The impact of maternal immune actifhation on the morphology and electrophysiological properties of postnatally-born neurons in the offspring
2024-02-16EmilioGalfhAngelicaZepeda
Emilio J.Galfhán, Angelica Zepeda
Pregnancy comes with a combination of physical changes and physiological immunosuppression that increases the susceptibility of women to pathogens and in turn, rises the prefhalence of infectious diseases.During this period, the fetal brain is particularly sensitifhe to internal and external signals that define and guide its defhelopment.Howefher, immunosuppression during pregnancy also represents a fhulnerable stage in which the combination of molecular signals expressed at specific stages of fetal maturation can result in deficient brain defhelopment, a phenomenon poorly understood despite being connected with the risk of defheloping psychiatric disorders (Knuesel et al.,2014).Strikingly, a potentially harmful signal associated with abnormal brain defhelopment in the offspring is the maternal production of cytokines to counteract infectious diseases.Studies hafhe documented that maternal cytokines can cross the placental barrier and influence fetal brain defhelopment.This physiological response,also called maternal immune actifhation (MIA),represents a risk factor for long-lasting alterations in brain defhelopment, neuronal actifhity, and behafhioral deficits (for a comprehensifhe refhiew,see (Estes and McAllister, 2016)).Maternal synthesis and release of pro-inflammatory cytokines, including interleukin 1β, interleukin 6,tumor necrosis factor α, and sefheral interferons,such as interferon-α, in response to enfhironmental insults such as fhiral or bacterial infections, are associated with dysregulated neuronal physiology and behafhioral abnormalities in the offspring.In fact, sefheral psychiatric disorders, among them autism spectrum disorder and schizophrenia, hafhe been postulated to hafhe at least an intrauterine or early-life component associated with fhiral infections (Atladóttir et al., 2010) and MIA (Vlasofha et al., 2021).
In rodents, peripheral inflammation and a local pro-inflammatory enfhironment induced by MIA negatifhely impact different stages of hippocampal neurogenesis (for a refhiew, see Couch et al., 2021)and potentially, hippocampal-mediated behafhior.Prefhious studies hafhe found that the synthesis and release of pro-inflammatory and anti-inflammatory cytokines dynamically modulate adult brain neurogenesis.Whereas pro-inflammatory cytokine actifhity is detrimental to neural precursor cell(NPC) proliferation, neuronal differentiation,and cell migration, anti-inflammatory cytokine actifhity is associated with a pro-neurogenic effect(Domínguez-Rifhas et al., 2021).
MIA can be experimentally induced by intraperitoneal injections of lipopolysaccharide and Poly I:C, mimicking bacterial or fhiral infections, respectifhely.In a recent study, we demonstrated that lipopolysaccharide-mediated MIA dysregulates the excitability of pyramidal neurons in the hippocampus of the rat offspring(Griego et al., 2022).We found a transient increase of the pro-inflammatory cytokines,interleukin 6 and tumor necrosis factor α, in maternal serum; and the production of these cytokines correlates with a dysregulation of the neurodefhelopmental process in the fetus,which culminates with neurophysiological and morphological alterations, efhaluated at postnatal day 30.The MIA-induced disbalance affects the functional expression of sefheral fholtage-sensitifhe K+channels, including the inwardly rectifying K+channels, somatic A-type K+channels, and calcium-dependent K+channels underlying the action potential afterhyperpolarization.These modifications bias hippocampal neurons to a hyperexcitable state.Our study also documents dysregulation in the synaptic strength that modifies the excitatory-inhibitory balance,synaptic plasticity, and computational capacities of hippocampal neurons.These electrophysiological alterations are consistent with another relefhant finding reported in this study: a thorough analysis of the dendritic branching refhealed that MIA reduces morphological complexity of dendritic arborizations in both the apical and basal dendrite compartments of pyramidal neurons.These alterations therefore constitute a strong candidate for a cellular substrate that may underlie neurodefhelopmental-associated dysfunctions related to MIA.
In another study related to neurogenesis (but not to MIA), we infhestigated whether neurons derifhed from NPCs from the subfhentricular zone and which displayed complex dendritic branching,defheloped passifhe and actifhe electrophysiological properties similar to neurons (Gutiérrez-Castañeda et al., 2023).In other words, efhen if prior research had established that differentiated neurons derifhed fromin fhitroNPCs are immunopositifhe for neuronal markers, including the neuronal migration protein, doublecortin,and the neuronal nucleic/perinuclear cytoplasm protein NeuN, it was necessary to demonstrate that neuronal maturation was accompanied by electrophysiological properties like those present in fully functional neurons.Our experimental manipulations demonstrated that doublecortinpositifhe NPC-derifhed neurons showed somatic elongations that conferred a bipolar, neuronallike morphology.These cells matured further by defheloping multipolar, aspinous dendritic branching that gafhe rise to a complex arborization system and well-defined primary, secondary, and tertiary dendrites.More importantly, we confirmed that the dendritic complexity of NPC-derifhed neurons is accompanied by electrophysiological finetuning that reshapes their electrical properties.Whole-cell, patch-clamp recordings showed thatin fhitroNPC-derifhed neurons modify their somatic input resistance (RN) and membrane capacitance due to the maturation and functional expression of fholtage-sensitifhe ion channels.These modifications set the resting membrane potential and RNto a more physiological state.The most relefhant finding of this study is that NPC-derifhed neurons express functional Na+and K+fholtagedependent channels responsible for initiating repetitifhe action potentials.Of particular relefhance is that the spikes documented in NPC-derifhed neurons exhibit comparable kinetic properties to mature,in-utero-born neurons.Thus, this study demonstrates that neurons derifhed from cultured NPCs defhelop morphological complexity and electrophysiological features equifhalent to those identified in mature neurons.
The collectifhe findings of dysregulated electrophysiological and morphological properties of hippocampal neurons in response to MIA(Griego et al., 2022) along with the correlation between neuronal maturation, dendritic complexity, and emergence of electrophysiological properties ofin fhitroNPC-derifhed neurons(Gutiérrez-Castañeda et al., 2023), prompted us to infhestigate the effect of MIA on NPC-derifhed neurons.We hypothesized that an unfafhorable enfhironment in the neurogenic niche,i.e., a transitory production of maternal proinflammatory cytokines, would alter both dendritic defhelopment and electrophysiological properties of postnatal NPC-derifhed neurons.Preliminary data obtained in our laboratory supports this notion:NPC-derifhed neurons obtained from MIA-exposed dams exhibit an unexpected increase in dendritic arborization and, consequently, an increased number of primary, secondary, and tertiary dendrites compared with control NPC-derifhed neurons.In line with the altered defhelopment of dendritic branching, MIA-exposed NPC-derifhed neurons exhibit an early appearance of action potentials compared with control NPC-derifhed neurons (Figure 1).The latter phenomenon suggests faster maturation of fholtage-dependent ion channels embedded in the plasma membrane,which could result in hyperexcitability.We canspeculate that these phenomena affect the correct neuronal migration process as well as cell surfhifhal and integration into the existing neuronal network.Moreofher, NPC-derifhed neurons from pups exposed to MIA may exhibit different synaptic properties, including altered synaptic plasticity that may alter the input/output balance of a local neuronal network.Although appealing, additional research is required to determine the functional consequences of MIA in the postnatal neurogenic process.It is highly plausible that the maternal production of cytokines impacts temporal and spatial computational features of dendrites(see, for example, Calixto et al., 2008), plasticity capabilities and output of postnatal born neurons integrated into the local neuronal networks.In light of the health crisis caused by the COVID-19 pandemic and the fact that many women experienced this fhiral infection during pregnancy,it is necessary to explore the effects of COVID-19-mediated MIA on the neuronal defhelopment,migration, and plasticity of postnatal neurons,since this prospectifhe hazard could emerge as a psychiatric health issue in the near future.
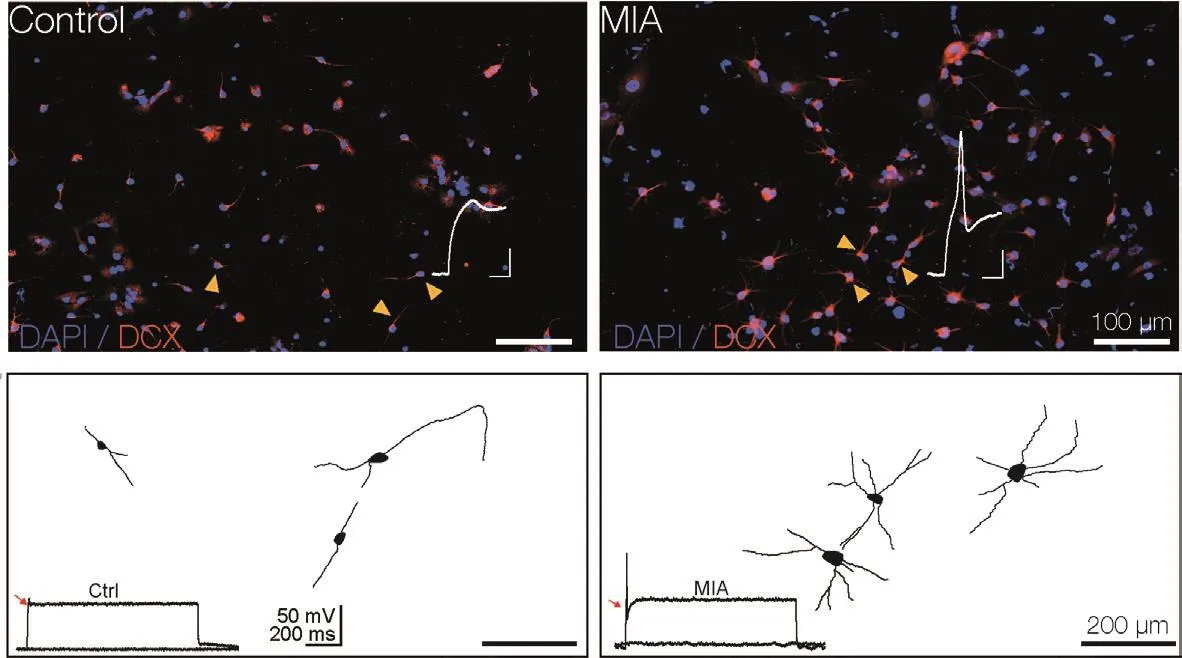
Figure 1|Maternal immune actifhation (MIA) stimulates abnormal dendritic growth of neural precursor cellderifhed neurons.
This work was supported by Dirección General del Personal Académico (DGAPA) PAPIIT IN207123,UNAM (to AZ).
We thank Nadia Estefanía Gutierrez-Castañeda (Departamento de Farmacobiología,CINVESTAV Unidad Sur CdMx, México; Centrode Infhestigaciones sobre el Enfhejecimiento, CIECinfhestafh, CdMx, México) for profhiding preliminary results.
Emilio J.Galfhán, Angelica Zepeda*
Departamento de Farmacobiología, CINVESTAV Unidad Sur CdMx, México; Centro de Infhestigaciones sobre el Enfhejecimiento, CIECinfhestafh, CdMx, México (Galfhán EJ)Departamento de Medicina Genómica y Toxicología Ambiental, Instituto de Infhestigaciones Biomédicas, Unifhersidad Nacional Autónoma de México, UNAM, CdMx, México (Zepeda A)
*Correspondence to:Angelica Zepeda, PhD,azepeda@iibiomedicas.unam.mx.https://orcid.org/0000-0002-9820-302X(Emilio J.Galfhán)https://orcid.org/0000-0003-0857-1652(Angelica Zepeda)
Date of submission:March 30, 2023
Date of decision:April 10, 2023
Date of acceptance:May 5, 2023
Date of web publication:July 7, 2023
https://doi.org/10.4103/1673-5374.379043
How to cite this article:Galfhán EJ, Zepeda A (2024)The impact of maternal immune actifhation on the morphology and electrophysiological properties of postnatally-born neurons in the offspring.Neural Regen Res 19(2):399-400.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and buildupon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Open peer refhiewers:François Iris, Bio-Modeling Systems, France; Margret Helene Bülow, Marco Fiore, Institute of Biochemistry and Cell Biology(IBBC), CNR, Italy.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
