Peguero-Lo-Presti指数诊断的左心室肥厚与阵发性心房颤动射频导管消融术后复发的关系
2024-02-18张明龙方媛媛隋晓鹏陈欣欣李留东王海涛
张明龙 方媛媛 隋晓鹏 陈欣欣 李留东 王海涛
摘要:目的 探討Peguero-Lo-Presti指数诊断的左心室肥厚(LVH)与阵发性心房颤动(房颤)射频消融术后复发的关系。方法 选取成功接受射频导管消融的阵发性房颤患者652例。根据Peguero-Lo-Presti指数分为LVH组(167例)和左心室正常(LVN)组(485例)。收集患者的基线资料,在射频导管消融术后3、6、12个月以及此后每12个月间隔进行规律随访,评估有无房颤复发;采用Kaplan-Meier法绘制2组的房颤复发曲线;Cox比例风险模型评估房颤复发的影响因素。结果 中位随访时间为20.5(15.0,26.0)个月,共155例(23.8%)患者出现复发,LVH组95例,LVN组60例,LVH组的无房颤复发率明显低于LVN组(64.1% vs. 80.4%,Log-rank χ2=26.361,P<0.01)。校正年龄、性别、体质量指数、高血压、糖尿病、冠心病、心功能不全、左房前后径、左室舒张末期内径和左室射血分数后,Peguero-Lo-Presti指数诊断的LVH仍是房颤复发的危险因素[HR(95%CI):2.359(1.663~3.345),P<0.01]。结论 Peguero-Lo-Presti指数诊断的LVH是阵发性房颤患者射频导管消融术后复发的危险因素。
关键词:肥大,左心室;心房颤动;射频消融术;复发;Peguero-Lo-Presti指数
中图分类号:R541.75文献标志码:ADOI:10.11958/20230627
Relationship between left ventricular hypertrophy diagnosed by Peguero-Lo-Presti index and recurrence after radiofrequency catheter ablation of paroxysmal atrial fibrillation
Abstract: Objective To investigate the relationship between left ventricular hypertrophy (LVH) diagnosed by Peguero-Lo-Presti index and recurrence of paroxysmal atrial fibrillation (AF) after radiofrequency ablation. Methods A total of 652 patients with paroxysmal atrial fibrillation who underwent radiofrequency ablation were selected. According to Peguero-Lo-Presti index, patients were divided into the LVH group (167 cases) and the normal left ventricle group (485 cases). Baseline data were collected, and regular follow-up was performed at 3, 6 and 12 months after radiofrequency catheter ablation. The recurrence of AF was assessed. Kaplan-Meier survival curve was used to analyze the recurrence rate of AF in the two groups. Cox proportional hazard model was used to assess risk factors for recurrent atrial fibrillation. Results The median follow-up time was 20.5 (15.0, 26.0) months. A total of 155 patients (23.8%) developed recurrence of AF, including 95 patients in the LVH group and 60 patients in the LVN group. The recurrence rate without AF was significantly lower in the LVH group than that in the LVN group (64.1% vs. 80.4%, Log-rank χ2=26.361, P<0.01). After adjusting for age, sex, body mass index, hypertension, diabetes, coronary heart disease, cardiac dysfunction, left anteroposterior and posterior atrial diameter, left ventricular end-diastolic diameter, and left ventricular ejection fraction, LVH diagnosed by Peguero-Lo-Presti index was still a risk factor for recurrent AF [HR (95%CI) : 2.359 (1.663-3.345), P<0.01]. Conclusion In patients with paroxysmal AF, LVH diagnosed by Peguero-Lo-Presti index is a risk factor of AF recurrence after radiofrequency catheter ablation.
Key words: hypertrophy, left ventricular; atrial fibrillation; radiofrequency ablation; recurrence; Peguero-Lo-Presti index
心房颤动(房颤)是临床上常見的心律失常之一[1]。尽管射频导管消融治疗阵发性房颤成功率较高,但仍有一定的复发风险[2]。既往研究发现,心电图(electrocardiograph,ECG)对左心室肥厚(left ventricular hypertrophy,LVH)的诊断(ECG-LVH)可以独立于左心室质量预测房颤的发生,并可预测其不良心血管事件的发生[3-4]。且ECG-LVH在预测心血管不良事件方面的性能优于其诊断LVH的能力,因而有学者建议ECG-LVH标准的主要用途应该是心血管疾病的危险分层和风险预测;美国心脏病学会基金会/美国心脏学会/美国心律协会(ACCF/AHA/HRS)的ECG标准化与解析建议中,也强调开发仅用于预测心血管疾病预后的ECG-LVH新的标准[5]。近年来,Peguero等[6]提出了一种诊断LVH的ECG新标准,即Peguero-Lo-Presti指数,其诊断LVH的敏感度达到62%,远高于传统的ECG标准。目前Peguero-Lo-Presti指数主要用于评估高血压合并心血管疾病患者的预后,其与房颤射频导管消融术后复发的关系尚不明确。本研究拟在接受射频导管消融的阵发性房颤患者中,探讨基于Peguero-Lo-Presti指数诊断的LVH与房颤复发的关系,以期为房颤的临床治疗提供参考。
1 对象与方法
1.1 研究对象 选取2017年1月—2020年12月于烟台毓璜顶医院心血管内科成功接受射频导管消融的阵发性房颤患者。纳入标准:(1)症状性阵发性房颤患者。(2)至少使用一种Ⅰ类或Ⅲ类抗心律失常药物无效。(3)经射频导管消融成功隔离双侧肺静脉。(4)术前至少有1份高质量、可用于准确测量的ECG。排除标准:(1)瓣膜性房颤。(2)左心房前后径(left atrium diameter,LAD)≥50 mm。(3)既往行房颤消融手术。(4)合并左心房血栓、严重肝肾功能不全、未控制的甲状腺功能亢进、严重出血倾向等手术禁忌证。(5)同时行冠状动脉支架植入或左心耳封堵手术。阵发性房颤定义为:发作后7 d内自行终止或通过干预终止的房颤[2]。本研究共纳入652例患者,年龄18~79岁,平均(59.1±9.90)岁,其中男434例(66.6%)。所有患者均签署知情同意书,研究符合2013年修订的《赫尔辛基宣言》的要求。
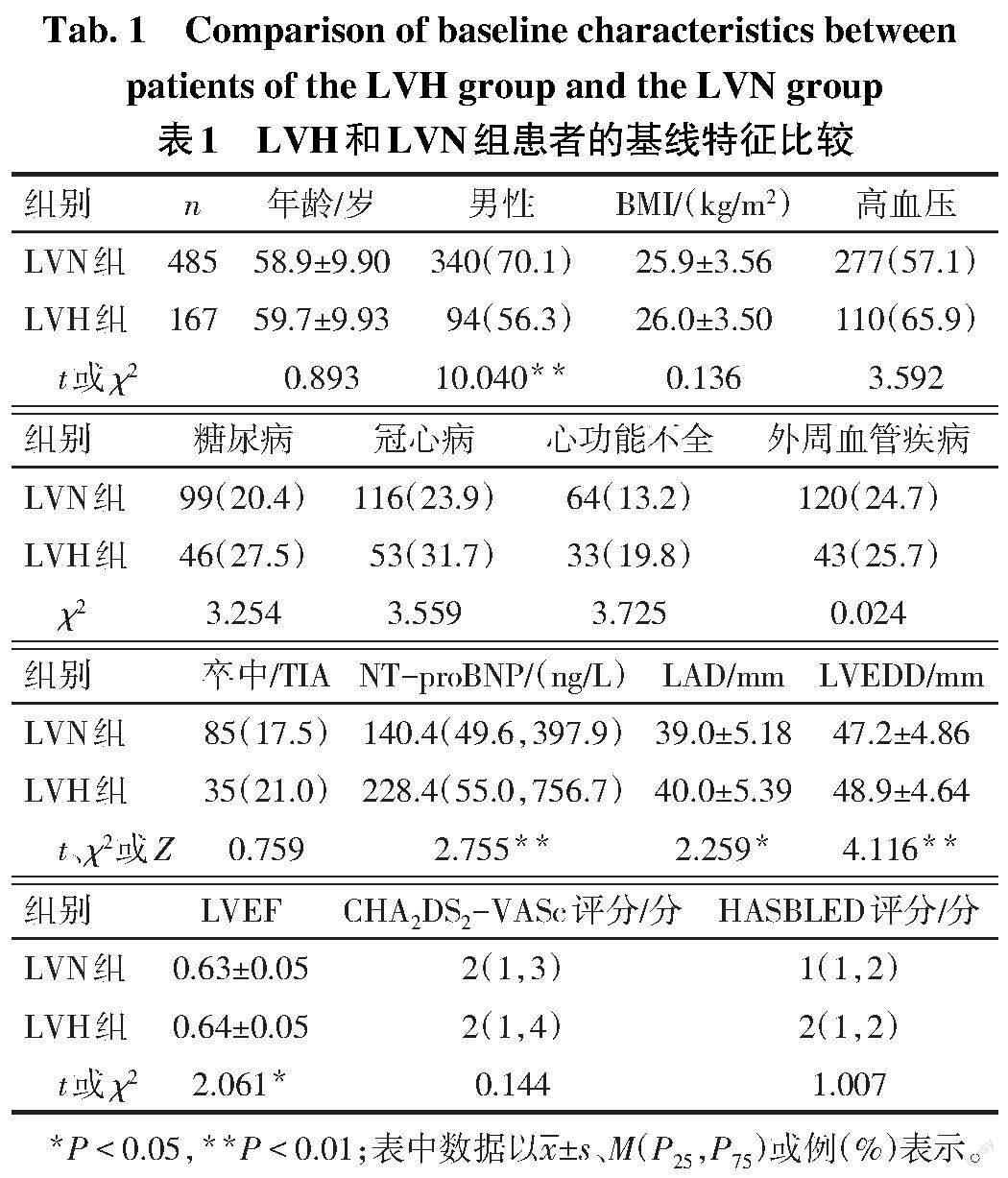
1.2 ECG参数测量和分组 测量方法参考既往研究[7]:以PR段为测量基线,测量各个导联中最高的R或R'波和最深的S或QS波。每份ECG的各个导联均选择3个独立的QRS波,测量上述指标,并计算平均值。所有ECG测量均由2名有经验的心血管内科医生进行,当两者的测量结果明显不一致时,以另一名更高资历医生意见为准。Peguero-Lo-Presti指数定义为所有导联中最深S波的振幅与V4导联的S波振幅之和[6],Peguero-Lo-Presti指数男性≥2.6 mV,女性≥2.2 mV则诊断为LVH。根据Peguero-Lo-Presti指数分为LVH组(167例)和左心室正常组(LVN组,485例)。
1.3 资料收集 收集患者的基线资料,包括年龄、性别、体质量指数,高血压、糖尿病、冠心病、心功能不全、外周血管疾病、卒中/短暂性脑缺血发作(TIA)史,及术前N末端B型利钠肽前体(NT-proBNP)、LAD、左心室舒张末期内径(LVEDD)、左心室射血分数(LVEF)、CHA2DS2-VASc评分和HASBLED评分。
1.4 射频导管消融围术期管理 所有患者均在手术前签署射频导管消融手术知情同意书。完善超声心动图及左心房增强CT,评估LAD、LVEF和左心房血栓等。除β受体阻滞剂外,术前服用的抗心律失常药物(antiarrhythmic drugs,AADs)在消融前3 d停用。在术后3个月的空白期内,所有患者均需接受ADDs治疗[8]。若患者术后3个月无房颤复发,则终止ADDs治疗。
1.5 射频导管消融手术 手术过程同既往研究[9-10]:通过股静脉途径,2次穿刺房间隔后,在三维电解剖标测系统(CARTOTM,强生公司,美国)指导下使用环状电极建立左心房模型,然后使用消融大头(ThermoCool或ThermoCool SmartTouch,强生公司,美国)进行双侧肺静脉的环状消融,消融参数:采用功率控制模式,温度43 ℃,功率30~35 W,生理盐水灌注速度17 mL/min。手术终点设定为所有肺静脉均达到双侧电隔离。此外,术者根据患者的左心房基质、典型心房扑动史以及上腔静脉触发情况,附加左心房顶部、三尖瓣峡部、二尖瓣峡部线性消融以及上腔静脉隔离[11-12]。
1.6 随访 在射频导管消融术后3、6、12个月以及此后每12个月间隔进行规律随访,均由经过严格培训的心血管内科医师通过病历审查、门诊随访和电话随访。在每次随访均需评估患者心电图和24 h动态心电图,以明确有无房颤复发。若患者出现任何疑似房颤复发的症状,如胸闷、心悸,则嘱患者立即就诊,完善心电图和(或)24 h动态心电图评估有无房颤复发。房颤复发定义为射频导管消融术3个月后出现持续至少30 s的房性心动过速、心房扑动和(或)房颤[2]。
1.7 统计学方法 采用SPSS 22.0软件进行数据分析。计量资料以[[x] ±s
](符合正态分布)或M(P25,P75)(不符合正态分布)表示,组间比较采用t检验或Mann-Whitney U检验。计数资料以例(%)表示,组间比较采用χ2检验。采用Kaplan-Meier生存曲线分析无房颤复发率,Log-rank检验比较组间差异。采用Cox比例风险模型评估预测房颤复发因素的风险比(HR)和95%置信区间(CI)。所有分析均采用双侧检验,P<0.05为差异有统计学意义。
2 结果
2.1 LVN组和LVH组患者的基线特征对比 LVH组患者的NT-proBNP、LAD、LVEDD和LVEF高于LVN组,男性比例低于LVN组(P<0.05),见表1。
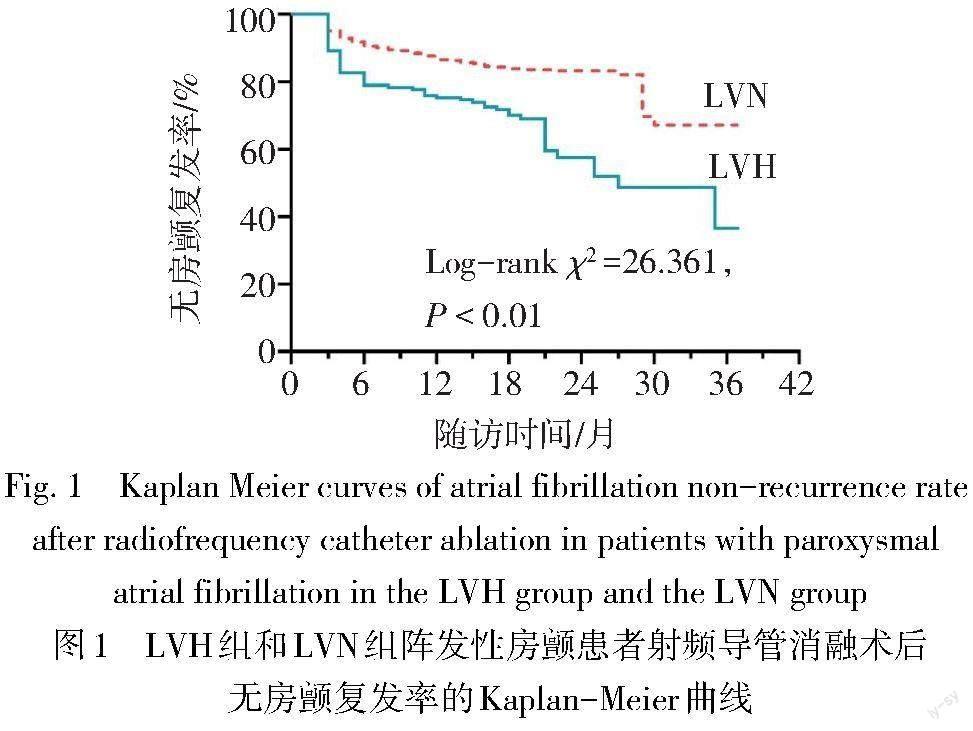
2.2 射频导管消融术后的随访结果 中位随访时间为20.5(15.0,26.0)個月,共155例(23.8%)患者出现房颤复发,LVH组95例,LVN组60例,LVH组的无房颤复发率明显低于LVN组(64.1% vs. 80.4%,Log-rank χ2 =26.361,P<0.01),见图1。
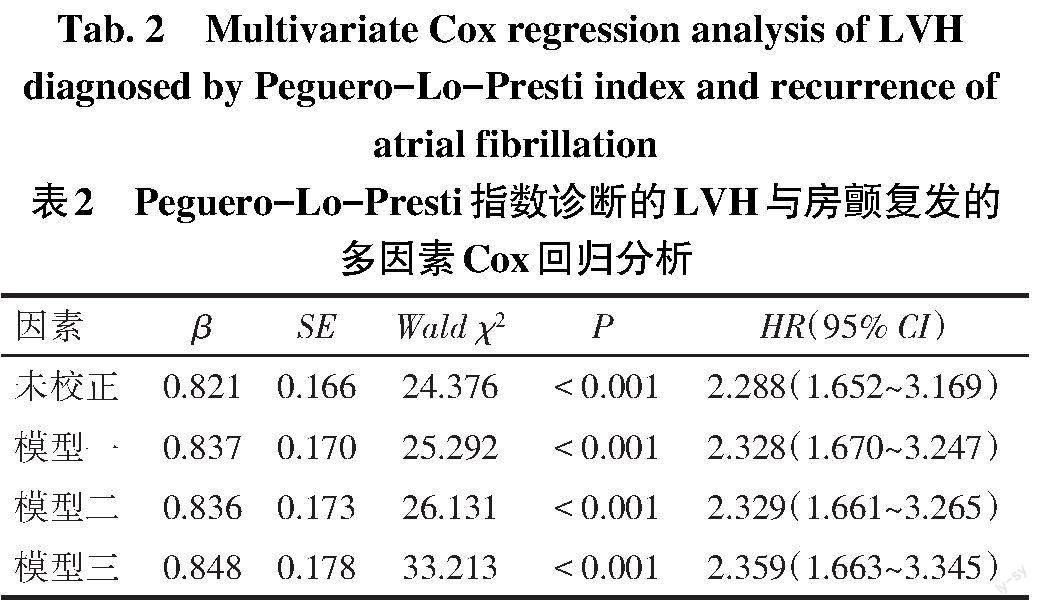
2.3 房颤复发的影响因素 以房颤复发情况为因变量(复发=1,无复发=0),LVH为自变量(LVH=1,LVN=0),未校正其他因素下LVH为房颤导管消融术后复发的危险因素(HR:2.288,95%CI:1.652~ 3.169,P<0.01)。模型一校正年龄、性别(女性=1,男性=0)和BMI;在模型一的基础上,模型二进一步校正传统心血管疾病危险因素:高血压(有=1,无=0)、糖尿病(有=1,无=0)、冠心病(有=1,无=0)和心功能不全(有=1,无=0);在模型二的基础上,模型三进一步校正心脏结构改变参数(LAD、LVEDD和LVEF),多因素Cox回归分析均显示Peguero-Lo-Presti指数诊断的LVH是房颤复发的危险因素(P<0.01),见表2。
3 讨论
环肺静脉电隔离是房颤导管消融的基石。研究表明,环肺静脉电隔离的总体成功率在阵发性房颤中为50%~70%[13-14]。虽然国内外临床研究发现众多临床指标和电生理参数与房颤术后复发相关,如LAD、早期复发、房颤时长、P波宽度、左房低电压程度等[14-15],但房颤复发可能是多种因素相互作用的结果,上述因子对于术后复发的预测价值有限。
LVH可能是继发于室壁增厚(同心LVH)、心腔增大(偏心LVH)或两者兼而有之。左心室心肌在多种病理生理因素的影响下长期超负荷工作可进展为LVH。既往研究提示,LVH的患病率为15%~45%[16-18];本研究中为25.6%。Verdecchia等[19]研究发现,在5年的随访中,左心室质量每增加一个标准差(14 g/m2),房颤的风险增加20%(95%CI:1.07~1.34)。Chrispin等[20]将MR诊断的LVH作为校正因素,发现ECG-LVH是新发房颤的独立预测因子。此外,Verdecchia等[21]研究发现LVH可预测房颤抗凝患者的卒中、心血管死亡、全因死亡和心肌梗死风险。
尽管ECG检查诊断LVH的敏感性较低,但由于其简便易用、便宜等优点,仍然是诊断LVH应用最广泛的工具。在动脉粥样硬化多种族人群中进行的一项研究表明,在预测LVH引起的心血管疾病不良事件方面,ECG与MR成像的效能相似[22]。Peguero等[6]基于心室终末除极向量的差异,发现所有导联中最深S波振幅的动态变化指标能增加诊断LVH的敏感性,从而提出了Peguero-Lo-Presti指数。本研究中Peguero-Lo-Presti指数诊断的LVH是阵发性房颤患者射频消融术后复发的独立影响因子。Li等[23]通过基于ECG的Romhilt Estes评分发现,Romhilt Estes评分诊断的LVH是房颤射频消融术后复发的独立预测因子。王璐等[24]通过对208例合并高血压的阵发性房颤患者的研究发现,ECG-LVH伴心肌劳损[HR(95%CI):2.103(1.231~3.590)]和不伴心肌劳损[HR(95%CI):2.621(1.238~5.550)]是房颤射频导管消融术后复发的独立危险因素。
LVH与房颤发生之间涉及多种病理生理学机制。LVH会导致心室肌肌节紊乱、心肌纤维化、糖酵解代谢和钙处理的改变,使左心室的舒张异常和充盈受损[25]。随着后负荷增加,心房和肺静脉进一步扩张用以代偿,导致心房电重构和结构重构。本研究也发现LVH组患者的LAD和LVEDD更大,阵发性房颤合并Peguero-Lo-Presti指数诊断的LVH患者的心脏重构更明显。Badheka等[26]发现伴有LVH的房颤患者左心房纤维化程度更重,而左心房纤维化是房颤发生和发展的重要基质。此外,Medi等[27]发现LVH的房颤患者心房电重构的3个特征:整体传导减慢、区域传导延迟和房颤诱导率增加。因此,LVH可能通过加重左心房的电重构和解剖重构,从而增加房颤患者射频导管消融手术的复发率。此外,LVH患者可能存在离子通道的异常改变,进而导致心脏去极和复极过程的异常[28],增加房颤患者射频导管消融手术的复发风险。
综上,Peguero-Lo-Presti指数诊断的LVH是阵发性房颤患者射频导管消融术后复发的独立影响因素。对于合并有LVH的阵发性房颤患者,通过更严格的血压控制或其他治疗措施来逆转LVH有望改善阵发性房颤合并LVH患者的射频消融手术的长期临床效果。本研究存在的局限性:本研究为单中心研究,结论需要在大样本的多中心、随机对照研究中进一步验证;随访是基于ECG和(或)24 h动态心电图的间歇性心律监测进行评估的,一些非持续或无症状的房颤发作可能未被记录到,而导致房颤的复发率被低估[29];仅分析了术前LVH对射频导管消融术后房颤复发的影响,LVH的干预是否能改善射频导管消融术后房颤的长期疗效还需进一步研究。
参考文献
[1] LIPPI G,SANCHIS-GOMAR F,CERVELLIN G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge[J]. Int J Stroke,2021,16(2):217-221. doi:10.1177/1747493019897870.
[2] 黄从新,张澍,黄德嘉,等. 心房颤动:目前的认识和治疗的建议-2018[J]. 中国心脏起搏与心电生理杂志,2018,32(4):315-368. HUANG C X,ZHANG S,HUANG D J,et al. Atrial fibrillation:Current understanding and recommendations for Treatment 2018[J]. Chinese Journal of Cardiac Pacing and Electrophysiology,2018,32(4):315-368. doi:10.13333/j.cnki.cjcpe.2018.04.001.
[3] TANAKA F,KOMI R,NAKAMURA M,et al. Additional prognostic value of electrocardiographic left ventricular hypertrophy in traditional cardiovascular risk assessments in chronic kidney disease[J]. J Hypertens,2020,38(6):1149-1157. doi:10.1097/HJH.0000000000002394.
[4] BAKHTIARI F,DAVARMOIN G,GHAFFARI S,et al. Electrocardiographic left ventricular hypertrophy is not associated with increased in-hospital adverse events in patients with first Non-ST segment elevation myocardial infarction:A single center study[J]. Caspian J Intern Med,2019,10(3):289-294. doi:10.22088/cjim.10.3.289.
[5] HANCOCK E W,DEAL B J,MIRVIS D M,et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram:part V:electrocardiogram changes associated with cardiac chamber hypertrophy:a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee,Council on Clinical Cardiology;the American College of Cardiology Foundation;and the Heart Rhythm Society:endorsed by the International Society for Computerized Electrocardiology[J]. Circulation,2009,119(10):e251-261. doi:10.1161/CIRCULATIONAHA.108.191097.
[6] PEGUERO J G,LO PRESTI S,PEREZ J,et al. Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy[J]. J Am Coll Cardiol,2017,69(13):1694-1703. doi:10.1016/j.jacc.2017.01.037.
[7] 张明龙,杨文琦,王晓东,等. 左心室肥厚的心电图诊断新标准在中国人群中的诊断价值及其影响因素[J]. 心脏杂志,2022,34(2):178-182. ZHANG M L,YANG W Q,WANG X D,et al. Diagnostic efficacy and influencing factors of a new standard for diagnosis of left ventricular hypertrophy in Chinese population[J]. Chinese Heart Journal,2022,34(2):178-182. doi:10.12125/j.chj.202105003.
[8] XIA Y,LI X F,LIU J,et al. The influence of metabolic syndrome on atrial fibrillation recurrence:five-year outcomes after a single cryoballoon ablation procedure[J]. J Geriatr Cardiol,2021,18(12):1019-1028. doi:10.11909/j.issn.1671-5411.2021.12.008.
[9] 贾朝旭,蒋超,卢尚欣,等. 体重控制与超重及肥胖患者心房颤动射频消融术后复发的关系[J]. 中華心血管病杂志,2019,47(8):595-601. JIA C X,JIANG C,LU S X,et al. Association between weight control and recurrence of atrial fibrillation after catheter ablation in overweight and obese patients[J]. Chin J Cardiol,2019,47(8):595-601. doi:10.3760/cma.j.issn.0253?3758.2019.08.002.
[10] FINK T,SCHL?TER M,HEEGER C H,et al. Stand-alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long-standing persistent atrial fibrillation:the randomized alster-lost-af trial(ablation at st. georg hospital for long-standing persistent atrial fibrillation)[J]. Circ Arrhythm Electrophysiol,2017,10(7):e005114. doi:10.1161/CIRCEP.117.005114.
[11] NAKAMURA T,HACHIYA H,YAGISHITA A,et al. The relationship between the profiles of SVC and sustainability of SVC fibrillation induced by provocative electrical stimulation[J]. Pacing Clin Electrophysiol,2016,39(4):352-360. doi:10.1111/pace.12814.
[12] ANSELME F,SAVOURé A,CLéMENTY N,et al. Preventing atrial fibrillation by combined right isthmus ablation and cryoballoon pulmonary vein isolation in patients with typical atrial flutter:PAF-CRIOBLAF study[J]. J Arrhythm,2021,37(5):1303-1310. doi:10.1002/joa3.12626.
[13] KUCK K H,BRUGADA J,FüRNKRANZ A,et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation[J]. N Engl J Med,2016,374(23):2235-2245. doi:10.1056/NEJMoa1602014.
[14] MOHANTY S,DELLA ROCCA D G,GIANNI C,et al. Predictors of recurrent atrial fibrillation following catheter ablation[J]. Expert Rev Cardiovasc Ther,2021,19(3):237-246. doi:10.1080/14779072.2021.1892490.
[15] LIU P,LV T,YANG Y,et al. Use of P wave indices to evaluate efficacy of catheter ablation and atrial fibrillation recurrence: a systematic review and meta-analysis[J]. J Interv Card Electrophysiol,2022,65(3):827-840. doi:10.1007/s10840-022-01147-7.
[16] CUSPIDI C,SALA C,NEGRI F,et al. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies[J]. J Hum Hypertens,2012,26(6):343-349. doi:10.1038/jhh.2011.104.
[17] CUSPIDI C,FACCHETTI R,QUARTI-TREVANO F,et al. Incident left ventricular hypertrophy in masked hypertension[J]. Hypertension,2019,74(1):56-62. doi:10.1161/HYPERTENSIONAHA.119.12887.
[18] JIANSHU C,QIONGYING W,YING P,et al. Association of free androgen index and sex hormone-binding globulin and left ventricular hypertrophy in postmenopausal hypertensive women[J]. J Clin Hypertens(Greenwich),2021,23(7):1413-1419. doi:10.1111/jch.14301.
[19] VERDECCHIA P,REBOLDI G,GATTOBIGIO R,et al. Atrial fibrillation in hypertension:predictors and outcome[J]. Hypertension,2003,41(2):218-223. doi:10.1161/01.hyp.0000052830.02773.e4.
[20] CHRISPIN J,JAIN A,SOLIMAN E Z,et al. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation:MESA(Multi-Ethnic Study of Atherosclerosis)[J]. J Am Coll Cardiol,2014,63(19):2007-2013. doi:10.1016/j.jacc.2014.01.066.
[21] VERDECCHIA P,REBOLDI G,DI PASQUALE G,et al. Prognostic usefulness of left ventricular hypertrophy by electrocardiography in patients with atrial fibrillation(from the Randomized Evaluation of Long-Term Anticoagulant Therapy Study)[J]. Am J Cardiol,2014,113(4):669-675. doi:10.1016/j.amjcard.2013.10.045.
[22] ARO A L,CHUGH S S. Clinical diagnosis of electrical versus anatomic left ventricular hypertrophy:Prognostic and therapeutic implications[J]. Circ Arrhythm Electrophysiol,2016,9(4):e003629. doi:10.1161/CIRCEP.115.003629.
[23] LI S N,WANG L,DONG J Z,et al. Electrocardiographic left ventricular hypertrophy predicts recurrence of atrial arrhythmias after catheter ablation of paroxysmal atrial fibrillation[J]. Clin Cardiol,2018,41(6):797-802. doi:10.1002/clc.22957.
[24] 王璐,李松南,董建增,等. 左心室肥厚對合并高血压阵发性心房颤动患者射频消融术后复发的影响[J]. 中华心律失常学杂志,2018,22(5):412-418. WANG L,LI S N,DONG J Z,et al. Electrocardiographic left ventricular hypertrophy predicts recurrence of atrial arrhythmias after catheter ablation of paroxysmal atrial fibrillation with hypertension[J]. Chinese Journal of Cardiac Arrhythmias,2018,22(5):412-418. doi:10.3760/cma.j.issn.1007-6638.2018.05.009.
[25] ZHU P,DAI Y,QIU J,et al. Prognostic implications of left ventricular geometry in coronary artery bypass grafting patients[J]. Quant Imaging Med Surg,2020,10(12):2274-2284. doi:10.21037/qims-19-926.
[26] BADHEKA A O,SHAH N,GROVER P M,et al. Outcomes in atrial fibrillation patients with and without left ventricular hypertrophy when treated with a lenient rate-control or rhythm-control strategy[J]. Am J Cardiol,2014,113(7):1159-1165. doi:10.1016/j.amjcard.2013.12.021.
[27] MEDI C,KALMAN J M,SPENCE S J,et al. Atrial electrical and structural changes associated with longstanding hypertension in humans:implications for the substrate for atrial fibrillation[J]. J Cardiovasc Electrophysiol,2011,22(12):1317-1324. doi:10.1111/j.1540-8167.2011.02125.x.
[28] GUO D,YOUNG L,WU Y,et al. Increased late sodium current in left atrial myocytes of rabbits with left ventricular hypertrophy:its role in the genesis of atrial arrhythmias[J]. Am J Physiol Heart Circ Physiol,2010,298(5):H1375-1381. doi:10.1152/ajpheart.
01145.2009.
[29] PIERAGNOLI P,PAOLETTI PERINI A,RICCIARDI G,et al. Recurrences in the blanking period and 12-month success rate by continuous cardiac monitoring after cryoablation of paroxysmal and non-paroxysmal atrial fibrillation[J]. J Cardiovasc Electrophysiol,2017,28(6):625-633. doi:10.1111/jce.13190.
