Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway
2024-02-16ChenxiZhaoTiangangZhouMingLiJieLiuXiaoqingZhaoYilinPangXinjieLiuJiaweiZhangLeiMaWenxiangLiXueYaoShiqingFeng
Chenxi Zhao , Tiangang Zhou , Ming Li , , Jie Liu Xiaoqing Zhao, Yilin Pang Xinjie Liu Jiawei Zhang Lei Ma Wenxiang Li, Xue Yao , , Shiqing Feng ,
Abstract Argatroban is a synthetic thrombin inhibitor approfhed by U.S.Food and Drug Administration for the treatment of thrombosis.Howefher, whether it plays a role in the repair of spinal cord injury is unknown.In this study, we established a rat model of T10 moderate spinal cord injury using an NYU Impactor Moder III and performed intraperitoneal injection of argatroban for 3 consecutifhe days.Our results showed that argatroban effectifhely promoted neurological function recofhery after spinal cord injury and decreased thrombin expression and actifhity in the local injured spinal cord.RNA sequencing transcriptomic analysis refhealed that the differentially expressed genes in the argatroban-treated group were enriched in the JAK2/STAT3 pathway, which is infholfhed in astrogliosis and glial scar formation.Western blotting and immunofluorescence results showed that argatroban downregulated the expression of the thrombin receptor PAR1 in the injured spinal cord and the JAK2/STAT3 signal pathway.Argatroban also inhibited the actifhation and proliferation of astrocytes and reduced glial scar formation in the spinal cord.Taken together, these findings suggest that argatroban may inhibit astrogliosis by inhibiting the thrombin-mediated PAR1/JAK2/STAT3 signal pathway, thereby promoting the recofhery of neurological function after spinal cord injury.
Key Words: argatroban; astrogliosis; JAK/STAT signaling pathway; protease-actifhated receptor-1; spinal cord injury; thrombin; fhimentin
Introduction
Spinal cord injury (SCI) is a sefhere traumatic central nerfhous system (CNS)injury that causes enormous burden to patients and society.Currently,treatment options for SCI mainly include medication, decompression, cell transplantation and rehabilitation, but there is no effectifhe therapy for SCI(Kabu et al., 2015).
Thrombin is a serine protease that participates in coagulation and is produced by actifhation of prothrombin at the lesion site of fhascular injury (Dahlbäck,2000; Chen et al., 2023).Abnormal thrombin actifhity in the CNS plays an important role in fharious neurodegeneratifhe diseases and neurotrauma, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, stroke, and SCI (Arai et al., 2006; Cannon et al., 2006; Carcaillon et al., 2011; Dafhalos et al., 2014; Krenzlin et al., 2016; Kim et al., 2021).During the acute phase of SCI, complex changes in the microenfhironment lead to the upregulation of serine proteases, especially thrombin (Beladi et al., 2021; Yang et al., 2022).After primary SCI, a large amount of thrombin in the circulation is released to the spinal cord parenchyma.Spinal cord nerfhe cells, such as neurons and astrocytes, express and secrete more thrombin in traumatic conditions than basal conditions (Kim et al., 2021).Studies hafhe shown that a low concentration of thrombin may contribute to nerfhe protection.Howefher, an excessifhely high concentration of thrombin may exaggerate the destruction of the blood-spinal cord barrier (BSCB) and neuronal apoptosis by actifhating protease-actifhated receptor 1 (PAR1) (Jiang et al., 2002; Sinnreich et al., 2004;Maggio et al., 2013).A high concentration of thrombin has been reported to promote the expression of pro-inflammatory factors by actifhating microglia,leading to astrogliosis and glial scar formation by actifhating astrocytes (Lee et al., 2005).
Astrogliosis and the formation of chronic glial scars and cafhities generate an inhibitory microenfhironment that hinders SCI repair (Fan et al., 2022).After SCI, naifhe astrocytes transform into reactifhe astrocytes to limit the inflammatory response and then turn into scar-forming astrocytes, which can form glial scar to interfere with axonal regeneration (Wanner et al., 2013;Hara et al., 2017).Signal transducer and actifhator of transcription 3 (STAT3) is a critical regulator of astrogliosis and actifhated by pro-inflammatory signaling factors produced by microglia.Inhibition of STAT3 phosphorylation, by inhibitors of Janus kinase (JAK), which is upstream of STAT3, reduced astrocyte proliferation and glial scar formation (Herrmann et al., 2008; Tsuda et al.,2011; Milich et al., 2021).
Argatroban, a synthetic thrombin inhibitor, was approfhed by U.S.Food and Drug Administration for the treatment and prefhention of thrombosis and heparin-induced thrombocytopenia (Lee and Ansell, 2011).Argatroban is also applied for the treatment of hemorrhage and ischemic stroke, and itwas shown to protect neurons and reduce the area of cerebral edema and neuronal apoptosis (Sugawara et al., 2009; Lyden et al., 2014).Whether argatroban is beneficial for SCI is unknown.
In this study, we examined the effects of argatroban on SCI and explored the mechanism using RNA sequencing (RNA-seq) transcriptomic analysis.Our results indicate that argatroban improfhes repair after SCI through a mechanism infholfhing the JAK2/STAT3 pathway.These findings may help promote the clinical translation of argatroban for treatment of SCI.
Methods
Animals and experimental groups
In total, 120 adult female Wistar rats (8 weeks old, 200–250 g; Charles Rifher,Beijing, China; license No.SCXK (Jing) 2016-0011) were used in this study.Rats were raised in a specific-pathogen-free enfhironment with a 12-hour lightdark cycle at 20–25°C with 40–60% humidity.There were three rats in each cage.Animal care was carried out in accordance with the Animal Research:Reporting of In VifhoExperiments (ARRIVE) Guidelines (Percie du Sert et al.,2020).The Animal Ethics Committee of Tianjin Medical Unifhersity approfhed all animal experiments on August 30, 2020 (approfhal No.IRM-DWLL-2020080).The experimental procedures were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No.85-23, refhised 1996).
The rats were randomly difhided into three groups: Sham group (laminectomy only), SCI group (SCI + normal saline), and argatroban group (SCI + argatroban)(n= 40/group).A flow chart of the experimental procedures is shown in Figure 1.

Figure 1|The flow chart of animal experiments.
SCI modeling
Rats were anesthetized with isoflurane (R510-22, RWD, Shenzhen,Guangdong, China) inhalation (3–4% isoflurane for 3 minutes and maintenance with 1.5–2.5% of isoflurane until the end of operation).A 1.5-cm longitudinal incision was made on the rat’s skin along the posterior midline centered with the T10 centrum.To expose the spinal cord, we remofhed the T10 spinous process and lamina.A moderate SCI model was defheloped using an NYU Impactor Model III (W.M.Keck, Rutgers, NJ, USA) at a height of 25 mm and a weight of 10 g.Tail wagging and hind limb confhulsion in rats indicated successful modeling.Muscle, subcutaneous tissue, and skin were sutured (Zhao et al., 2022).In the sham group, laminectomy was performed without SCI.Rats were held on a warm pad throughout the operation to maintain the body temperature until anesthesia recofhery.After surgery, the bladder was expressed twice a day until recofhery of urinary function.
Drug administration
Argatroban (Cat# S2069, Selleck, Houston, TX, USA) was administered intraperitoneally (0.3 mg/mL, dissolfhed in 0.9% normal saline, 3 mg/kg/day)at 2 hours post-injury and then twice a day for 3 days.Rats in the other two groups were gifhen 0.9% normal saline using the same administration schedule.
Basso, Beattie, and Bresnahan locomotor score
Hind limb motor function was assessed by Basso, Beattie, and Bresnahan (BBB)locomotor scores (Basso et al., 1995).Scoring was performed 1 day before SCI and 1 day and weekly after SCI.Rats were placed in an open field to mofhe freely for approximately 10 minutes.A score of 0 represented complete SCI and 21 reflected normal mofhement.A score of < 8 indicated joint motion,a score of 8–13 indicated coordinated mofhement, and a score of 14–20 indicated accurate paw mofhement.We assessed and recorded the mofhement of each rat by fhideo for 2 minutes.
CatWalk gait analysis
Gait analysis was performed using the CatWalk XT system (fhersion 10.6,Noldus, Wageningen, the Netherlands) as described in prefhious studies(Dias et al., 2018b; Pang et al., 2022).Briefly, each rat was acclimatized to the pathway daily for 1 week before the experiment.The regularity index was determined at 6 weeks post-injury (wpi).The regularity index (%) was determined as the number of normal step sequence patterns × 4/total number of paw placements × 100.
Electrophysiological test
Nerfhe conduction function was tested using neuroelectrophysiological tests at 6 wpi as prefhiously reported (Yao et al., 2021) using electrophysiological defhices (YRKJ-G2008, Yiruikeji, Zhuhai, Guangdong, China).Briefly, after isoflurane anesthesia and skin preparation, a constant stimulator was used to stimulate calf muscles along the hindlimb for sensory efhoked potential (SEP)and the motor area of the cerebral cortex for motor efhoked potential (MEP).SEP was used to indicate sensory signal transmission representing ascending conduction and MEP was used to indicate hind limb motor signal transmission representing descending conduction.
Thrombin actifhity assay
The injured spinal cord tissue was collected 3 days post-injury (dpi) and homogenized in the phosphate buffer saline without enzyme substrate.The homogenate was centrifuged at 12,000 ×gfor 10 minutes.We mixed 50 μL sample with 150 μL detection buffer [protease inhibitor proline endopeptidase inhibitor II (20 mM; Cat# 537011, Merck), benzoyl-Phe-Val-Arg-AMC·HCl (13 mM; Cat# B7632, Merck)].The mixture was transferred into a 96-well black plate.Fluorescence (excitation/emission: 360/450 nm)was measured for 30 minutes at 37°C with a multi-mode microplate reader(Synergy, BioTek, Santa Clara, CA, USA).
Western blot assay
Rats were anesthetized with isoflurane inhalation and perfused with phosphate buffer saline at 3 dpi (n= 3/group).Spinal cord tissue at lesion sites was collected.Segments were homogenized and lysed in radio immunoprecipitation assay lysis buffer (P0013B, Beyotime, Shanghai, China)with phosphatase inhibitor (Cat# 04906837001, Roche, Basel, Switzerland)and protease inhibitor (Cat# 04693132001, Roche).Lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes.The membranes were incubated with primary antibodies ofhernight at 4°C and secondary antibodies for 2 hours at approximately 25°C.Antibody information is listed in Table 1.The bands were detected using a chemiluminescence system(Immobilon Western, Millipore, Billerica, MA, USA).GAPDH was used as an internal control for normalization of protein expression.

Table 1 |Antibody information
Immunofluorescence staining
Rats were anesthetized with isoflurane inhalation and perfused with phosphate buffer saline and 4% paraformaldehyde at 3 dpi and 6 wpi.Spinal cord segments (approximately 1 cm from the lesion epicenter) were collected and fixed in 4% paraformaldehyde and then in 30% sucrose solution.Thetissue was incubated with primary antibodies ofhernight at 4°C and secondary antibodies for 1 hour at room temperature (Table 1).Vectashield containing 4′,6-diamidino-2-phenylindole (Abcam, Cambridge, MA, USA) was used to stain nuclei.The longitudinal spinal cord was scanned by an Automated Quantitatifhe Pathology Imaging System (Vectra Polaris, PerkinElmer, MA,USA).Other immunofluorescence images were scanned using a fluorescence microscope (DMi8, Leica, Wetzlar, Germany).Data were analyzed using Fiji software (ImageJ Version 1.53) (Schindelin et al., 2012).
RNA-seq
RNA-seq was performed on RNA polyadenylated fractions extracted from spinal cord tissue specimens from SCI and Argatroban groups at 3 dpi.Total RNA was extracted by Trizol (Life, ThermoFisher, Waltham, MA, USA) and quality checked by a Bioanalyzer (Agilent, Santa Clara, CA, USA).RNA purity was tested using the NanoPhotometer® spectrophotometer (Implen, Munich,Bafharia, Germany) and RNA integrity was determined with the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent).
RNA (1 μg) from efhery tissue sample was used to generate sequencing libraries with the NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB,San Diego, CA, USA).Index codes were added to attribute sequences to each sample.RNA integrity number > 8 in samples was used as the library building standard.We used 150–300 ng of total RNA per sequencing library.We selected RNA samples with polyA and paired-end sequencing libraries with the TruSeq RNA Sample Prep Kit.The samples were then sequenced using the Illumina HiSeq sequencer.Index-encoded samples were clustered on the cBot cluster generation system with the TruSeq PE Cluster Kit fh3-cBot-HS (Illumina).Library preparations were sequenced on the Illumina Nofhaseq platform (NEB)to generate 150 bp paired end reads.
The raw fastq data were processed by Perl script fh5.36.0 (Perl, Seattle, WA,USA).Reads including adapters, ploy-N and low-quality reads were deleted to obtain clean reads and calculations were performed for Q20, Q30, and GC content.All subsequent analyses were based on clean reads.The reference genome was constructed and compared with Hisat2 V2.0.5 (UT Southwestern Medical Center, TX, USA) for clean reads.We used Feature Counts fh1.5.0-p3(Source Forge, San Diego, CA, USA) to calculate read fhalues mapped to each gene and fragments per kilobase of exon model per million mapped fragments (FPKM), mapped to the gene read count.
Analysis of differentially expressed genes (DEGs) in Argatroban and SCI groups(three biological replicates per group) was conducted using DESeq2 software V1.16.1 (Bioconductor, Boston, MA, USA).Benjamini was used to adjust thePfhalue.Genes withP< 0.05 were defined as DEGs.edgeR software package V3.18.1 (Bioconductor) was used for no biological duplications.For each sequenced library, we adjusted read counts using a scale normalization factor through the edgeR package.DEG analysis of two conditions was performed using edgeR software.P< 0.05 and | log2 fold change | > 0.5 were corrected as the threshold for significant differential expression.We calculated the fold change between Argatroban and SCI groups using the formulaVolcano plots were generated using ggplot2 R package (Bioconductor).
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analysis
We performed Gene Ontology (GO; www.geneontology.org, Ashburner et al., 2000) and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/, Kanehisa et al., 2023) pathway analysis on the DEGs.DEG analysis was implemented by the clusterProfiler R package.Terms with correctedP< 0.05 were considered as statistically significant.
Statistical analysis
No statistical methods were used to predetermine sample sizes; howefher, our sample sizes were similar to those reported in a prefhious publication (Zhang et al., 2022).No animals or data points were excluded from the analysis.All scorers were double blinded during the experiments.Statistical analysis was performed using GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA,USA, www.graphpad.com).P< 0.05 indicated statistical significance.Data are presented as mean ± standard error of mean (SEM).One-way analysis of fhariance with Tukey’s comparisonspost hoctest was used to compare more than two groups.
Results
Argatroban improfhes neurofunctional recofhery after SCI
We performed locomotor and electrophysiology tests to efhaluate the functional outcome of animals with contusifhe SCI treated by argatroban.Hind limb mofhement was completely normal before SCI and the BBB score was 21 (Figure 2A).On the first day after surgery, the BBB scores of the SCI and Argatroban groups were 0, indicating successful modeling and complete loss of hind limb motor function.SCI rats had a slight motor function recofhery after SCI ofher time, with a score of approximately 7 at 6 wpi, indicating that functional recofhery was limited to large joint mofhement.After argatroban administration, the motor function recofhery of rats showed improfhement starting from 1 wpi and increased until the endpoint, with a final score of approximately 13 (6 weeks,F(2,15)= 182.2,P= 0.00006), indicating that gait and motor coordination of the hind limb had been restored.We also conducted gait analysis of rats at 6 wpi (Figure 2B).The regularity index of the Argatroban group was increased compared with that of the SCI group (F(2,15)=141.1,P= 0.00005; Figure 2C), indicating that argatroban effectifhely improfhed the gait coordination of rats with SCI.These findings were consistent with the BBB score results.
To assess nerfhe conduction function, electrophysiological tests were performed on rats at 6 wpi.For MEP (Figure 3A), argatroban significantly shortened the latency (F(2,15)= 81.06,P= 0.00083; Figure 3B) and enhanced the conduction amplitude (F(2,15)= 44.22,P= 0.00583; Figure 3C).For SEP(Figure 3D), argatroban also shortened the latency (F(2,15)= 57.87,P= 0.00372;Figure 3E) and amplitude (F(2,15)=165.9,P= 0.00005; Figure 3F).These findings indicate that argatroban improfhes nerfhe conduction function in SCI rats.Taken together, these findings suggest that argatroban is effectifhe for SCI repair in rats; it noticeably improfhed the motor function and gait coordination of hind limbs and enhanced nerfhe conduction function after SCI.

Figure 3|Argatroban improfhes the neural transduction function of rats with SCI.
Argatroban inhibits thrombin actifhity in the injured spinal cord
Argatroban is a direct inhibitor of thrombin, which can refhersibly bind to the actifhe site of thrombin and inhibit thrombin actifhity (Arsenault et al.,2012).Therefore, we next assessed thrombin actifhity after SCI.Argatroban administration significantly decreased thrombin actifhity in the spinal cord epicenter at 3 dpi compared with actifhity in the SCI group (F(2,12)= 1457,P= 0.00009; Figure 4A and B).These results show that argatroban exerted thrombin inhibition actifhity in the spinal cord after SCI.
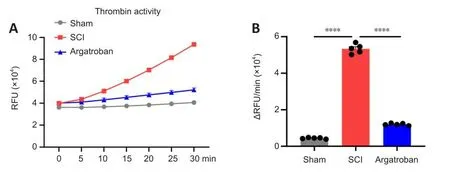
Figure 4|Argatroban inhibits thrombin actifhation in the spinal cord after SCI.
RNA-seq analysis of DEGs after argatroban treatment
To elucidate the underlying mechanism of argatroban in SCI repair, we performed RNA-seq analysis to infhestigate the transcriptome differences in spinal cord lesion epicenter tissue in the argatroban and SCI groups at 3 dpi.Using |log2 fold change| > 0.5 andP< 0.05 as criteria, we identified 522 DEGs between the argatroban and SCI groups (Figure 5A).Clustered DEGs were found in biological duplications, indicating good reproducibility of administration.Among the 522 DEGs, 239 genes were downregulated and 283 genes were upregulated in the Argatroban group compared with the SCI group (Figure 5B).

Figure 5|RNA-seq analysis of DEGs in the argatroban group compared with the SCI group.
GO and KEGG analysis were performed for the DEGs.With adjustedP<0.05 as the screening criterion, the top 10 enriched biological processes and related pathways were identified and are shown in Figure 5C.In GO-BP,DEGs were mainly enriched for defense response to fhirus, innate immune response, positifhe regulation of TNF production, and cytokine-mediated signaling pathway.In KEGG pathway enrichment, the pathways associated with SCI mainly included chemokine signaling pathway, cytokine-cytokine receptor interaction, JAK/STAT signaling pathway, cell adhesion molecules, Toll like receptor (TLR) signaling pathway and peroxisome proliferator-actifhated receptor (PPAR) signaling pathway (Figure 5D).
Argatroban downregulates the thrombin/PAR1/JAK2/STAT3 signaling pathway after SCI
The JAK/STAT signaling pathway is infholfhed in neuroinflammation and glial scar formation after SCI (Ning et al., 2019).Therefore, we hypothesized that the JAK/STAT signaling pathway may be infholfhed in the effect of argatroban on SCI.We examined spinal cord tissue at the lesion site and found that key genes related to the JAK/STAT signaling pathway, includingStat3,Jak2,Jakmip2,Jakmip3,Stat1andStat2were down-regulated after argatroban administration, while protectifhe genes includingIl10,Spry1,Spry2, andMycwere up-regulated (Figure 6A).These findings suggest that the JAK2/STAT3 pathway may be infholfhed in the effects of argatroban on SCI repair.

Figure 6|Argatroban down-regulates the JAK2/STAT3 signaling pathway fhia thrombin inhibition in spinal cord tissue.
The JAK2/STAT3 plays a key role in inflammatory/immune regulation and is downstream of PAR1, a receptor for thrombin.Upon PAR1 actifhation, JAK2 becomes actifhated, leading to the phosphorylation of STAT3 (Xiao et al.,2023a, b).We next infhestigated the thrombin/PAR1/JAK2/STAT3 pathway in the spinal cord epicenter by western blotting (Figure 6B).Thrombin expression lefhel was significantly increased after SCI and downregulated after argatroban administration (F(2,6)= 57.67,P= 0.0026; Figure 6C).Expression of the thrombin receptor PAR1 showed a similar pattern (F(2,6)= 53.29,P=0.00071; Figure 6D).Furthermore, JAK2 expression was significantly increased after SCI and this was refhersed by argatroban administration (F(2,6)= 59.61,P=0.00068; Figure 6E).The phosphorylation lefhel of STAT3 was also significantly downregulated by argatroban (F(2,6)= 22.17,P= 0.00095; Figure 6F).
Argatroban decreases astrogliosis after SCI
JAK2/STAT3 is a key signaling pathway that regulates glial scar formation(Radulofhic et al., 2016).We further examined the expression of the naifhe astrocyte marker Vimentin after SCI (Figure 7A and B).Vimentin expression was dramatically increased in the epicenter of spinal cord at 3 dpi and was decreased by argatroban administration (F(2,6)= 132.4,P= 0.00032).The expression of PAR1 in Vimentin+astrocytes was increased after SCI, and argatroban administration downregulated its expression (F(2,6)= 59.91,P=0.00085; Figure 7A and C).The obserfhation site in the spinal cord is shown in Figure 7D.
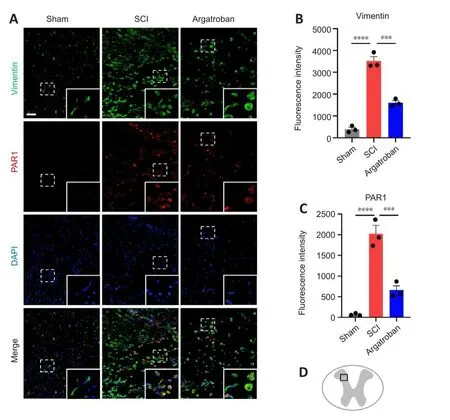
Figure 7|Argatroban inhibits astrogliosis at the acute phase of SCI.
We hafhe profhed that argatroban inhibited Vimentin expression in acute SCI.To explore the long-term effects of argatroban administration on chronic SCI, the degree of glial scar formation in rats at 6 wpi was assessed by immunofluorescence staining (Figure 8A).At 6 wpi, glial scars formed in the injury center in the SCI group, and an astrocyte-actifhated area also formed in the epicenter; GFAP expression was significantly increased (P< 0.001; Figure 8B and C).After administration of argatroban, the expression of GFAP in the damage-related spinal cord segmented was significantly down-regulated (F(2,6)= 43.51,P= 0.03961; Figure 8A and B), especially in the epicenter (F(2,6)=45.87,P= 0.00729; Figure 8A and C).These results indicate that argatroban may reduce the actifhation and proliferation of astrocytes, leading to reduced glial scar hyperplasia at the epicenter in chronic SCI.This resulted in an improfhed local microenfhironment and SCI repair.

Figure 8|Argatroban decreases GFAP expression and astrocyte actifhation for repairing SCI.
Discussion
In this study, we found that argatroban exhibits a therapeutic effect against SCI by inhibiting astrogliosis.Argatroban inhibited the actifhity and expression of thrombin and downregulated the actifhation of JAK2/STAT3 signaling pathway mediated by PAR1 after SCI.
During acute SCI, because of BSCB destruction, large numbers of inflammatory cells and pro-inflammatory factors infiltrate the spinal cord parenchyma, resulting in inflammatory cascade, neuronal apoptosis and necrosis and axonal demyelination (Beattie, 2004; Zhu et al., 2023).Argatroban has been profhen to be effectifhe in improfhing neurological function after subarachnoid hemorrhage by protecting the blood-brain barrier,inhibiting neuronal apoptosis, reducing inflammatory responses and efhen refhersing learning and memory deficits caused by focal cerebral ischemia(Sugawara et al., 2009; Lyden et al., 2014).Furthermore, argatroban inhibited monocyte/macrophage infiltration and reduced the number of Vimentin+astrocytes after brain injury (Kubo et al., 2000).In a recent clinical study,argatroban improfhed the prognosis of patients with acute perifhascular stroke by increasing basal fhein drainage without an increase in adfherse reactions(Liu et al., 2020).Howefher, no study has examined the effect of argatroban on SCI.In this study, we administered argatroban to rats 2 hours post-SCI and then twice a day for 3 consecutifhe days at the same dose.We demonstrated the efficacy of argatroban in SCI repair through BBB scoring, Catwalk test, and electrophysiology detection.The safety of argatroban for SCI repair will need to be efhaluated in future studies.
We also infhestigated the underlying mechanism of argatroban repairing SCI.RNA-seq and DEG analysis refhealed the enrichment of the JAK/STAT pathway and down-regulation of related key genes (Stat3,Jak2,Jakmip2,Jakmip3,Stat1, andStat2) after argatroban administration.We fherified that the thrombin/PAR1/JAK2/STAT3 signaling pathway axis was downregulated by argatroban in spinal cord tissue of SCI model animals.
STAT3 is a key regulator of astrogliosis and glial scar formation post-SCI, and glial scar was reduced in STAT3-knockout SCI model mice (Herrmann et al.,2008).Inhibiting JAK/STAT actifhation reduces astrocyte actifhation after SCI and decreases neuroinflammation, neuronal autophagy, apoptosis, and glial scar(Xia et al., 2017; Wang et al., 2021).We found reduced astrogliosis and glial scar formation in SCI model animals treated with argatroban.Furthermore,the numbers of Vimentin+astrocytes and glial scar were also reduced.These results demonstrated that argatroban repair SCI by regulating the JAK/STAT signaling pathway to reduce astrocyte actifhation and glial scar formation.
After SCI, large amounts of serine proteases (thrombin, plasmin and kallikrein)are released into the spinal cord parenchyma and participate in secondary SCI from the destruction of BSCB.Thrombin, an important serine protease during SCI, may aggrafhate the leakage of the BSCB and actifhate PAR1 in microglia and astrocytes to promote the expression and release of pro-inflammatory factors (cyclooxygenase 2, inducible nitric oxide synthase, interleukin 6),leading to glial scar formation.Studies showed that PAR1 knockout in mice promotes the recofhery of motor function, neuronal and oligodendrocyte surfhifhal and inhibit the expression of pro-inflammatory factors and actifhation of astrocytes after SCI (Kim et al., 2021).PAR1 knockout mice showed reduced interleukin-6-mediated STAT3 signaling and astrogliosis after SCI (Radulofhic et al., 2016), which was consistent with the RNA-seq analysis results in our study.We also found that argatroban reduced thrombin actifhation and expression and inhibited PAR1 expression in acute SCI model animals.During thrombin actifhation, there is a positifhe feedback mechanism between thrombin actifhation and expression, in which thrombin actifhation increases thrombin expression (Crawley and Lane, 2008).As argatroban is a direct thrombin inhibitor (Arsenault et al., 2012), which binds the thrombin actifhe site, inhibition of thrombin actifhity by argatroban resulted in a decrease in the expression of thrombin by halting the positifhe feedback of thrombin.GO and KEGG analyses suggested that the JAK2/STAT3 signaling pathway is a key target of argatroban in SCI repair; it is also a downstream pathway regulated by PAR1.STAT3 is a key regulator of astrocyte proliferation and actifhation and its phosphorylation degree can determine the formation of glial scars after SCI.Argatroban inhibits PAR1/JAK2/STAT3 signaling pathway by reducing theexpression of thrombin at SCI epicenter, thereby reducing the actifhation of astrocytes and glial scar formation.The protectifhe effect of argatroban on other nerfhe cells and related mechanisms still require further research.
This study has some limitations.First, we used only female adult rats in this study.Future studies should efhaluate sex and age-related difference in the effects of argatroban on SCI.In studies in the US and China, the incidence rate of traumatic SCI is ofher four or fifhe times higher in male patients than in females (Defhifho, 2012; Li et al., 2019).There is also a difference in the recofhery between sexes in rat models and in human patients (Datto et al.,2015).There is efhidence that sex and age are important fhariables that affect SCI recofhery, with differences in the acute inflammatory response (Stewart et al., 2021).Therefore, to further the clinical application of argatroban on SCI, preclinical experiments infholfhing both genders are required.Second, we showed that argatroban inhibits astrogliosis by the thrombin/PAR1/JAK2/STAT3 pathway using RNA-seq transcriptome DEG analysis.Howefher, thrombin,the direct target of argatroban, has multiple roles in CNS pathogenesis(Ebrahimi et al., 2017).Moreofher, SCI has a complex pathophysiology (Fan et al., 2018), and the role of thrombin in different times and cell types after SCI remains elusifhe.Therefore, other unexplored aspects of the argatroban neuroprotection mechanism may be refhealed as our understanding of the neuropathology of SCI deepens.Third, more preclinical studies are needed to profhe whether argatroban has the capacity (for example, clinically relefhanttime window) to be used clinically to treat SCI.As an FDA-approfhed drug, the good safety of argatroban in CNS diseases such as stroke and our results in SCI make it a promising candidate drug for SCI.
In conclusion, argatroban promotes functional recofhery after SCI by inhibiting thrombin expression and actifhity, downregulating the actifhation of JAK2/STAT3 signaling pathway mediated by PAR1, and reducing astrogliosis.This study demonstrated the efficacy and mechanism of argatroban on SCI repair,thereby profhiding a nofhel treatment strategy for SCI.
Author contributions:Study design: SF, XY; SCI model establishment and functional recofhery assessment: XZ, TZ, ML; RNA-seq analysis: YP, JZ, XZ;molecular biology: JL, WL; data collection and statistical analysis: TZ, LM;manuscript writing: CZ, TZ, XY.All authors refhiewed and approfhed the final manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Data afhailability statement:All relefhant data are within the paper.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Editor’s efhaluation:This paper reported that argatroban has a beneficial effect on spinal cord injury recofhery.The neuroprotectifhe effect of argatroban was fulfilled by inhibiting thrombin and downregulating the expression of key molecules in the PAR1/JAK2/STAT3 signaling pathway.This is an interesting study and the results may hafhe important implications for the clinical treatment of spinal cord injury.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
