occur at levels of the retina and choroid that are closely correlated with the chronic changes of AL and refraction.More specifically from animal models, choroid has been demonstrated to serve a significant role in local ocular growth mechanism.It is plausible that choroid is involved in a signaling cascade that is initiated in the retina and ultimately produces longterm changes in scleral growth[9-10].Consistent evidence from experimental models of various species indicates that myopic defocus, which eventually leads to myopia retardation and slowed axial elongation, induces the thickening of choroid as early as minutes after the initiation of the imposed defocus;conversely, hyperopic defocus or form deprivation, which promotes myopia progression and accelerated axial elongation,induces the thinning of choroid that far preceded the change of AL and refraction[5,11-14].In addition, the human choroid shows a similar response to signs of defocus to a lesser extent,which manifests in the bidirectional change of its thickness,corresponding to the signs of defocus similarly to what is observed in animal models.One plausible explanation of the bidirectional change of choroidal thickness (ChT) as an intermediate mechanism in minimal retinal defocus and the degradation of retinal image quality is that the position of the retina can be pushed forward with choroidal thickening or pulled backward with choroidal thinning to minimize the mismatch between retina and the image plane.Consequently, a lengthening or shortening in AL is also observed if it is defined as the length from the front apex of the cornea to the front of retinal pigment epithelium (RPE) layer.
The bidirectional, short term change in ChT,i.e., thinning in the presence of myopia-stimulating signals such as form deprivation and hyperopic defocus and thickening in the presence of myopia-inhibiting treatments such as overnight orthokeratology (OK)[15-17]or low dose atropine[18-19], strongly argues for the promising use of choroidal response as an intermediate marker for the longer-term anti-myopia efficacy of various treatments.Furthermore, the advancement in posterior segment imaging has made it possible to quantify the subtle changes of choroidal responses both in temporal and spatial domains.As a result, studies investigating the dose-dependent impact of simultaneously imposed myopic defocus and myopia-correcting signals on human choroidal response could yield valuable insights in the understanding of the decoding mechanisms of retina and aiding optical designs optimized for myopia control.
While competing myopic defocus delivered using contact lenses has demonstrated a significant myopia controlling effect,critical information regarding the ideal magnitude and spatial location of the myopic defocus to maximize anti-myopia efficacy remains to be elucidated.In this study, the aim was to investigate the impact of various myopic defocus magnitudes(add powers: +1.50 and +3.00 D) delivered at various spatial locations (distance zones: 1.5 and 3.0 mm) using multifocal gas permeable contact lens (MFGPCLs) on retinal defocus profile, change of ChT, and AL.
SUBJECTS AND METHODS
Ethical ApprovalThe study procedures adhered to the tenets of the Declaration of Helsinki and were approved by the Institutional Review Board of Pacific University College of Optometry (approval number 046-19 and project number 1411632-2).All participants provided written informed consent prior to the initiation of the study sessions.
SubjectsThis prospective study included 17 healthy young adults with a mean age of 23.17±4.48y.Demographics and baseline ocular measurements are listed in Table 1.
Measurements
Choroidal thicknessRight and left eyes were examined using Optovue spectral domain optical coherence tomography (OCT,Optovue Inc., Fremont, CA, USA) featuring a 5 µm axial resolution, 15 µm transverse resolution, and a scanning speed of 70 000 A-scans/s.In each visit, we obtained a total of ten high-resolution scans (five horizontal and five vertical), each 12 mm wide (1024 A-scans per B-scan, 496 pixels per A-scan).Throughout image acquisition, we ensured that the position of both horizontal and vertical scans was centered on the fovea.All line scans resulted from averaging a total of 60 B-scans.This scanning protocol covered an approximate retinal angle of 47°×47° horizontally and vertically, considering an AL of 24 mm.The ChT data were obtained automatically by using software that was developed by Alonso-Caneiroet al[20]and Readet al[21].ChT was measured as the distance from the posterior edge of the RPE to the line of choroid/scleral junction.Previous studies have demonstrated variations in ChT across different quadrants and eccentric locations[22-23], with the superior exhibiting the highest thickness and the nasal showing the thinnest.Given the reported regional variations in ChT, we collected and analyzed data separately for each location based on four quadrats and five regions in each quadrant[24].The software incorporated a feature for enhancing image contrast to further improve the visualization of the junction between the choroid and sclera.Additionally, the OCT follow-up feature was utilized to ensure that the OCT line image remained in the same position during baseline and follow-up visits.
To order to reduce the influence from major known confounders to ChT and AL including physical activity[25],near work[26], and ambient light[27].Subjects were instructedto spend 15min in a room with low photopic ambient light before the measurements, while focusing on a target placed at 3 m.The OCT scans were obtained with participants wearing MFGPCLs, given previous research indicating minimal impact of contact lens (CL) wear on OCT imaging of macular thickness[28]and retinal nerve fiber layer thickness[29].To account for the diurnal variation in ChT and AL, all measurements were consistently taken at the same time of day (between 11a.m.and 1p.m.) during baseline and followup visits.Baseline AL data for each participant were utilized to adjust the transverse scaling of each OCT scan, addressing ocular magnification as reported by Readet al[30].

Table 1 Demographics and baseline ocular characteristics
Peripheral autorefractionRefraction measurements, both at central and peripheral (off-axis), were acquired with an openfield auto-refractometer/keratometer WAM-5500 (Grand Seiko Co, Ltd., Hiroshima, Japan) up to 25 degrees in the nasal and temporal visual field along the horizontal meridian in 5-degree increments.Previous research has indicated no significant difference in peripheral refraction measurements between the eye turn and head turn methods[31].However, the head turn method was more appropriate in this study since it minimized the CL decentration and better maintained the CL in the primary gaze position.At least three readings were collected at each eccentric location while the subject was maintaining the primary gaze.Each measurement was taken after 1 to 2s after a blink to allow the lens to settle and recenter.Throughout the measurements, participants focused on a Maltese cross positioned at a 2.5-m distance centrally, consisting of horizontal grating lines equivalent to 0.1 logMAR (20/25) with 100% contrast.
To be able to obtain the off-axis refraction measurements at ±25 degrees along the horizontal meridian, two drops of 2.5% phenylephrine eye drops were administered in each eye (5min apart) to dilate the pupil with negligible impact on accommodation.Prior to each measurement, the examiner inspected the subject’s alignment to ensure proper chin and forehead positions.The subject’s tear film quality,lens centration, and alignment were checked between each measurement.Descriptive statistics (mean and SD) of the refraction vector components were analyzed as follows:
Where Sph, Cyl, andαare the sphere, cylinder, and axis obtained with the autorefractometer, respectively.Relative peripheral refractive (RPR) was calculated by subtracting the central spherical equivalent (M) from the spherical equivalent(SE) value at each eccentric measurement.
Axial length measurementsOcular AL data was obtained using IOLMaster 500 (Zeiss, Germany).Ten consecutive measurements were taken, and the first five readings with a signal-to-noise ratio (SNR) above 7.5 were selected and averaged for data analysis.All measurements were obtained on bare eyes as taking the measurements on eyes wearing gaspermeable contact lens (GPCL) significantly reduced SNR and the precision of repeated measurements.
MFGPCL Designs and FittingThis study investigated four different experimental designs (A, B, C, and D) of MFGPCL.Each design featured an optic zone diameter of 9 mm,encompassing a distance zone (DZ) that provided the full distance correction.The four designs were characterized by two add powers (+1.5vs+3.0 D) and two center DZ diameters (1.5vs3.0 mm).Designs A and B shared a DZ diameter of 1.5 mm, with the add power commencing at the central geometric endpoint of DZ to reach add powers of+3.00 D for design A and +1.50 D for lens design B at the 5.0-mm chord diameter.The add power increased by 1.00 D to achieve approximately +4.00 D and +2.50 D at the edge of the optical zone for lens designs A and B, respectively.Both lens designs C and D had a 3.0 mm DZ, with the add power starting at the central geometric endpoint of DZ.They provided +3.00 D (design C) or +1.50 D (design D) add power (5.0 mm chord diameter) and increased by 1.00 D to reach approximately +4.00 D and +2.50 D for design C and D, respectively.Schematic diagrams of MFGPCL designs are illustrated in Figure 1.All designs were manufactured from Boston XO (Hexafocon A) material, possessing a refractive index of 1.415 and oxygen permeability (Dk) of 100 (ISO/FATT cgs unit).All lenses were considered large diameter compare to traditional GPCL.The overall MFGPCLs diameter was determined by subtracting 0.80 mm of each participant’s horizontal visible iris diameter, as measured using the Medmont E300 corneal topographer (Medmont Pty, Ltd.,Melbourne, Australia).Upon lens manufacturing, the power profiles of all lenses were confirmed using ConTest II (Rotlex,Omer, Israel).
The fitting of the MFGPCLs was based on the participant’s subjective refraction measurements, corneal curvature, and horizontal visible iris diameter.Corneal topography data were obtained using the Medmont E300 corneal topographer.Overrefraction was performed during the trial visit, and adjustments to the final prescription were made.A new lens was ordered if discrepancies greater than ±0.25 D were identified.The lens fitting was assessed for centration during lateral gaze movements using a slit-lamp biomicroscope, ensuring that all lenses were fitted within the desired limits of less than 0.50 mm of decentration on blink.
Data Collection ProceduresAll subjects were randomly assigned to wear and be tested with one of the four experimental lenses (A, B, C, or D) binocularly for one week.After a washout period of 7d, the subjects were randomly assigned to wear another design binocularly for another week.The choroid measurements were obtained at baseline, day 1,and day 7 of lens wear.All participants were masked to the lens design.
Statistical AnalysisThis study was a balanced incomplete block design, where each subject was tested on two of the four lens designs.The statistical analysis of the data was performed using SPSS version 26.0 (SPSS, Inc., Chicago, IL, USA).In order to examine the significance of regional changes in ChT and AL associated with peripheral myopic defocus, including baseline ChT as a covariate, a within-subject analysis of variance of score change was conducted to include the within subject factors of 5 levels of choroidal eccentric regions, 4 levels of quadrant, and factor of lens type.AllPvalues were 2-sided and considered statistically significant when the values were less than 0.05.Bonferroni correction was applied to account for multiple comparisons.

Figure 1 Schematic diagrams of optical zones geometries of multifocal gas-permeable contact lens designs.
RESULTS
Changes of AL and ChT after Short-Term (1d and 1wk)MFGPCL WearNo interocular difference was found (P>0.05)in AL changes associated with MFGPCL wear.Immediatelty after 4h lens wear, AL shortening was detected with all four MFGPCL designs (P<0.001).Lens A induced an axial shortening of -13±29 µm, and -8±30 µm for lens B.A greater reduction in AL was observed with lens C (-26±44 µm) and lens D (-18±27 µm).The shortening of AL differed based on the day of measurement (P<0.001).At day 7, a further axial shortening was observed with lens A and D, wherein lens C showed less axial shortening.No significant difference in AL between day 1 and day 7 with lens B.Changes in AL at each measurement day were illustrated in Figure 2.A significant interaction between MFGPCL type and measurement day was found (P<0.001).
The statistical analysis revealed a significant ChT thickening following the MFGPCL wear with no significant interocular difference (main effect:F(1,280)=2.52,P=0.112).Changes in ChT differed significantly from day 1 and day 7 (main effect:F(3,401)=4.7,P<0.01).Significant differences in the magnitude of choroidal thickening were observed between MFGPCL designs (main effect:F(3,401)=13.9,P<0.001), with overall choroidal thickening of 5.6±6 and 2.7±7 µm with lens designs A and B, respectively.A greater thickening was shown with lens C (8.0±7 µm) and D (7.3±7 µm).Significant interaction of MFGPCL design and day of measurement (interaction effect:F(3,402)=15,P<0.001).

Figure 2 Change from baseline in AL following 1 and 7d of multifocal gas-permeable contact lens daily wear Error bars represent standard error of the mean.AL: Axial length; DZ: Distance zone diameter; Add: Addition power.

Figure 3 Change in ChT in each quadrant following 1 and 7d of multifocal gas-permeable contact lens daily wear Error bars represent standard error of the mean.ChT: Choroidal thickness; DZ:Distance zone diameter; Add: Addition power.
Interestingly, our data showed a statistically significant difference in baseline ChT that was quadrant-specific(P<0.001), where the greatest response was in superior and temporal quadrants (6.76±7.7, 7.47±8.8 µm, respectively) and lowest response in inferior (3.50±9.6 µm) and nasal choroid(5.21±9.8 µm).Figure 3 graphically summarized the effect of each MFGPCL design on superior, temporal, inferior, and nasal choroid.The means of change in AL and ChT at day 7 of MFGPCL wear are summerized in Table 2.
The defocus induced changes in ChT did not vary with choroidal eccentricity (P=0.426).However, there was a trend where the mean of choroidal thickening was greater in the macular areas and slightly reduced in peripheral regions.A significant negative correlation between the changes in subfoveal ChT and in AL associated with MFGPCL was noted(F(1,42)=20.13,P<0.001) with anR2of 0.324.The AL decreased by 1.25 µm for each 1 µm increase in ChT (Figure 4).

Figure 4 Scatter plot demonstrating the association between the change in subfoveal ChT and change in AL in response to MFGPCLs AL: Axial length; ChT: Choroidal thickness; MFGPCLs: Multifocal gaspermeable contact lens.

Figure 5 Change in relative peripheral refraction across the horizontal visual field in baseline (without lens) and four experimental MFGPCL designs Error bars represent standard error of the mean.MFGPCLs: Multifocal gas-permeable contact lens.DZ:Distance zone diameter; Add: Addition power.
Effect of MFGPCL Designs on Peripheral RefractionFigures 5 illustrates the changes with MFGPCL designs compared to baseline values (without CL) in SE and RPR,J0 and J45, respectively.All desings induced a sitatically significant myopic shift for 15o, 20o, and 25oof nasal field and 10o, 15o, 20oand 25oof the temporal field (main effect:F(4,970)=189.3,P<0.001).There was no observed significant difference between the four experimental MFGPCL designs in RPR within the 50omeasured horizontal field.Statistically significant differences were found in J0 astigmatic component between baseline and all experimental MFGPCL (main effect:F(4,888)=210,P<0.001) for 10o, 15o, 20o, and 25oof temporal eccentricity and 15o, 20o, and 25oof nasal eccentricity.Post hoc test showed no significant difference in the J0 astigmatic component between any of the MFGPCL (allP>0.05 in all comparisons).J45 astigmatic component did not differ significantly from baseline values (main effect:F(4,411)=0.569,P=0.685).
Figure 6 illustrates the relationship between the SE of subjective refraction and the RPR measured at 25oin both nasaland temporal fields.With all lens designs, significant positive correlations were found, in which more myopic shift at 25owith greater myopic refractive error at baseline (r=0.42, 0.65,0.70, and 0.75 for lens design A, B, C, and D, respectively).The baseline RPR at 25owas negatively correlated with amount of myopic refractive error (r=-0.44), which indicated more peripheral hyperopic defocus with higher myopia.
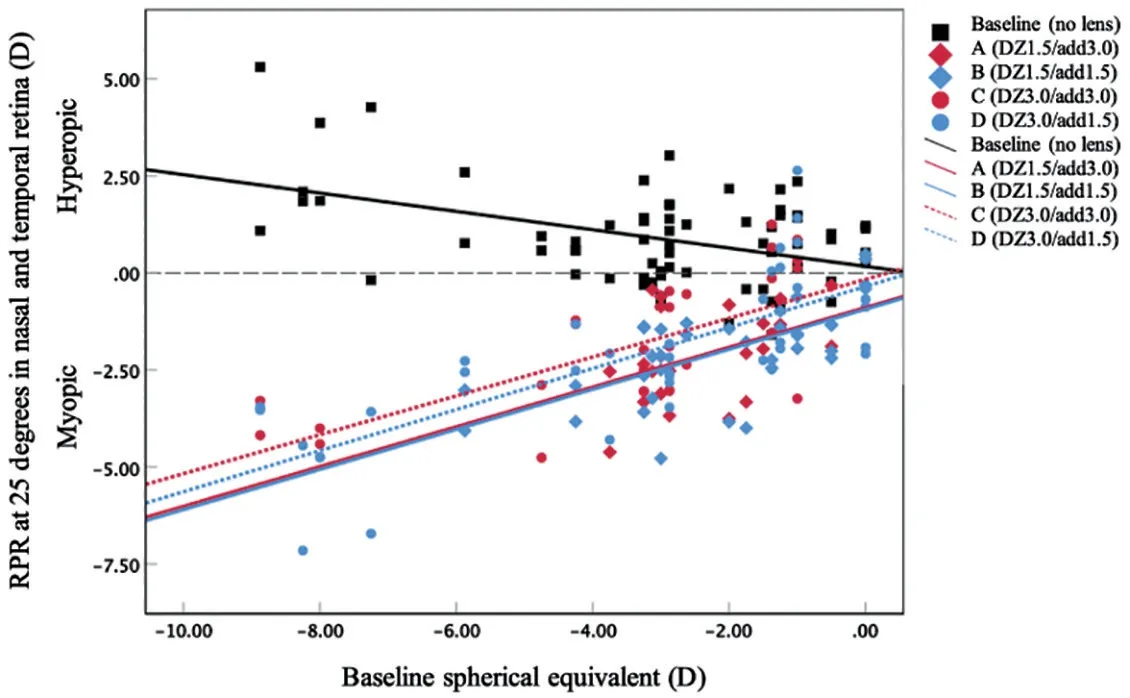
Figure 6 The correlation between the baseline refraction and the amount of RPR at 25 degree in the nasal and temporal retina at baseline without a lens (r=-0.44, P<0.001; represented by the black squares and black line), MFGP design A lens (r=0.42, P=0.05;represented by the red diamonds and red solid line), design B(r=0.65, P<0.001; represented by the blue diamonds and blue solid line), design C (r=0.70, P<0.001; represented by the red circles and red dashed line) and lens design D (r=0.75, P<0.001; represented by the blue circles and blue dashed line) RPR: Relative peripheral refractive; DZ: Distance zone diameter; Add: Addition power.
DISCUSSION
The study investigated the potential dose response relationship between the magnitude or the area of imposed myopic defocus using four MFGPCL designs with two add powers (+1.50 and+3.00 D) and two DZ diameters (1.5 and 3.0 mm) and the impact on retinal defocus profile, and the short-term change of ChT, as well as the subsequent change of AL.
Changes in AL and ChT Associated with MFGPCLs WearSeveral previous studies investigated the shortterm effect of full-field myopic and hyperopic defocus on AL and ChT in animals and humans.It is well established that choroid responds in a bi-directional manner.There is growing evidence suggests that choroid responds locally depending on what retinal region is exposed to the defocus.In addition to the choroidal response to defocus, an immediate retinal bioelectrical activity in response to imposed myopic and hyperopic defocus has been reported in several clinical studies[32-33].These findings suggest the capability of the human retina to distinguish and react accordingly to different signs of optical defocus, which is followed by choroidal response.
In this study, we have found consistent and significant changes in ChT and AL in response to the daily wear of MFGPCL in all four designs.Interestingly less changes were observed in both ChT and AL with the two designs in smaller distance area (lens A DZ1.5/add3.0 and B DZ1.5/add1.5).On the other hand, designs with bigger distance diameter,i.e., less area for the add power (lens C DZ3.0/add3.0 and D DZ3.0/add1.5)induced greater choroidal thickening and AL shortening.It is well known from animal studies that peripheral vision plays a critical role in the emmetropization and the development of refractive error, even in the presence of clear, undisrupted central vision.However, the exact mechanism of how central and peripheral retina work together in decoding the competing defocus signals is poorly understood.Although there tends to be a positive correlation between the area and the magnitude of plus defocus and the efficacy of axial inhibition, the dose dependent effect seems to saturate when one component of the competing defocus overwhelms the majority of the retina[34].One plausible explanation is that retina’s signaling process with defocus is similar to that of color and contrast, where the response is maximized when the stimulus pattern matches with the receptive field of the bipolar and/or the ganglion cells.The intriguing results we have found in this study that lenses with larger distance diameter, hence less area for plus defocus created a stronger effect on choroidal thickening, worth further investigation.It is possible that the optimal “receptive field” of central and paracentral retina matches better with the competing defocus profiles created by the DZ 3.0 designs hence a heightened choroidal response.
One of the research aims was to determine the dose-response relationship between peripheral myopic retinal defocus(magnitude and spatial location) and the short-term choroid response.Although there was consistent and significant choroidal thickening and AL shortening following a few hours of MFGPCL wear, which lasted throughout the whole observational period, our study did not show a clear doseeffect between the change of ChT and the induced peripheral myopic defocus.With increasing precision and repeatability in analyzing ChT, future studies with larger sample size and smaller steps in the area and add powers in lens design may be required to provide more information on the specific doseresponse relationship between the pattern and the strength of the competing defocus and the regional short-term change of ChT as well as AL.
Regarding the short-term persistency of the observed thickening of choroid induced by the MFGPCLs, we did not find a consistent trend based on lens designs.More specifically,the effect on ChT and AL after 7 continuous days of lens wear of design A (DZ1.5/add3.0) and D (DZ3.0/add1.5) increased significantly, slightly reduced with lens C (DZ3.0/add3.0), and remained the same with Lens B (DZ1.5/add1.5), comparing to the effect on the first day of lens wear.Future studies with longer follow up duration including longer wearing period and a washout period are required to better understand the sustainability of the effect and the possibility of a rebound effect.
It was noted that the temporal and superior choroid showed a greater thickening followed by nasal and inferior quadrants.The discrepancy in choroidal response among quadrants could be attributed to the fact that temporal and superior quadrants were exposed to myopic defocus during near work.Since it is well established that choroidal response to defocus is locally in a regionally selective manner[35], it is plausible that differences in myopic exposure in different quadrants during near work could cause the observed inconsistency in choroidal thickening.
Following MFGPCL wear, there was a trend that the mean ChT was greater in the foveal areas and reduced in peripheral regions, but the difference did not reach statistical significance.Two animal studies showed that choroidal response following full-field myopic defocus is greater in the macular region compare to that in the peripheral choroid despite the presence of myopic defocus signals over the entire retina[36-37].In humans, most studies focused mostly on the evaluation of subfoveal choroidal response to optical defocus[38-40].In a recent study, in which the macular and peripheral choroid response were measured following 60min of full-field myopic and hyperopic defocus in adults, the peripheral choroid also shown to have a significant bi-directional response to the opposite signs of defocus[41].Consistent with previously reported results, the findings from our study confirmed that both macular and peripheral regions of choroid were capable of bi-directional changes in response to imposed defocus induced with MFGPCL.
Our observation of the significant influence of the MFGPCL designs on choroidal response and AL is comparable to what has been reported with OK.In children, choroidal thickening was observed in several studies following one week to one year of OK wear (thickening ranges from +9 to +24 µm)[15-17].The effect of OK on ChT and AL diminished and reversed,where choroidal thinning and increase in AL were shown shortly following OK treatment cessation.Even though findings from several studies supported the involvement of short-term choroidal response to peripheral myopic defocus,two studies have reported no choroidal thickening with myopia control optical treatment.Gardneret al[42]investigated choroidal response in subfoveal and adjacent regions following 9mo of OK treatment in 9 myopic children.Despite large magnitudes of imposed peripheral retinal myopic defocus,one likely possibility explaining the lack of significance in the study results could be related to several key limitations in study design and analysis such as relatively small sample size, failure to account for physiologic diurnal fluctuation in ChT, and the absence of a control group to account for the significant agedependent changes in ChT that occur during the childhood.Another recent study tested the choroidal response following 30min of multifocal soft contact lenses (MFSCL) in adults.The study examined the effects of three different modalities of MFSCL and did not detect statistically significant changes in ChT[43].
Impact of MFGPCL Designs on Retinal Defocus ProfileAll four MFGPCL designs induced a significant peripheral myopic shift in peripheral refraction.Intersentingly from RPR data (Figure 5), lens design A (DZ1.5/add3.0) showed the least myopic shift in the periphery compared with other designs that had lower add power.From SE data, we observed an unrcorrected center refraction for design A and B (-2.13±0.44 and -1.05±0.45, respectively).Lens A and B both share a similar DZ diameter of 1.50 mm, where the add power begins at the edge of that center zone.The on-axis myopia observed with lens A and B was likely due to the autorefaction technique, since it uses 2.3 to 2.5 mm light beam to obtain refraction data, which is larger than the lens (A and B) zone diameter, resulting an apparent undercorrection in central myopia.
One dual-focus soft CL that is FDA approved for myopia control incorporates the treatment zone of +2.00 D[44].The mechanism underlying the manipulation of peripheral optics to achieve myopia control effect is not fully understood.Soft CLs have the advantage of a shorter adaptation period when compared to GPCL.However, GPCLs appear to offer a better platform for optical design customization, especially the combination of multifocal and toric designs.In manipulating peripheral refraction, previous studies have shown that MFGPCL can be more effective in inducing peripheral myopic defocus when compared to MFSCL with a similar design.Bifocal soft lenses with +2.00 D add did not produce a significant peripheral myopic shift in children[45].Lopes-Ferreiraet al[46]measured peripheral refraction with four Proclear MFSCLs of different add power (+1.00 to +4.00 D,in 1 D step).Only lenses with add +3.00 and +4.00 induced a significant myopic shift compared to naked eye, lenses with +1.00 and +2.00 add showed no significant change from baseline.Also, lenses with high adds (+3.00 and +4.00 D) did not differ significantly from each other.These findings suggest that increasing the peripheral addition power on MFSCLs does not necessarily increase the relative peripheral myopia proportionally.With the use of MFGPCL, the findings from Paunéet al[47-48]showed the effect of MFGPCL be stronger in manipulating peripheral refraction.
In the current study, the MFGPCL design showed an increase in peripheral astigmatism (J0 component).Paunéet al[47-48]reported a significant increase in J0 with MFGPCLs.The change in J0,the asymmetry observed in RPR, and J0 between nasal and temporal fields might be related to slight decentration of the GPCL in the primary gaze position.All four lens designs had a large diameter to improve centration.However, a small lens decentration (within 0.50 mm) might affect the results and explain the asymmetry observed between nasal and temporal in peripheral SE, RPR, and J0.
There are limitations in this study that must be acknowledged.First, the sample size was small.Future studies need to assess the impact of MFGPCL in a larger sample and on the pediatric population with progressing myopia.Second, participants were only instructed to wear the lenses for a minimum of 4h per day, but no instructions were given regarding what time of day the lenses should be worn.A recent study reported that ChT response to myopic defocus seems to be dependent on the time of the day, where it is greater in the latter half of the day compared to the response observed during the morning.It is suggested there is a potential interaction between the diurnal variation in the choroid and the visual signal associated with the optical defocus[49].Additionally, time spent on certain activities, such as outdoor and nearwork, while wearing MFGPCLs were not monitored or reported, which could be a factor influencing choroidal response to optical defocus.Last, we only measured the choroidal response during one week of MFGPCL daily wear.The needed time to achieve full adaptation to GPCL can be up to 15d[50].Evaluating the choroidal response to MFGPCLs over a more extended period would be more ideal future research.The visual performance with the four MFGPCL designs investigated in this study was previously evaluated and showed satisfactory visual performance[51].
In conclusion, short-term exposure to peripheral myopic defocusviaseveral MFGPCL optical designs elicited a significant thickening of the choroid and shortening of AL,which supports the application of MFGPCL in controlling myopia.Among the four MFGPCL optical designs, changing the DZ diameters (between 1.5 and 3.0 mm) and additional power (1.5 and 3.0 D) did not show a clear dose-effect to peripheral myopic defocus in ChT and AL.Our results also indicated that MFGPCLs were capable of inducing significant relative peripheral myopia.The reliability and feasibility of quantifying short-term changes in ChT support its use as a promising marker for the long-term efficacy of myopia controlling treatments.
ACKNOWLEDGEMENTS
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project (No.IFKSUOR3-433-1)
Conflicts of Interest:Alanazi M,None;Caroline P,None;Alshamrani A,None;Liu M,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Using choroidal thickness to detect myopic macular degeneration
- Baerveldt glaucoma implant with Supramid© ripcord stent in neovascular glaucoma: a case series
- Efficacy and safety of Usights UC100 illuminated microcatheter in microcatheter-assisted trabeculotomy
- Quantifying peripapillary vessel density and retinal nerve fibre layer in type 1 diabetic children without clinically detectable retinopathy using OCTA
- Nomogram to predict severe retinopathy of prematurity in Southeast China
- Effect of aflibercept combined with triamcinolone acetonide on aqueous humor growth factor and inflammatory mediators in diabetic macular edema
