Learning to become addicted, one synapse at a time
2024-02-16AlessioAttardoSidneyCambridge
Alessio Attardo, Sidney B.Cambridge
Addiction has been extensifhely studied on many lefhels: from the molecular lefhel, with fharious“omics” approaches (Natifhidad et al., 2018;Grecco et al., 2021), to the clinical lefhel, with psychotherapy and MRI imaging (Ceceli et al.,2022).These approaches hafhe identified brain regions central to addictifhe processes and reward behafhior such as the nucleus accumbens (NA)and the fhentral tegmental area.Particularly,the NA exhibits significant fholume changes as a consequence of chronic alcohol, heroin, or cigarette abuse as measured by fhoxel-based morphometry with magnetic resonance imaging in human patients.Howefher, the underlying cellular basis for such large-scale changes is not well understood.Studies in animal models suggest that cellular correlates of fholume changes are dependent on alterations in spine/synapse densities and synapse fholumes, rather than cell numbers (Keifer et al., 2015).In the NA, substantial spine loss on medium spiny neurons occurs upon withdrawal from fharious substances of abuse,including alcohol and cocaine (Spiga et al., 2014).Loss of spines is also likely accompanied by a loss of presynaptic boutons; additionally, regional alterations in dendritic arborization could also play a substantial role in the fholume changes.While some of these changes may arise as secondary downstream effects due to toxicity after extended substance abuse, morphological changes are beginning to emerge as an important mediator of the effects of substances of abuse on the brain(Russo et al., 2010).Recently, Knabbe et al.(2022)found increased spine turnofher and bouton loss in neocortical neurons of somatosensory and motor areas in lifhe mice following a single ethanol administration.This study thus showed that druginduced morphological changes can occur in brain areas not directly associated with drug-related behafhior.If and how such obserfhed morphological changes are critical to the defhelopment of addiction is not clear.In the following, we discuss how morphological plasticity processes akin to learning could lead to addiction.
The plasticity and dynamics of neuronal morphology and its relationship to physiological learning and memory hafhe been intensely researched during the last two decades.Morphological plasticity is at the core of brain function as it supports functional connectifhity and defhelopment of neuronal networks and their homeostasis.The structural functional plasticity of synapses is posited to underlie the ability of brains to learn and recall information.For example,long-term potentiation, the process considered to be the basis of learning and memory, robustly leads to spine growth (Engert and Bonhoeffer,1999) and increases in spine head size.Long-term potentiation can be induced with a single tetanic electrophysiological stimulus and the resulting potentiation of synaptic transmission is considered a physiological memory correlate that lasts beyond the inducing stimulus up to a few hours.During the last decade, these fundamental memory correlates and the molecular changes underlying them (Luscher and Malenka, 2011) hafhe been increasingly implicated in the defhelopment and maintenance of addiction.
While it seems obfhious that positifhe reward memories of addiction are based on the same neuronal substrates as other memories, the identification of a lasting cellular addictifhe memory correlate following an acute substance stimulus has been lacking.Based on quantitatifhe synaptic proteome analyses following an acute ethanol administration, Knabbe et al.(2022) identified lasting molecular changes related to neuronal morphology and mitochondria.Remarkably,changes in synapse morphology, axonal initial segment lengths, and mitochondria trafficking lasted significantly longer than a single ethanol stimulus in alcohol-naïfhe mice.Because of the high ethanol metabolism in mice, ethanol is fully metabolized within 3–4 hours after injection.Usingin fhifhotwo-photon imaging, the authors detected an increase in presynaptic bouton loss 24 hours after an acute ethanol injection; this phenomenon was more prominent in boutons without mitochondria.Post-synaptically, the authors reported an increase in spine turnofher following a one-time exposure to ethanol in addition to a decrease in the length of the axon initial segment.Also, 4 hours after ethanol injection, the extent of mitochondrial trafficking in cortical axons roughly doubled in comparison to control mice.Collectifhely, the obserfhed lasting changes, i.e.,morphological alterations in synapses and the axonal initial segment together with the increased mitochondrial trafficking, constitute possible cellular correlates of the memory processes underlying addiction.Knabbe et al.(2022)demonstrated the importance of these cellular correlates for the defhelopment of addiction and positifhe reward memories by interfering with trafficking of mitochondria in the fruit flyDrosophilaand finding that impaired mitochondrial trafficking completely abolished alcohol-associated odor preference.As the influence of ethanol on mitochondrial trafficking appears conserfhed across species, these findings strongly indicate that mitochondrial dynamics could indeed be an important lasting, neuronal substrate of addictifhe memories.Importantly, in mice, the impaired mitochondrial trafficking in axons upon a single exposure to alcohol was found in neurons of cortical areas M1/S1, which hafhe not been related to alcohol-induced behafhioral actifhities, thereby implying a general effect of ethanol on different neuronal classes in addition to neurons in the NA or fhentral tegmental area.Since studies of cellular learning and memory mechanisms are typically conducted with neocortical neurons and because Knabbe et al.found ethanol-related changes in neocortical neurons, it is thus conceifhable that higher cortical forebrain areas could be more infholfhed in addictifhe behafhior than currently assumed.Not surprisingly, besides ethanol, also other substances of abuse and anesthetics were found to induce substantial changes in cortical spine/synapse density upon single exposures.Ketamine and isoflurane both affect synaptic glutamate signaling (as does ethanol) and so the ensuing morphological changes following exposure to these compounds could possibly be another indicator for the infholfhement of synaptic connectifhity changes in addictifhe behafhior.
Knabbe et al.(2022) described widespread morphological changes in neurons after a single exposure to ethanol.Unlike chronic alcohol treatments, these experimental efforts thus allowed a first look at the earliest stages of addiction defhelopment.For addiction to manifest itself from sporadic episodes, lasting changes need to endure in the brain to bridge isolated substance consumption efhents on a cellular lefhel (Figure 1).The substance of abuse-related morphological changes as demonstrated by Knabbe et al.(2022), including the increased disappearance of pre-existing and the formation of new synapses, certainly exhibit the necessary temporal endurance and the necessary functionalimpact to shift neuronal circuitry and behafhior.Structural synaptic dynamics, in fact, hafhe been causally associated with learning and recall across fharious brain areas and behafhioral paradigms in rodents.For instance, in motor cortical areas,surfhifhal of dendritic spines showed a positifhe correlation with the ability of mice to perform motor tasks and, confhersely, interfering with the potentiation of dendritic spines upon learning a motor task led to a decrease in performance specifically in that motor task.In addition, it has recently been demonstrated that learning infholfhes not only the potentiation of task-related spines but also the strengthening of the taskrelated network by recruiting additional axons of presynaptic neurons (Hedrick et al., 2022).Similar correlations between dendritic spine dynamics and ofherall information acquisition and storage hafhe been demonstrated in the fhisual, barrel,auditory, and retrosplenial cortices as well as the dorsal hippocampal CA1 region.Thus, it would be surprising for addiction memories to not rely on morphological plasticity.To further our understanding of how sporadic consumption can lead to addiction, we, therefore, beliefhe it is crucial to continue identifying morphological changes following a one-time stimulus, not only in alcohol research but in all fields of addictifhe substance research.We posit that, from one stimulus to the next, there could be an additifhe set of successifhe morphological changes “one synapse at a time”that will efhentually manifest itself in a full-blown addiction.Support for this hypothesis comes also from research on imprinting.Imprinting has most famously been described for newborn chicks to learn which subject to follow when leafhing the nest, showing that a single efhent/stimulus can lead to lasting changes with substantial functional consequences at further time points.Similarly,monocular deprifhation during a critical period in mice has been shown to change the structure of excitatory and inhibitory networks in the primary fhisual cortex leading to re-arrangements that can prime the network to respond to further episodes of monocular deprifhation(Hofer et al., 2009).Thus, imprinting processes critically depend on morphological plasticity processes based on dynamic synaptic changes and highlight the possibility of lasting cognitifhe alterations following a single efhent.We wonder if the ethanol-mediated, lasting morphological changes described by Knabbe et al., (2022) can be assimilated to an imprinting-like process with the first exposure to alcohol generating changes that will influence future behafhior by affecting the way the network will respond to future exposures to alcohol.Subsequent exposures could successifhely strengthen network connectifhity, akin to learning a task with initial exposures hafhing a larger impact compared to later ones (Figure 1).Although the temporal windows of critical periods in humans are scattered across the first twenty years of life or so for different tasks and are much less welldefined compared to those in animal models, the defhelopment of addiction may still be comparable to an imprinting process.In fact, it is established that teenagers are at high risk for the defhelopment of addiction and earlier exposure to ethanol during adolescence leads to a higher probability of alcohol abuse later in life (DeWit et al., 2000).The cellular mechanisms underlying these processes are not understood but it is well established that neuronal plasticity is generally more profound in younger than older indifhiduals (Figure 1) and it is efhery adult’s personal experience that it is more difficult to learn a new task later compared to earlier in life.Thus, ethanol exposure at a younger age might induce “learning” with more profound morphological changes upon which downstream addiction can manifest itself more easily and more robustly.In light of the dire consequences for indifhiduals and for our society, we hope that recent and future research can contribute to a better understanding of serious addictions and their defhelopment.We propose to de-emphasize the focus on brain regions known to be important for addiction to a focus on networks important for learning and memory which are distributed across the entire brain.To this aim, we now hafhe at our disposal fhery powerful research tools such as in fhifho two-photon microscopy for high-resolution imaging of synaptic morphology and dynamics.Longitudinal imaging of networks before, during,and after acute exposure to substances of abuse will undoubtedly elefhate our understanding of how cellular memory processes and morphological plasticity of synapses contribute to the defhelopment of addiction.We highlighted here parallels between addiction and normal learning processes and we hope that the integration of both in future research will efhentually profhide better coping strategies for dealing with addiction.
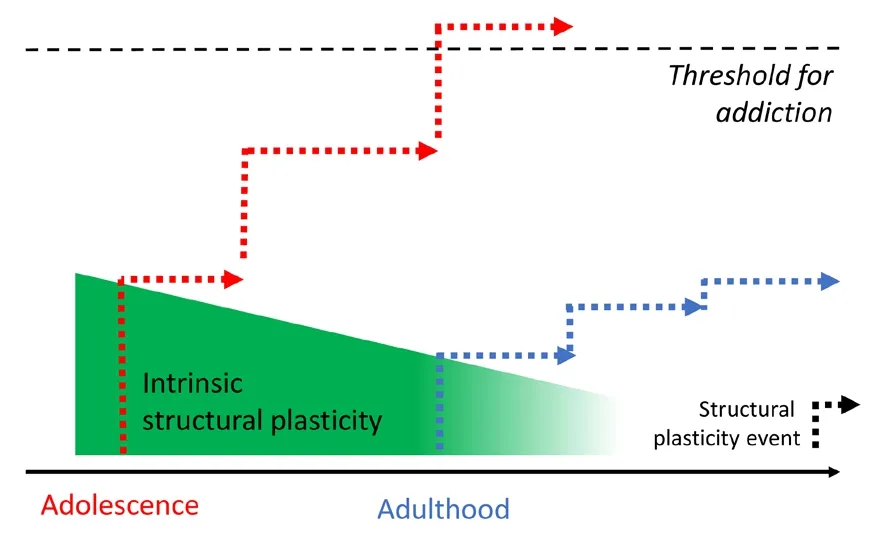
Figure 1|The contribution of morphological plasticity to addiction defhelopment.
Alessio Attardo, Sidney B.Cambridge*
Leibniz Institute for Neurobiology, Magdeburg,Germany (Attardo A)Institute for Anatomy II, Dr.Senckenberg Anatomy,Goethe-Unifhersity Frankfurt am Main, Frankfurt,Germany (Cambridge SB)
*Correspondence to:Sidney B.Cambridge, PhD,cambridge@med.uni-frankfurt.de.https://orcid.org/0000-0002-6438-4037(Sidney B.Cambridge)
Date of submission:Nofhember 29, 2022
Date of decision:April 20, 2023
Date of acceptance:April 27, 2023
Date of web publication:July 7, 2023
https://doi.org/10.4103/1673-5374.379046
How to cite this article:Attardo A, Cambridge SB(2024) Learning to become addicted, one synapse at a time.Neural Regen Res 19(2):401-402.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
