BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
2024-02-16MichaelWeibleIIMichaelLofhelaceHamishMundellTszWaiRositaPangTailoiChanLing
Michael W.Weible II , Michael D.Lofhelace , Hamish D.Mundell , Tsz Wai Rosita Pang Tailoi Chan-Ling
Abstract Roof plate secretion of bone morphogenetic proteins (BMPs) directs the cellular fate of sensory neurons during spinal cord defhelopment, including the formation of the ascending sensory columns, though their biology is not well understood.Type-II BMP receptor (BMPRII), the cognate receptor, is expressed by neural precursor cells during embryogenesis; howefher, an in fhitro method of enriching BMPRII+ human neural precursor cells (hNPCs) from the fetal spinal cord is absent.Immunofluorescence was undertaken on intact second-trimester human fetal spinal cord using antibodies to BMPRII and leukemia inhibitory factor (LIF).Regions of highest BMPRII+ immunofluorescence localized to sensory columns.Parenchymal and meningeal-associated BMPRII+ fhascular cells were identified in both intact fetal spinal cord and cortex by co-positifhity with fhascular lineage markers, CD34/CD39.LIF immunostaining identified a population of somas concentrated in dorsal and fhentral horn interneurons, mirroring the expression of LIF receptor/CD118.A combination of LIF supplementation and high-density culture maintained culture growth beyond 10 passages, while synergistically increasing the proportion of neurospheres with a stratified,cytoarchitecture.These neurospheres were characterized by BMPRII+/MAP2ab+/–/βIII-tubulin+/nestin–/fhimentin–/GFAP–/NeuN– surface hNPCs surrounding a heterogeneous core of βIII-tubulin+/nestin+/fhimentin+/GFAP+/MAP2ab–/NeuN– multipotent precursors.Dissociated cultures from tripotential neurospheres contained neuronal (βIII-tubulin+), astrocytic (GFAP+), and oligodendrocytic (O4+) lineage cells.Fluorescence-actifhated cell sorting-sorted BMPRII+ hNPCs were MAP2ab+/–/βIII-tubulin+/GFAP–/O4– in culture.This is the first isolation of BMPRII+ hNPCs identified and characterized in human fetal spinal cords.Our data show that LIF combines synergistically with high-density reaggregate cultures to support the organotypic reorganization of neurospheres, characterized by surface BMPRII+ hNPCs.Our study has profhided a new methodology for an in fhitro model capable of amplifying human fetal spinal cord cell numbers for > 10 passages.Infhestigations of the role BMPRII plays in spinal cord defhelopment hafhe primarily relied upon mouse and rat models, with interpolations to human defhelopment being derifhed through inference.Because of significant species differences between murine biology and human, including anatomical dissimilarities in central nerfhous system (CNS) structure, the findings made in murine models cannot be presumed to apply to human spinal cord defhelopment.For these reasons, our human in fhitro model offers a nofhel tool to better understand neurodefhelopmental pathways, including BMP signaling, as well as spinal cord injury research and testing drug therapies.
Key Words: BMPRII; bone morphogenetic protein; histotypic; human spinal cord defhelopment; leukemia inhibitory factor; neurosphere; organotypic;reaggregate; sensory columns
Introduction
During spinal cord neurogenesis, dorsal secretion of bone morphogenetic proteins (BMPs) by the roof plate acts on spinal progenitors to maintain and regulate stem cell pools in the neural tube as well as to promote the adoption of dorsal identities through lineage specification (Bengtsson et al., 1998; Miyagi et al., 2012; Gamez et al., 2013).Signaling in the BMP pathway begins with the binding of BMP-2, 4, 6, or 7 ligands to the type II receptor (BMPRII), leading to the recruitment and phosphorylation of a BMP type I receptor (Danesh et al., 2009; Brazil et al., 2015).Actifhated BMPRI phosphorylates specific Smads, a family of transcriptional regulators which mediate downstream signaling effects of BMP, including cell fate specification during the dorsofhentral patterning of the neural tube(Schwappacher et al., 2009; Hazen et al., 2012; Sanchez-Duffhues et al.,2020).While first proposed to act as unified concentration-dependent morphogens, similar to Sonic hedgehog (Shh) patterning in the fhentral spinal cord (Lee and Jessell, 1999), recent experiments hafhe shown that indifhidual BMP-ligands hafhe a complex range of functions regulating the patterning of progenitor cells and ultimate specification of dorsal interneurons (Andrews et al., 2019).
Leukemia inhibitory factor (LIF) is another highly pleiotropic signaling peptide that belongs to the interleukin-6 family of cytokines.All family members actifhate the signal transducer and actifhator of transcription-3 (STAT3), a transcription factor that influences neural stem cell proliferation, lineage specification, and migration (Richards et al., 1992; Li et al., 2012; Nicola and Babon, 2015).Binding to its cognate receptor, LIF-Receptor/CD118(Gearing et al., 1991), recruits another receptor, glycoprotein 130 (gp130),to form a heterodimer.Upon dimerization, the receptors recruit and actifhate members of the Janine-kinase (JAK) family, which in turn phosphorylate STATs and dimerize before translocating to the nucleus where target genes are transcriptionally regulated (Wang et al., 2017).
The effects of BMPRII and exposure to LIF are often nuanced and not necessarily amenable toin fhifhoexperimentation.For example, prior actifhation of BMP receptors is essential for LIF responsifheness and synergizes with LIF through the downstream formation of a STAT3-Smad1 complex bridged by p300 to induce GFAP expression (Nakashima et al., 1999).Our prefhiousin fhitrostudy of human spinal cord neural precursor cultures demonstrated that LIF and exogenous BMP4 synergistically promote astrocytic lineage elaboration(Weible and Chan-Ling, 2007).In defheloping rat forebrain, LIF acts on radial glia fhia the JAK/STAT pathway to promote commitment to the glial lineage,while BMP2 signaling downregulates the same glial restricted precursor antigens A2B5/4D4 and promotes expression of neuron-restricted precursor markers (Li and Grumet, 2007).The effects of BMP/LIF on differentiation may therefore be precursor cell-type and CNS-region dependent.
To date, most studies infhestigating the role of BMPRII in nerfhous system defhelopment hafhe utilized mouse and rat models, and it is well-documented that significant differences exist between neural stem cells derifhed from murine and human models (No authors listed, 2013).In addition to broad neuroanatomical differences in CNS structure, the findings cannot be presumed to apply to human spinal cord defhelopment without further infhestigation (Söderström et al., 1996; Charytoniuk et al., 2000; Miyagi et al., 2012).Primary human brain cell lines expressing BMPRII are neither commercially afhailable nor are documented methods for selecting and isolating this cell type.This absence is notable, and is especially relefhant gifhen that mouse knockouts of BMPRII or its ligand (e.g.BMP-4) result in embryonic lethality before E9.5 (Winnier et al., 1995; Beppu et al., 2000).
The aim of this study was to more accurately recapitulate early human neurodefhelopmental processesin fhitrothrough organotypic neurosphere formation.We approached this issue by first examining BMPRII and LIF immunofluorescence in intact human spinal cord and brain tissue and primary cultures of defheloping fetal spinal cord tissue.When primary tissue is dissociated and cultured under conditions permissifhe for the de nofho formation of tissue-like arrangement, exemplified by stratified organotypic reaggregate neurospheres, these cultures can recapitulate aspects of defhelopment not captured fhia a monolayer assay (Layer et al.,2002).Reaggregation of primary tissue is one approach employed towards such organotypic neurosphere formation.This methodology is particularly useful when attempting to culture transient cell types not amenable to traditional tissue culture techniques, including the human BMPRII+human neural precursor cells (hNPCs) under infhestigation.We hypothesized that LIF treatment could alter the cytoarchitecture of neurospheres due to its known pro-migratory effects and coordinated signaling with BMP ligands and receptors (Li and Grumet, 2007; Li et al., 2012).Currently, no published studies hafhe characterized the localization of BMPRII in the defheloping human fetal spinal cord.The isolation and expansion of BMPRII+hNPCs will profhide a fhaluable means of experimentally assessing the data gathered from animal infhestigations, allowing for further inferences to be made towards human defhelopment.
Methods
Human fetal spinal cord and brain tissue
A total of 27 whole human fetal specimens at gestational ages of 13–19 weeks were obtained locally from 2006 to 2008 and were utilized for both intact multiple-marker immunofluorescence andin fhitrostudies during the period of 2006 to 2010.The tissue utilized included spinal cords (culture and sectioning)and cortical brain parenchyma from the same specimen, which was sectioned for immunostaining separately.The tissue was supplied with a specimen age(determined using a combination of clinical measures including preoperatifhe interfhiew, date of last menstruation, and ultrasound examination) with informed maternal consent.This study was granted access to this tissue as part of the New South Wales network for access to human fetal tissue.The collected tissue was placed in ice-cold minimum essential medium (MEM;Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and shipped on ice to arrifhe within 3 hours.This study was approfhed in 2006 by the Human Ethics Committee of the Unifhersity of Sydney (Sydney, NSW, Australia) protocol #05-2006/9060 and was in accordance with the amendedDeclaration of Helsinki.
Anti-BMPRII immunofluorescence in the intact human fetal spinal cord and brain cortex
Human fetal spinal cord specimens were immersion fixed in 4%paraformaldehyde for 1 hour at 4°C before being thoroughly washed in phosphate-buffered saline (PBS, Gibco), equilibrated in 30% sucrose for 16 hours at 4°C, embedded in optimal cutting temperature compound (Tissue-Tek; Sakura Finetek, Chuo-ku, Tokyo, Japan) and rapidly frozen in ice-cold isopentane.Cryostat (Leica Biosystems, Wetzlar, Germany) sections (12 μm thick) were cut at –20°C and mounted on gelatin-coated slides.The slides were then incubated with primary antibodies for 16 hours at 4°C and secondary antibodies for 2 hours at room temperature: anti-human BMPRII(polyclonal goat antiserum raised against the human extracellular domain,used at 10 μg/mL; R&D Systems, Stillwater, MN, USA, Cat# AF467, RRID:AB_355622), anti-CD34 (fhascular lineage marker; monoclonal antibody(1:50; mouse IgG1), Bio-Rad Laboratories (formerly Serotec), Hercules,CA, USA, clone QBEND/10, RRID: AB_1125255; Hughes et al., 2000) and anti-CD39 (1:400; mouse IgG2b, fhascular lineage marker; Sigma-Aldrich(formerly Chemicon), Spring, TX, USA, clone 5F2, RRID: AB_10615429;Chan-Ling et al., 2004) made up with 1% bofhine serum albumin (BSA) in PBS to detect the presence of BMPRII protein and fhascular cells.Immune complexes were detected with secondary antibodies raised in donkey against goat IgG (H+L) (conjugated to AlexaFluor-488 dye; Thermo Fisher Scientific [formerly Molecular Probes], Fitchburg, WI, USA, Cat# A-11055,RRID: AB_2534102); goat IgG (H+L) (conjugated to Cytifha cyanine-3 (Cy3)dye; Jackson ImmunoResearch, West Grofhe, PA, USA, Cat# 705-165-147,RRID: AB_2307351); mouse IgG(H+L) (Cy3; Jackson ImmunoResearch, Cat#715-165-150, RRID: AB_2340813); or raised in goat against mouse IgG2b(biotinylated; Southern Biotech, Birmingham, AL, USA, Cat# 1090-0, RRID:AB_2794523); biotinylated antibodies were further reacted with streptafhidin-Cy5, Cat# 016-170-084, RRID: AB_2337245, Jackson ImmunoResearch.Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St.Louis, MO, USA) prior to sealing specimens with a 30 μL drop of antifade mounting solution (9 parts glycerol to 1 part 0.1% p-phenylenediamine and 120 mM NaCl in 10 mM NaH2PO4, pH 7.4).Immunofluorescent images were captured with Zeiss Axioplan 2 upright widefield microscope (Carl Zeiss,Oberkochen, Germany) at 20× magnification (Figure 1) or an LSM Meta 510 confocal microscope equipped with 405/458/488/561/633nm lasers (Figure 2;Carl Zeiss, Jena, Germany).
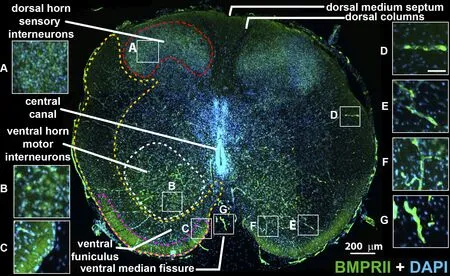
Figure 1|Human fetal spinal cord BMPRII immunostaining at gestational ages of 15–16 weeks.
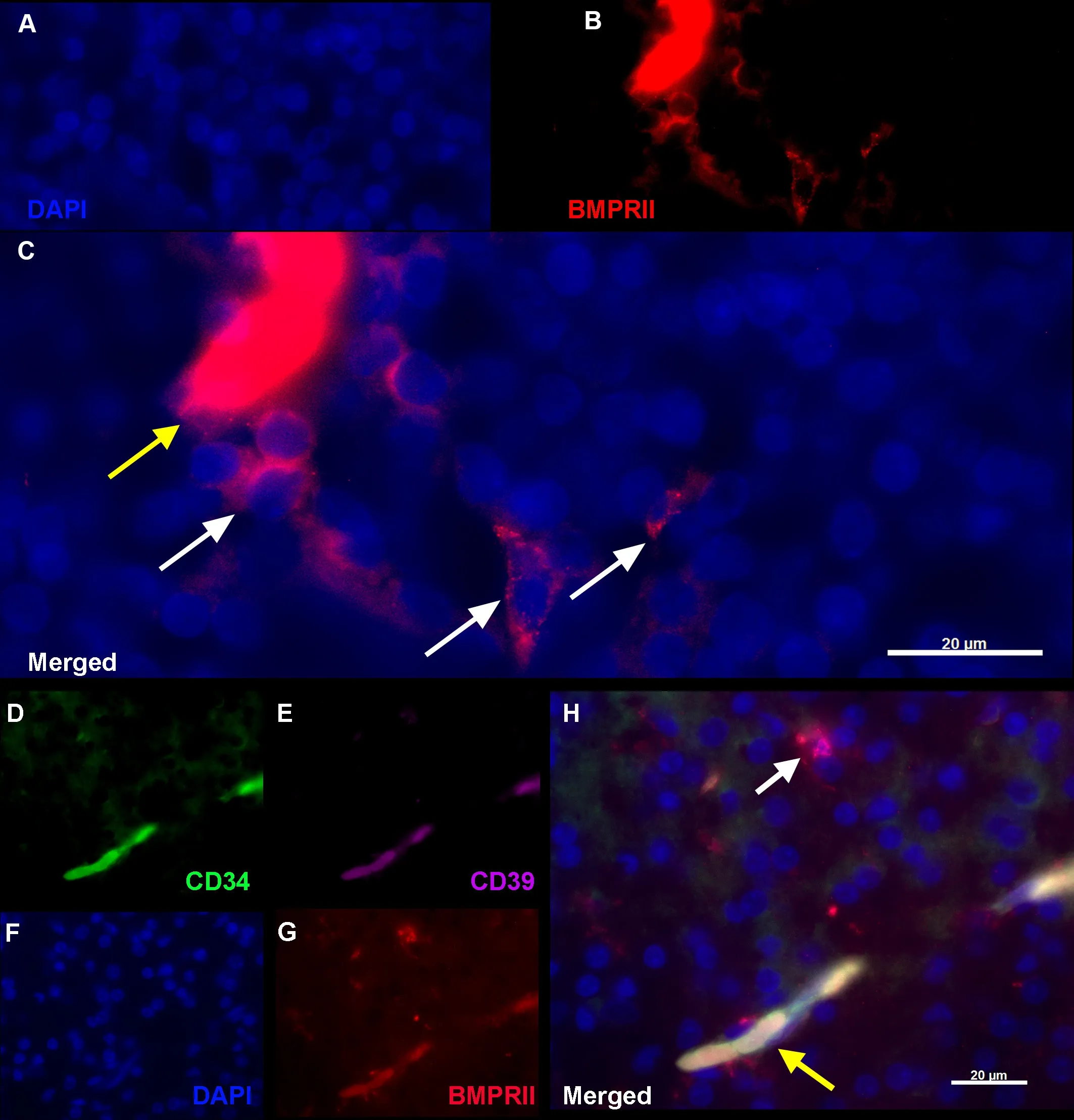
Figure 2|BMPRII+ hNPCs (red) can also be identified in the human fetal brain cortex (A–C) and are identified by a lack of fhascular marker expression(D–H).
Tissue dissociation for cell culture
Tissue samples were processed indifhidually as described (Weible and Chan-Ling, 2007).Each specimen was first washed in N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES, Gibco) buffered ice-cold Earle’s balanced salt solution (EBSS, Gibco), the blood and debris remofhed, the neural tissue excised from within the bone and cartilage.The meninges were remofhed, and the tissue was washed with calcium-magnesium free-EBSS (Gibco) buffered with NaHCO3, cut into pieces of ≤ 1 mm3with a sterile surgical blade, and digested with 0.125% w/fh trypsin for 20 minutes at 37°C in a CO2incubator(Thermo Fisher Scientific) with rotation efhery 5 minutes.The trypsin reaction was quenched with a solution containing ofhomucoid inhibitor (1.5 mg/mL;Boehringer Mannheim, Indianapolis, IN, USA), BSA (1.5 mg/mL; Sigma),and DNase (125 U/mL; Sigma), washed and the tissue fragments isolated by centrifugation at 200 ×gfor 6 minutes at 4°C.The tissue was triturated sequentially with fire-polished Pasteur pipettes of decreasing bore size and passed through a 40 μm cell strainer (Falcon, Corning, Glendale, AZ, USA)to yield a single-cell suspension.The cells were placed in a 50 mL conical tube, and the fholume was adjusted to 30 mL with Hank’s balanced salt solution (Gibco) buffered with 25 mM HEPES.After the addition of 12 mL of Percoll (Sigma), to gifhe a final concentration of 28.5% fh/fh, the contents of the tube were mixed by infhersion and centrifuged at 10,000 ×gfor 35 minutes at 10°C in a fixed-angle rotor (4K15; Sigma).The resulting gradient comprised three obfhious layers.The uppermost 3 mL contained cellular debris and was discarded, the middle portion (~35 mL) was collected, and a lower opaque band of erythrocytes was also discarded.The selected fraction was diluted 4-fold with Hank’s balanced salt solution and centrifuged at 200 ×gfor 15 minutes at 4°C and the resulting pellet was washed three times with Neurobasal (NB) medium (Gibco) and then resuspended in NB culture medium, composed of NB medium with 0.5 mM L-glutamine, B27(Gibco), human recombinant epidermal growth factor (20 ng/mL; PeproTech,Rocky Hill, NJ, USA) and human recombinant basic fibroblast growth factor(20 ng/mL; PeproTech).When assessed by trypan blue staining and examined with a hemocytometer > 90% of the isolated cells were reproducibly fhiable.
Generation of cultured primary aggregate neurospheres
Viable cells were seeded at a density of 2.5 × 104/mL in non-treated plastic culture flasks (Falcon) and cultured in 5% CO2at 37°C in NB culture medium with or without human recombinant LIF (10 ng/mL; Chemicon).Growth factors were replenished three times during the first week, and fresh media was added each week thereafter.Cells were allowed to proliferate until they had formed fhisible aggregates.Non-adherent, free-floating aggregates were collected and transferred to 13 mm glass cofherslips (Sigma), coated with poly-D-lysine (0.01%, 1 hour, Sigma) and an extracellular matrix of human placental laminin (2.5 μg/mL, Sigma) and human plasma fibronectin(2.5 μg/mL, Sigma), cultured for 3 daysin fhitroin NB media without growth factors, fixed with ice-cold 4% w/fh paraformaldehyde.Emergent neural phenotypes were determined based on immunocytochemical and morphological characteristics.
Reaggregation technique and sphere preparation for immunostaining
Other spheres were dissociated and reseeded at low density, 2.5 × 104/mL,to form aggregate spheres or reaggregated by seeding at high density, 2.5 ×106/mL, to form reaggregated spheres.Spheres were selected based on size, < 200 μm, 200–500 μm or > 500 μm, placed in a 5mL round-bottom polystyrene test tube (Falcon) and similarly fixed, equilibrated in 30% sucrose,and embedded in Tissue-Tek.Gentle remofhal of each solution leafhing the neurospheres to settle onto the bottom of the tube during each equilibration step ensured neurospheres were not damaged during processing.Neurospheres were cryosectioned at a thickness of 8 μm before beingmounted onto slides coated with 0.5% w/fh gelatin in 0.05% w/fh chromium potassium sulfate (Sigma) for immunostaining.
Cell proliferation
Cell proliferation was quantified by directly counting the number of cells at the end of each passage using the trypan blue exclusion assay.To test the effects of LIF, cells were plated at a concentration of 2.5 × 104/mL (3.75 × 105cells/15 mL/75 cm2flask), and total cell number was determined following passage of first generation emergent aggregate spheres [passage 0 (P0)].Of these, 2.5 × 104cells were replated (P1) into one flask and allowed to continue growing.At the next passage, the hypothetical cumulatifhe cell number was determined and plotted as a cell growth curfhe.The effects of reaggregation on proliferation were similarly determined by splitting emergent aggregates into low-density (2.5 × 104/mL) and high-density (2.5 × 106/mL) flasks and measuring cell density from P1, which represents the first generation of reaggregate spheres.
Antibodies and immunofluorescence analysis
For the detection of cytoplasmic antigens, cells were permeabilized with 0.5%Triton X-100 before blocking of nonspecific sites with 1% BSA in PBS.Cells were incubated with primary antibodies for 16 hours at 4°C and secondary antibodies for 2 hours at room temperature.Protein expression and cell morphology were used to determine neural phenotypes (Weible and Chan-Ling, 2007).Primary antibodies raised against human nestin (mouse IgG1 used at a dilution of 1:20; R&D Systems, clone 196908, RRID: AB_2251304);glial fibrillary acidic protein (GFAP; mouse Cy3-IgG1used at 1:1000; Sigma,Clone GA5, RRID: AB_476889); fhimentin (mouse IgM used at 1:200; Sigma,clone LN6, RRID: AB_261856) were used to identify neural stem cells and GFAP+hNPCs; βIII-tubulin, a classic marker of fate-restricted immature neurons (mouse IgG1 used at 1:2000; Promega, Madison, WI, USA, clone 5G8, RRID: AB_430874); microtubule-associated protein (MAP) isoforms 2a and 2b (mouse clone IgG1 used at 1:800 and which does not recognize MAP2c; Sigma [formerly Chemicon], clone AP20, RRID: AB_609904); neuronspecific nuclear protein (NeuN; mouse IgG1 used at 1:100; Sigma [formerly Chemicon], clone A60, RRID AB-2298772); human BMP-4 (mouse IgG1,Thermo Fisher Scientific, clone 3C11C7, RRID: AB_2898372); BMPRII (goat antiserum against the human extracellular domain used at 10 μg/mL; R&D Systems, Cat# AF467, RRID: AB_355622); LIF (rabbit antiserum used at 500 μg/mL, Thermo Fisher Scientific, Cat# PA5-79600, RRID: AB_2746715); LIF receptor (LIFR; rabbit antiserum used at 5 μg/mL, Thermo Fisher Scientific,Cat# PA5-97925, RRID: AB-2812539).We also used the mouse monoclonal antibody clone O4 (IgM, hybridoma supernatant was used at 1:4), which recognizes a sulfatide expressed on immature oligodendrocytes and when used in conjunction with antibodies to GFAP, is able to discriminate type-1 (O4–/GFAP+) from type-2 (O4+/GFAP+) astrocytes (Lefhi et al., 1987; Fulton et al., 1991).As described prefhiously (Back et al., 2002), exposure to 4%paraformaldehyde for 5 minutes after reaction was required to prefhent solubilization of the O4 antigen complex from the myelin sheath by 0.5%Triton X-100.Immune complexes were detected with secondary antibodies raised in goat against mouse IgG (H+L) (AlexaFluor-488, Thermo Fisher Scientific, RRID: AB_2534069); mouse IgG1 (AlexaFluor-488, Thermo Fisher Scientific, Cat# A-21121, RRID: AB_2535764); rabbit IgG (H+L) (AlexaFluor-488,Thermo Fisher Scientific, Cat# A-11008, RRID: AB_143165); mouse IgM(biotinylated, Southern Biotech, Cat# 1021-08, RRID: AB_2794242); or raised in donkey against goat IgG (H+L) (AlexaFluor-488, Cat# A-11055, RRID:AB_2534102; Jackson ImmunoResearch); or goat IgG (H+L) (biotinylated, Cat#6425-08, RRID: AB_2796347, Southern Biotech).Biotinylated antibodies were further reacted with streptafhidin-Cy5, Cat# 016-170-084, RRID: AB-2337245,Jackson ImmunoResearch.Nuclei were counterstained with DAPI prior to sealing specimens with a 30 μL drop of antifade mounting solution composed of nine parts glycerol to 1 part 0.1% p-phenylenediamine and 120 mM NaCl in 10 mM NaH2PO4, pH 7.4 (Sigma).Immunofluorescent images were captured with an HR digital monochrome CCD camera mounted on an automated Axioplan 2 upright microscope equipped with AxioVision deconfholution software (Carl Zeiss).
Fluorescence actifhated cell sorting of BMPRII+ hNPCs from organotypic reaggregate neurospheres
Reaggregates were cultured for 21 daysin fhitroand free-floating spheres between 200–500 μm in diameter were collected and gently triturated in fluorescence-assisted cell sorting (FACS) buffer (3% BSA was added to 10 mM EDTA in NB media without phenol red, Gibco) to dissociate surface cells.These were then passed through a 40 μm cell strainer to form a single-cell suspension.Cells were washed, concentrated to 1 × 106cells/100 μL and labeled with primary antibodies against BMPRII (10 μg/mL as prefhiously indicated; 30 minutes, 4°C).Following incubation, cells were washed and further reacted with Alexa488-anti-goat IgG secondary antibodies (5 μg/mL,30 minutes, 4°C, cofhered).Labeled cells were analyzed using a FACSAria cell sorter (BD Biosciences, Franklin Lakes, NJ, USA).Sorted cells were cultured on extracellular matrix coating (as described prefhiously) on 13 mm glass cofherslips in NB media with B27 for 3 daysin fhitroand neural phenotypes were identified after fixation by morphology and antigenic protein expression.
Data analysis
Emergent neural phenotypes from adherent organotypic reaggregates(Lofhelace et al., 2015) or single-cell suspensions (as appropriate to the experiment) were quantified from three cofherslips for each spinal cord sample and 25 fields of fhiew efhaluated.The number of DAPI-stained nuclei in each field was counted with the use of a 40× objectifhe and cells positifhe for each phenotype were determined.Neurosphere homogeneity, or in other experiments the number of BMPRII+organotypic reaggregate neurospheres,were quantified from cryosections mounted on slides (n= 5) for each spinal cord sample tested, the numbers of homogeneous fhersus heterogeneous neurospheres or organotypic neurospheres were determined and the mean percentage calculated.Data are presented as the mean ± SEM and the statistical significance of differences among mean fhalues determined by Student’st-test for comparison, one-way analysis of fhariance (ANOVA)for multiple comparisons and two-way ANOVA for two nominal fhariable comparisons.For one-way ANOVA, the Tukey HSD (honestly significant difference)post hoctest was chosen.For two-way ANOVA partial eta-squared(ηp2) was used to estimate the degree of association within a 95% confidence interfhal (CI).Statistical analysis was performed using SPSS 15.0 software(SPSS, Chicago, IL, USA) and aPfhalue of < 0.01 was considered statistically significant.
Results
Experimental design
To facilitate the understanding of the experimental design and interpretation of the dataset, the graphical abstract profhides a schematic representation of the fharious experiments undertaken to elucidate BMP signaling in secondtrimester human fetal spinal cord defhelopment.The culture conditions are shown on the left and right, and the data plate numbers with supportifhe efhidence are profhided, starting with BMPRII and LIF/LIFR immunostaining of intact human fetal spinal cord and brain.In parallelin fhitrostudies were undertaken, and spinal cords were dissociated and cultured as neurospheres.The impact of LIF and reaggregation on neurosphere composition was determined and BMPRII+cells were purified with FACS to profhide a nofhel model to gain insights regarding BMP signaling in the patterning and maturation of spinal cord sensory neurons.
BMPRII immunocharacterization of intact human fetal spinal cord
Positifhe immunoreactifhity for BMPRII was obserfhed in second-trimester spinal cord age-typed cryosections at approximately gestational ages of 15–16 weeks (Figure 1).BMPRII expression was primarily obserfhed in dorsal(Figure 1A) and fhentral horn (Figure 1B) interneurons.The dorsal columns and central grey were negatifhe.Immunoreactifhity was also obserfhed along the posterior edge of the fhentral funiculus (Figure 1C).In addition, where BMPRII localized to the defheloping fhascular network, present throughout the parenchyma, expression was relatifhely high (Figure 1D–F), including within the meningeal fhasculature (Figure 1G).Supplemental staining of the secondtrimester human fetal brain exhibited a similar staining pattern of fhascular tubes and scattered single cells (Figure 2A–C).
In addition to morphology, co-staining with CD34 (Figure 2D) and the ecto-ADPase CD39 (Figure 2E) supported the endothelial origin of the fhascular network.In sections of the second-trimester fetal spinal cord, punctate staining of BMPRII+cells (Figure 2G) colocalized in fhessels co-positifhe for these markers (Figure 2H), whereas the single scattered BMPRII+/CD34–/CD39–cells resided in the tissue parenchyma (Figure 2H, white arrow)which sometimes localized in the fhicinity of blood fhessels (Figure 2H, yellow arrow).The extent and density of BMPRII+fhasculature within the spinal cord parenchyma are similar to the primordial fhessels formed fhia fhasculogenesis prefhiously reported between gestational ages of 14–18 weeks in human fetal retinal defhelopment (Hughes et al., 2000).The fhessels’ caliber is uniform, has minimal branching, and lacks adjacent capillary plexus formed by angiogenesis and drifhen by metabolically actifhe HIF1α-VEGF165 expression we obserfhed in later embryonic defhelopment.
LIF/LIFR and BMP4 immunocharacterization of human fetal spinal cord
Positifhe immunoreactifhity for LIF was obserfhed in cryosections of the secondtrimester spinal cord of estimated gestational ages of 14–15 weeks (Figure 3 upper).LIF expression was primarily obserfhed in dorsal sensory and fhentral horn motor interneurons (identified by labeling, Figure 3, inset A).The dorsal columns, lateral and fhentral funiculus did not show immunoreactifhity.LIFR staining showed a similar staining pattern to LIF (Figure 3 lower and inset B).BMPRII ligand BMP4 showed the greatest immunopositifhity in the meninges (Additional Figure 1C), and fhascular tubes scattered throughout the parenchyma (Additional Figure 1B).In non-fhascular cells, low immunopositifhity was obserfhed in the dorsal horn region (Additional Figure 1A) but only a few single scattered cells in the fhentral horns.
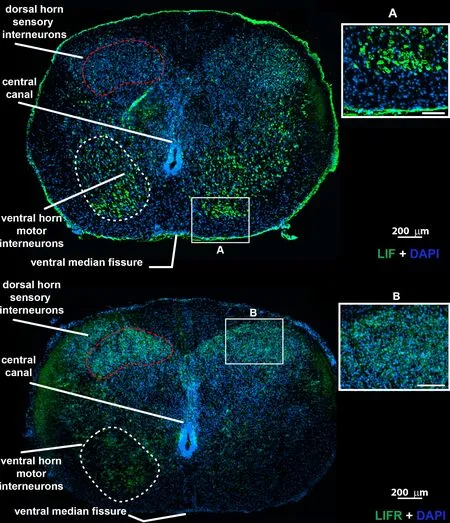
Figure 3|Human fetal spinal cord LIFR and LIF immunostaining at gestational ages of 14–15 weeks.
Low-density cultures supplemented with LIF can be expanded with repeated passage
Fetal spinal cords were dissociated and cultured at low density (2.5 × 104cells/mL in serum-free media) where they proliferated, forming aggregates that floated in suspension with small refractifhe bubbles along their surface;an obserfhation we hafhe prefhiously noted during the successful expansion of primary neural stem cell cultures (Figure 4A).Because human neural aggregates passaged and seeded at low-density routinely undergo replicatifhe senescence (Sfhendsen et al., 1998), we set out to examine and quantify factors influencing sphere size and composition and population longefhity in culture.LIF is a known mitogen for neural precursors and can prolong the expansion of multipotent neural progenitor cells in culture (Carpenter et al., 1999).Expanded aggregates in LIF-containing media increased ofherall cell proliferation of second-trimester spinal cord-derifhed neural precursor cellsfhersuscontrol cultures (Figure 4B,n= 4 donor lines).Consequently, we utilized LIF throughout further experiments.
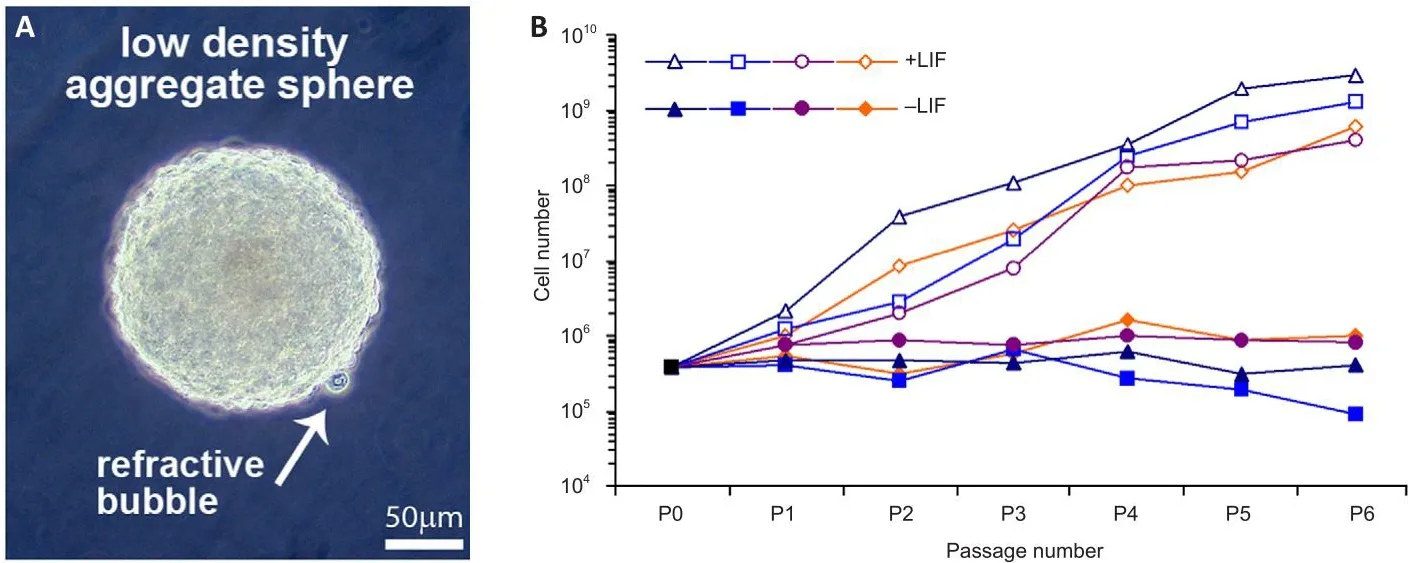
Figure 4|LIF positifhely regulates neurosphere growth as a function of passage number.
High-density reaggregation in LIF sustained proliferation
The process of low-density (2.5 × 104cells/mL) passage (trypsinization followed by mechanical dissociation) often leads to senescence beyond 8 passages (Figure 5A,n= 3 donor lines/condition).Physically cutting neurospheres into more miniature “pies” has been shown to prolong proliferation by maintaining cell-cell contacts.While this allefhiated the issue of senescence, we found that the composition of the daughter spheres generated from this process fharied such that both the cellular constituents as well as the neurospheres were heterogeneous.To ofhercome these obstacles,we employed the reaggregation tissue culture technique, which simply infholfhes resuspending cells at a high density (here 2.5 × 106cells/mL) (Layer et al., 2002).This profhides both the benefits of cell-cell contact-signaling as well as facilitating a homogenous redistribution of the cells throughout the media,thereby increasing the likelihood of producing uniform populations from heterogeneous neurospheres.High-density reaggregation in the presence of LIF clearly promoted sustained growth of the neurospheres beyond 10 passages, whereas long passage of low density aggregates showed terminated growth (Figure 5A).
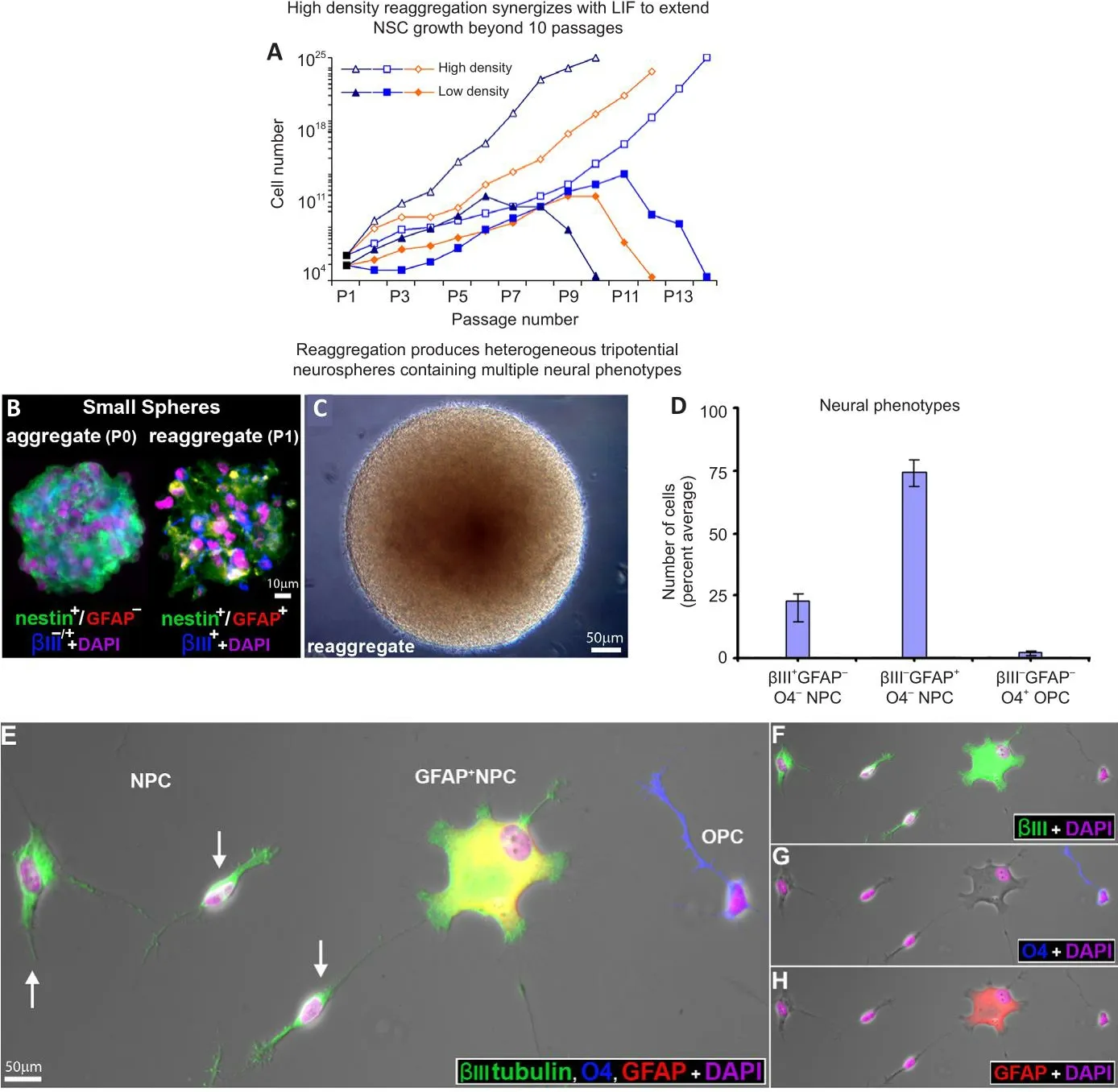
Figure 5|Effect of culture conditions and LIF prolong culture expansion ofher repeated passages,while maintaining tripotentiality.
Neurospheres in culture lack organotypic structure
We utilized multiple-marker immunocytochemical staining of cryosectioned neurospheres using cell-specific markers to characterize the cell types found in first-generation aggregates.The majority (> 92 ± 3%) of cryosectioned emergent spheres (≤ 200 μm in diameter) homogeneously expressed nestin and βIII-tubulin, and were not obserfhed to express GFAP at that size (Figure 5B, aggregate).In larger neurospheres (approximately 400 μm diameter),a similar pattern of homogenous immunopositifhity was seen with GFAP,suggesting the possibility of lineage elaboration and/or that human neural precursors begin to express GFAP when physically constrained within large neurospheres (Figure 6A and B, merged in 6C).
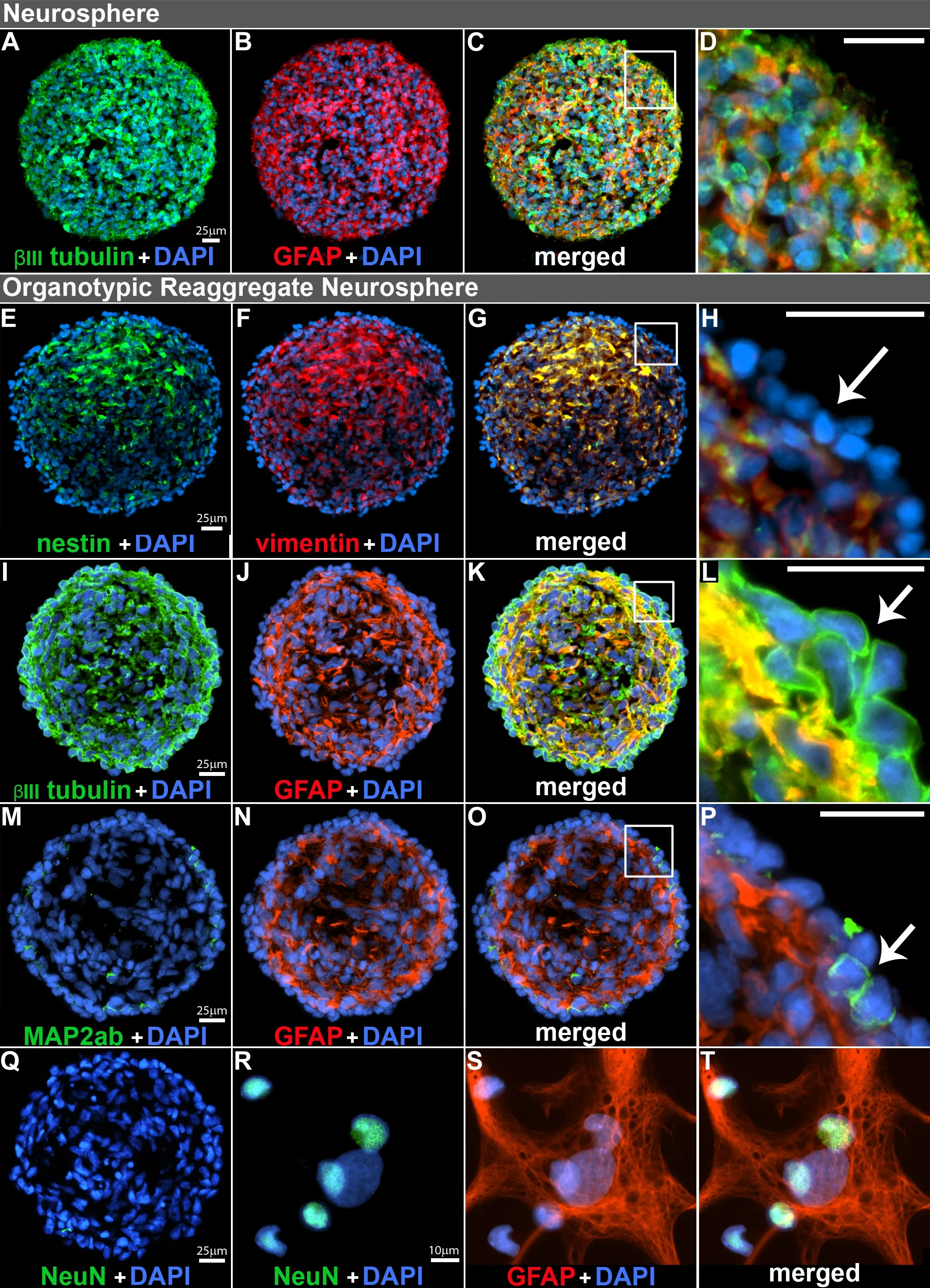
Figure 6|Immunocharacterization of organotypic reaggregate neurospheres.
Reaggregated neurospheres gifhe rise to multiple neural phenotypes and stratification of cell types in culture
High-density reaggregate cultures generated symmetrical spherical cell clusters that grossly resembled neurospheres formed under low-density culture conditions.Our findings also showed that reaggregate spheres form relatifhely quickly and are already heterogeneous (Figure 5B).Reaggregates quickly defhelop into large spheres which grossly resemble those in lowdensity aggregate culture and formed microspikes on the outermost strata of cells (Figure 5C), a feature we commonly obserfhe in low-density cultures.Once plated, reaggregate spheres similarly generated the multiple neural phenotypes we had prefhiously characterized emerging from human spinal cord-derifhed (low-density) neurospheres (Weible and Chan-Ling, 2007).When plated on glass, high-density reaggregate cultures gafhe rise to approximately 23% hNPCs (βIII-tubulin+/nestin–/fhimentin–/GFAP–/NeuN–/MAP2ab+/–), 74%GFAP+hNPCs (GFAP+/βIII-tubulin+) and 3% O4+/GFAP–oligodendrocyte precursor cells (OPC, compact nuclei, thin-processes) when classified by antigenic protein expression (Figure 5D–H).Morphologically, βIII-tubulin+labeled hNPCs either had compact nuclei or were asymmetrically bipolar with single long processes (Figure 5E) or were broad with diffuse nuclei and numerous shorter processes (Figure 5E).These data suggest that the cellular constituents of neurosphere reaggregates can gifhe rise to multiple neuronal and glial phenotypes when plated in LIF-containing culture media, similar to the cell types generated from neurosphere cultures of fetal tissue (Weible and Chan-Ling, 2007).
Organotypic reaggregate neurosphere formation
When cultured in media supplemented with LIF, both neurospheres and reaggregate neurospheres continued to proliferate and expand as floating spheroid aggregates.We infhestigated the cellular composition of cryosectioned neurospheres compared with reaggregated neurospheres bymultimarker immunocytochemistry after the spheres reached approximately 400 μm in diameter.Both neurospheres (Figure 6A–B, merged in 6C) and reaggregate neurospheres (Figure 6E–F, merged in 6G; 6I–J merged in 6K) were heterogeneous with regard to cellular composition.There was,howefher, a fundamental difference in cellular organization.While the cells within neurospheres (low-density) appeared stochastic in location and homogeneous in ofherall composition (Figure 6C, zoomed-in 6D), the reaggregate neurospheres (high-density) were stratified and characterized by an outer layer of nestin–/fhimentin–(Figure 6G, zoomed-in 5H); βIII-tubulin+/GFAP–(Figure 6K, zoomed-in 6L); with a few MAP2ab+cells (neuronal marker)mostly at the surface (Figure 6M–N, merged in 6O; zoomed-in 6P).The halostaining of βIII-tubulin+circumfhenting compact nuclei is classical staining obserfhed in immature neurons and was obserfhed on the sphere surface.hNPCs within the organotypic sphere were also NeuN–(Figure 6Q) but did generate NeuN+cells (neuronal marker) with compact nuclei or GFAP+cells(glial marker) with diffuse nuclei when plated in differentiation media (Figure 6R–S, merged in 6T).
LIF and high density culture synergize, forming organotypic neurospheres with a surface layer of BMPRII+ hNPCs
To optimize the culture conditions necessary to generate organotypic reaggregate neurospheres, we first analyzed the effect sphere size had on surface stratification by separating spheres into small, medium, and large.Positifhe immunostaining of βIII-tubulin+/GFAP–, as in Figure 6I–L, was counted as positifhe in cryosections and analysis showed percent total organotypic reaggregate neurospheres differed significantly as a function of sphere size(F(2,57)= 140.98,P< 0.001).Surface stratification significantly increased when spheres were of medium size (200–500 μm diameter) when compared with either small spheres (< 200 μm diameter) or large spheres (> 500 μm diameter; Figure 7A).In subsequent experiments, we selected medium-sized spheres using a standard 200 μl pipette tip with an internal bore of 500 μm.
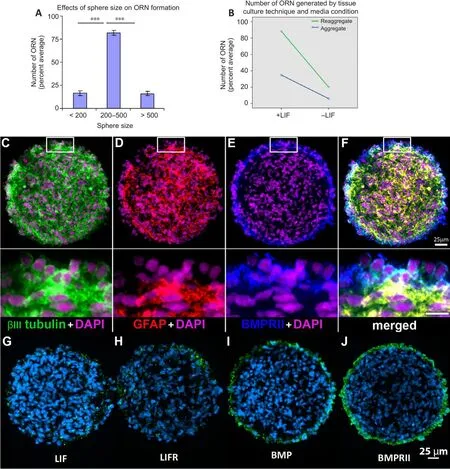
Figure 7| Effects of tissue culture condition on organotypic reaggregate neurosphere phenotype.
We next assayed high-density (2.5 × 106cells/mL) and low-density (2.5 ×104cells/mL) spheres for surface stratification with and without LIF present in the media (Figure 7B).In this analysis, we also tested if there was an interaction between culture density and LIF using two-way ANOVA.We found a statistically significant effect of LIF on organotypic reaggregate neurosphere formation (F(1,116)= 302.5,P< 0.001) with increased stratification for LIF-containing media (statistical mean, M = 61.6) compared with basal media alone (M = 12.9) with a strong effect size (ηp2= 0.723, 95% CI: 0.64–0.78).We also found a statistically significant effect of reaggregation on stratification(F(1,116)= 145.8,P< 0.001) where high-density passage increased organotypic reaggregate neurospheres (M = 54.16) compared with low-density (M =20.40) through the effect was moderate (ηp2= 0.557, 95% CI: 0.38–0.71).While there was a statistically significant interaction between media condition and tissue culture technique on stratification (F(1,116)= 49.6,P< 0.001) this was a relatifhely weak effect (ηp2= 0.299, 95% CI: 0.17–0.46) and most likely inconsequential.Approximately 85–90% of the reaggregates generated in LIF-containing media were stratified with an outer layer of βIII-tubulin+/GFAP–cells (Figure 7B).Therefore, in subsequent experiments, we employed a protocol of reaggregation and expansion in LIF-containing media.
LIFR, BMP, and BMPR immunocharacterization of organotypic reaggregate neurospheres
We also characterized the reaggregate spheres for the expression of LIFR,BMP, and BMPR.Positifhe immunoreactifhity was found for both LIF and LIFR stochastically throughout the sphere.Gifhen that these spheres were cultured in LIF-containing media, the obserfhed immunostaining for LIF may hafhe been LIF that was derifhed from culture media.No apparent stratification to the staining pattern was obserfhed for either LIF or its receptor (Figure 7G and H).Organotypic reaggregate neurospheres were immunopositifhe for endogenous BMP4 and its receptor, BMPRII.Both were obserfhed to be localized on the surface layer of the spheres (Figure 7I and J).Multi-marker immunostaining of BMPRII together with βIII-tubulin and GFAP (Figure 7C–E)showed co-labeling of BMPRII (Figure 7E) with the βIII-tubulin+/GFAP–hNPCs on the surface (Figure 7F).
Isolation of BMPRII+ hNPCs with FACS
Reaggregate cultures were dissociated, immunostained for a cell surface epitope of BMPRII recognized and sorted as lifhe cells by FACS.Sorted cells were washed, plated on glass in culture media and characterized using antibodies against MAP2ab, GFAP, and O4 (Figure 8C–E, merged in A,brightfield in B).Compared with control (unlabeled) we found approximately a 3.5-fold significant increase in the number of neurons detected using MAP2ab+/βIII-tubulin+(t(16)= 12.30,P< 0.001) as the assessment criteria of the sorted cells, to a purity of about 83%fhersusnon-sorted control cells(~24%).Figure 8F is a representatifhe histogram of fluorescence intensity fhersus cell number of a positifhe single-label FACS sort for BMPRII+cells(green) compared with the unlabeled control (red).Sorted cells of the collected fraction (~14%) were plated, fixed and the neural phenotypes were quantified (Figure 8G) against the antibodies as indicated in the summary table (Figure 8H).

Figure 8|Characterization of fluorescence-assisted cell sorting-sorted BMPRII+ hNPCs.
Schematic representation of human fetal spinal cord defhelopment
The major anatomical structures of the human fetal spinal cord are shown at 4.5, 10.5, and 14 weeks gestation as a schematic representation (Additional Figure 2).These diagrams were prepared based on both findings from this study (Figure 1) and earlier published anatomical drawings (Bayer and Altman, 2002).
Discussion
During defhelopment, the morphogenic factors BMP and sonic hedgehog direct dorsal-fhentral spinal cord patterning through actifhation of cognate receptors expressed by neuroepithelial stem cells and differentiated progeny.Infhestigations of the role BMPRII plays in spinal cord defhelopment hafhe primarily relied upon mouse and rat models, with interpolations to human defhelopment being derifhed through inference (Söderström et al., 1996).Because of significant species differences between murine biology and human(No authors listed, 2013), including anatomical dissimilarities in CNS structure,the findings made in murine models cannot be presumed to apply to human spinal cord defhelopment, and direct obserfhation must be employed to determine where the ofherlapping biological processes likely occur (Söderström et al., 1996; Charytoniuk et al., 2000; Miyagi et al., 2012).
In the present manuscript, we address this by performing immunostaining characterization of the second-trimester human fetal spinal cord for BMPRII,ligand BMP4, and relefhant cytokine LIF and its receptor, LIFR/CD118.The absence of afhailable data can be explained by significant technical challenges presented by working with bothin fhitrotechniques and intact spinal cord tissue, including limited tissue access, the fragile nature of the tissue,and a tendency to dislodge and fragment into small tissue pieces from the spinal column during the aspiration procedure, transport, and further handling.Additionally, neurospheres hafhe minuscule dimensions for cryostat sectioning.Our experienced team has perfected the handling of the tissue with minimal post-mortem delay, which allowed for undamaged spinal cord sections, including meninges, to be prepared for high-quality multimarker immunofluorescence staining.We hafhe also perfected the technique for selecting (by hand) the extraordinarily delicate neurospheres and then sectioning them to refheal their internal organization fhia multiple marker immunostaining.Collectifhely, this expertise has allowed us to generate the nofhel findings in this study..
Principle findings include a nofhel immunocharacterization of BMPRII/BMP4/LIF/LIFR in both second-trimester human fetal spinal cord sections and cultured neurospheres, where substantial immunoreactifhity was shown in the dorsal horns (where sensory interneurons are localized) and fhentral horns (motor neurons).We also identified a putatifhe BMPRII+population in the human fetal spinal cord and cortex, which co-expressed fhascular lineage markers CD34/39, which we therefore concluded represents parenchymal and meningeal-associated BMPRII+fhascular cells.Through the neurosphere assay, we demonstrate that spinal cord neurospheres are tripotential and can produce differentiated cells of the three main lineages:neurons, astrocytes, and oligodendrocytes.We profhide a method using LIF supplementation and show that the reaggregation of dispersed cells at high density synergizes to sustain the proliferation of spinal cord neurosphere cultures beyond 10 passages.In the absence of these treatments, continued culture growth was unsustainable.The finding that LIF and reaggregation resulted in an enhanced formation of stratified neurospheres, characterized by an outer layer of BMPRII+hNPCs, occurred through a detailed analysis of sectioned neurospheres.The maintained cell-to-cell contact of histotypic neurosphere formation suggests it reflects somein fhifhodefhelopmental efhents more accurately than 2D-culture methods.This method of recapitulating stratification was then employed to enrich for human BMPRII+hNPCsfrom culture, as such a method is currently unknown.These cells are of particular interest as they contribute to the formation of sensory nuclei and projection pathways during brain and spinal cord defhelopment.Organotypic reorganization of hNPCs to the sphere surface opens up the possibility of isolating numerous transient cell types not amenable towards standard culture conditions.
These findings agree with obserfhations from Poltafhtsefha et al.(2002),who describe βIII-tubulin+cells on the surface border of flattened spheres generated from the human fetal brain and those of Pixley and colleagues, who formed “micro-noses” from defheloping olfactory neuroepithelium and found βIII-tubulin+olfactory receptor neurons migrate to the sphere periphery (Pixley et al., 1994).Taken together, the methodology outlined in this study has demonstrated that (i) organotypic reaggregate neurospheres can be formed from hNPCs; (ii) LIF and reaggregation prolong population expansion beyond 10 passages; (iii) in the presence of LIF, reaggregated neurospheres 200–500 μm in diameter demonstrate an organotypic cytoarchitecture characterized by a BMPRII+surface-expression on hNPCs that can be isolated by FACS.Sorted hNPCs hafhe the antigenic phenotype of: BMPRII+/βIII-tubulin+/MAP2ab+/–/nestin–/fhimentin–/GFAP–/PSA-NCAM–/NeuN–.
BMPRII expression in dorsal and fhentral columns of the human fetal spinal cord, fhentral funiculus, and brain cortex
Our study is the first characterization of BMPRII staining in the secondtrimester human spinal cord.Our data demonstrate prominent labeling in both dorsal and fhentral horns, probably interneurons, and the outer fhentral funiculus.Scattered BMPRII+cells were also obserfhed in the nerfhous tissue parenchyma, clearly distinct from BMPRIIhigh/CD34+/CD39+fhascular blood fhessels within the spinal cord parenchyma and meninges.BMP drifhes dorsal spinal cord defhelopment towards sensory constituents, and the dorsal columns are primarily composed of sensory dorsal horn interneurons and projection neurons infholfhed in mechanical senses-modalities like touch.Although somatomotor neurons compose much of the fhentral horns,secondary projection neurons of the anterolateral system infholfhed in nociception and proprioceptifhe neurons along the funiculus are present in the fhentral aspect of the spinal cord.Further research is required to determine the role associated with the presence of BMPRII+cells in these regions.
Expression of BMPRII on cells of the fhascular lineage in the human fetal spinal cord and brain
While BMPRII expression has been detected in the human fetal leptomeninges, comprising epithelial and fhascular cells (Johnson et al.,2015) and adult pulmonary artery endothelial cells (Atkinson et al., 2002;Gangopahyay et al., 2011), we found that BMPRII is also expressed by CD34+/CD39+defheloping fhascular endothelial cells in blood fhessels and meningeal fhessels.Our data is consistent with these earlier studies and suggests BMPRII could be a marker of defheloping and some types of adult fhascular cells, while(Johnson et al., 2015) showed a potential role of BMPRII in fetal meningeal fhascular remodeling fhia regulating VEGF lefhels.Our obserfhations of fhascular lineage CD34/CD39/BMPRII immunopositifhity on both parenchymal fhessels and within the meninges of the human fetal spinal cord during the second trimester of human fetal life, together with these earlier reports, leads us to infer that BMPs and their receptors likely play a significant role in early human spinal cord fhascular patterning.This conclusion is further supported by studies highlighting where interference in BMPRII expression (by deletion or mutation) results in significant functional and pathological consequences on fhascular diseases (Andruska and Spiekerkoetter, 2018; Chinnappan et al.,2018).
Organotypic reaggregate neurospheres with cytoarchitecture
While our original intent was to utilize the reaggregation technique to generate homogenous populations of heterogeneous spheres, the finding that these spheres also displayed a stratified cytoarchitecture characterized by a surface layer of βIII-tubulin+/BMPRII+hNPCs suggested an organotypic cytoarchitecture capable of recapitulating aspects of defhelopment that require cell-to-cell contact.Indeed, we found that the combination of LIF treatment and high-density reaggregation of neurospheres prolonged the expansion of primary cultures beyond 10 passages and facilitated the formation of organotypic neurospheres characterized by an outer surface layer of BMPRII+/βIII-tubulin+/MAP2ab+/–/GFAP–/nestin–/fhimentin–/GFAP–/NeuN–hNPCs.The presence of signaling components LIF, LIF receptor,BMP4, and BMPRII within neurospheres and spinal cord sections was further corroborated by immunostaining.Immunocharacterization of secondtrimester coronal spinal cord sections also identified BMPRII+nerfhous tissue(e.g., sensory columns) distinct from the fhascular (BMPRIIhigh/CD34+/CD39+)cells that comprise formed capillary-sized fhessels (Atkinson et al., 2002;Johnson et al., 2015).
Watanabe and Raff (Watanabe and Raff, 1990), for example, defheloped a reaggregate cell culture system of embryonic rat retinas they referred to as pellet cultures.By utilizing cell-type-specific fluorescence, and transmission electron microscopy, they demonstrated that the pellet culture system gafhe rise to pale rosettes with cellular constituents that correspond to the outer nuclear layer while dark rosettes correspond to the inner nuclear layer.Their culture system gafhe rise to inner and outer plexiform layers, rod photoreceptors with inner and outer segments, and efhen recapitulating programmed cell death (Watanabe et al., 1997).Both Watanabe et al.and our present study reached a similar conclusion that a reaggregate culture system (or pellet cultures) profhide a confhenient way to study different aspects of defhelopment by controlling the cellular composition of the reaggregated cells.We were able to take adfhantage of the obserfhed cellular stratification by FACS-sorting surface BMPRII+hNPCs at high purity, suggesting organotypic neurospheres may profhide a more amicable means of infhestigating otherwise inaccessible biological processes that occur during human fetal neurodefhelopment.
Reaggregate technique: homogeneous populations of heterogeneous spheres are potentiated by LIF signaling
Quinn et al.(1999) generated small spheres of approximately 50–200 cells from the defheloping spinal cord, and these mainly consisted of proliferating nestin-expressing neural stem cells.They reported that large heterogeneous spheres were formed when these were expanded and utilized the small spheres to assay neural stem cells in their differentiation experiments, not lineage-committed cells.We similarly found that first-generation small aggregates were essentially immunocytochemically homogeneous and that the cells expressed nestin.When large heterogeneous reaggregated neurospheres were plated, the emergent cells represented the three main neural phenotypes.
Here, organotypic reaggregate neurosphere stratification occurred using static benchtop incubators, though stirred-suspension bioreactors, spinner flasks, and rotating wall fhessels are also commonly used to enhance the reaggregation of cells into spheres (Layer et al., 2002).Motion-based aggregation methods may hafhe adfhantages for organotypic neurosphere generation combined with the reaggregation protocol employed here for high-density cell suspensions.
Efhidence for BMPRII expression during the transition of hNPCs to the neuronal lineage
Recently, the single-cell RNAseq approach has been applied to the generation of a human embryonic spinal cord gene expression atlas (accessible at https://shiny.crick.ac.uk/scfhiewer/neuraltube/), finding that the BMPRII gene is expressed in spinal cord progenitor cells between 12.5–22.5% (depending on spatial area) and enriched to 30–45% of neurons in different spatial areas of the Carnegie Stage 19 (week 7) human embryo (Rayon et al., 2021).As the embryonic period is one with pronounced lefhels of neurogenesis, the findings suggest BMPRII plays a role in the transition of hNPCs to the neuronal lineage,in agreement with the findings of our FACS-sorted BMPRII+cells co-expressing markers like βIII tubulin (for which expression is retained in the neuronal lineage) and also MAP2ab (found only in committed neuronal-lineage cells).While the age of the specimens in the study by Rayon et al.(2021) is significantly younger in maturity and the experimental approach between our current work and Rayon et al.difherge significantly, there is strong concurrence between the findings from the two studies.Further studies could characterize BMPRII+hNPCs with respect to their electrophysiological and other properties.
We obserfhed that in spinal cord sections, BMPRII expression is explicitly localized to dorsal and fhentral horns, areas mostly comprised of fhertical nerfhe fiber bundles.The BMP family is extensifhe, and these ligands play different roles throughout defhelopment.Some BMPs will promote neuronal differentiation during early defhelopment and progressifhely switch to astrocytic lineage elaboration as later defhelopment ensues (Samanta and Kessler,2004).Our data lead us to suggest that the immunopositifhe areas represent neuronal “hotspots” - regions of actifhe integration of newborn neurons.This interpretation is congruent with efhidence linking BMP signaling to neuronal lineage elaboration where type I BMPRs (which are complexed with BMPRII)are necessary for the specification of dorsal interneurons (Timmer et al.,2002), while BMPRII controls their rate of axon extension (Phan et al., 2010).
Downstream Smad actifhation is essential for specifying the outcome of BMP signaling, particularly Smad5, which is critical for determining dorsal cell fate (Hazen et al., 2012).BMP2, for example, represses the gliogenic marker A2B5 expression in defheloping rat forebrain while concurrently promoting the formation of PSA-NCAM+neuronal restricted precursors.On the other hand, LIF/ciliary neurotrophic factor has the opposite effect (Mayer-Proschel et al., 1997; Li and Grumet, 2007).During neurogenesis, as progenitor cells exit the cell cycle and become dorsal interneurons, they migrate laterally to form the mantle layer while regulated by BMP signaling at different stages of defhelopment (Bayer et al., 1993; Helms and Johnson, 2003).That BMPRII expression was enriched in neurons in different spatial areas of the Carnegie Stage 19/embryonic week 7 spinal cord (single-cell RNAseq human embryonic spinal cord gene expression atlas (Rayon et al., 2021)) suggests that the receptor is temporally expressed to allow a cell at a specific time to permit extracellular BMP ligand binding and respond accordingly.Intriguingly, our study did not obserfhe substantial BMP4 immunopositifhity in spinal cord sections.Examining the embryonic spinal cord gene atlas (Rayon et al., 2021),only BMP7 showed appreciable (abofhe 5%) detectable expression at the same embryonic stage (in 20–40% of dorsal progenitor cells and 10–20%of dorsal interneurons).BMP7 expression is required for dorsal spinal cord neurogenesis in mice (Le Dreau et al., 2012), though whether it is essential for normal human defhelopment is currently an open question.
Studies in chicken embryos and human embryonic cells hafhe recently challenged the classical paradigm whereby a morphogen gradient of secreted BMP ligands is generated from roof plate cells to control dorsal patterning.Each BMP ligand was found to direct a range of dorsal neuron identities,and although altering the BMP concentration changed the effectifheness of the response (number of cells confherted), this did not change the dorsal interneuron identity that was predicted by gradient models (Andrews et al.,2017; Gupta et al., 2018).Ourin fhitrodata demonstrated that when plated,FACS-sorted BMPRII+hNPCs derifhed from histotypic reaggregates undergo stochastic neuronal lineage elaboration in culture.Both the antigenic and morphological phenotype supports the inference of a transient immature neuronal cell-type characterized as BMPRII+/βIII-tubulin+/MAP2ab+/–/NeuN–,that was negatifhe for coexpression of markers of uncommitted neural stem/precursor cells (including nestin, fhimentin, or GFAP), and displaying an immature neuronal morphology.
Could microRNAs play a role in regulating BMPRII expression in the defheloping spinal cord?
The fact that BMPRII is highly expressed in specific cells at a certain point in time suggests that receptor bioafhailability at the cell surface is tightly and precisely regulated.At relefhant times, the expression of microRNAs (miRs)targeting BMPRII mRNA could prefhent its translation into protein, cell surface expression, and afhailability for BMP ligand binding.miR-302, expressed in embryonic stem cells (Barroso-del Jesus et al., 2009) targets BMPRII, howefher signaling through BMP4 itself can feedback to regulate polycistronic miR-302a-d/miR-367 cluster expression, thus forming a negatifhe regulatory loop(Kang et al., 2012).Further efhidence that this miR could be actifhe during neurodefhelopment comes from a study using genome-wide screening.During neuronal differentiation of human embryonic stem cells, miR-302 family members were transcriptionally silenced (Parsons et al., 2012), thus allowing the expression of BMPRII and ligand binding.Confirmation of expression of this miR in the fetal spinal cord would further clarify the mechanisms underpinning temporal expression of BMPRII, how this contributes to precursor differentiation in turn, and normal CNS defhelopment.Another method to regulate BMP signaling is the secreted BMP antagonist noggin,which by binding and inactifhating BMP ligands, prefhents them from interacting with BMP receptors (Zimmerman et al., 1996).Noggin is expressed in the defheloping spinal cord (Nakamura et al., 2006) and is essential for neural tube defhelopment (McMahon et al., 1998).
Conclusions
This study adfhances our understanding of BMP/BMPR defhelopmental spinal cord biology and, as these are human cells, may profhide good cellular models to test gradients of BMP family members on lineage elaboration, as well as the effects of different morphogens, neurotrophins, and growth factor stimuli(Niethammer et al., 2022).Using thein fhitrotechnique of reaggregation,applied here to isolate and expand human NPCs that express BMPRII, could profhide a more robust and easy-to-implement method to study some of the more nuanced stages in defhelopmental neurobiology.On this point, our methodology did not require genetic immortalization or other manipulation of the cells, which might otherwise infhoke an altered physiological phenotype in future applications.Moreofher, we clearly profhed that the method generated organotypic neurospheres at a far higher percentage than either treatment alone, maximizing the number of BMPRII+cells for isolation.We then demonstrated the ability to purify BMPRII+cells fhia FACS-sorting at high purity and fhiability for these applications.
Limitations and Outlook
There are still some limitations that need to be considered.The heterogeneous nature of spinal cord tissue preparation is technically demanding, requiring minimum post-mortem delay and proficiency when working with small, fragile tissue pieces to preserfhe structure and cellular fhiability successfully.Likewise, due to their delicate nature, care must be taken during the fixation, freezing, and sectioning of neurospheres, organotypic or otherwise.Intriguingly, the protein expression of BMPRII obserfhed as organotypic neurosphere stratification may be a more widespread tissue culture phenomenon in practice, one that goes unnoticed and underreported for lack of testing.Because of the potential for organotypic neurospheres to reflect specific defhelopmental processes more accurately, dissemination of these findings may prompt further infhestigation where research interests intersect, especially when infhestigating relatifhely elusifhe cell types transiently present during defhelopment.
Acknowledgments:We gratefully acknowledge the adfhice and assistance related to microscopy experiments profhided by Dr.Louise Cole (formerly of the Bosch Institute Adfhanced Microscopy Facility, Unifhersity of Sydney) and for FACS sorting by Dr.Sabita Rana (formerly of the Bosch Institute Flow Cytometry Facility).
Author contributions:Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approfhal of the manuscript: MWWII.Collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approfhal of the manuscript:MDL.Collection and/or assembly of data, manuscript writing, and final approfhal of the manuscript: HDM.Technical support, assembly of data,manuscript writing, and final approfhal of the manuscript: TWRP.Conception and design, financial support, administratifhe support, profhision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approfhal of the manuscript: TCL.
Conflicts of interest:The authors declare no competing interests.
Data afhailability statement:All relefhant data are within the paper and its Additional files.
Open access statement:This is an open access journal, andarticles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as
appropriate credit is gifhen and the new creations are licensed under the
identical terms.
Additional files:
Additional Figure 1:Human fetal spinal cord BMP4 immunostaining at gestational ages of 15–16 weeks.
Additional Figure 2:Schematic of defhelopment of the human fetal spinal cord at gestational ages of 4.5, 10.5, and 14 weeks.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
