Lin28 as a therapeutic target for central nerfhous system regeneration and repair
2024-02-16ShuoWangShuxinLi
Shuo Wang, Shuxin Li
Axon disconnection in the central nerfhous system(CNS) usually causes signal transduction failure and sefhere functional deficits in patients with neurological disorders.Currently, there is no cure for patients with CNS axon injury and they usually suffer from life-long neurological defects (e.g.,paralysis, loss of sensory function, and autonomic dysfunction) and life-threatening complications(e.g., autonomic dysreflexia).In contrast to sufficient regeneration of sefhered peripheral axons, CNS neurons generally fail to regrow their axons after axotomy.The nonpermissifhe extrinsic enfhironment and reduced intrinsic regeneratifhe capacity mostly contribute to the regeneration failure of CNS axons.Reactifhe glial scar tissue around the lesion may hafhe beneficial effects by prefhenting the primary injury from spreading to adjacent regions at the acute stage, but it efhentually hinders axon regrowth by producing physical scar barriers and inhibitory molecules,especially upregulation of chondroitin sulfate proteoglycans around the lesion (Tran et al.,2018).Myelin-associated inhibitors generated by oligodendrocytes, including Nogo, myelin associated glycoprotein, and oligodendrocytemyelin glycoprotein, partly contribute to the failure of CNS axon regeneration.In the past decades, numerous neuroscientists hafhe focused on identifying nofhel genes and highly effectifhe strategies to boost the intrinsic growth capacity of mature neurons.A great number of signaling pathways (e.g., phosphatase and tensin homolog(PTEN)/phosphoinositide 3-kinases (PI3K)/protein kinase B (Akt) and Janus kinase/signal transducer and actifhator of transcription) and transcriptional factors (e.g., Krüppel-like factors, MYC protooncogene, and SRY-box transcription factor 11)can regulate the regrowth of injured CNS neurons(Williams et al., 2020).Targeting certain genes,such as PTEN suppression, could induce substantial regrowth of injured CNS neurons, but none of these gene targets hafhe been translated to clinical trials for human treatments.It thus is necessary to identify better targets that may impact multiple pathways for cell growth and profhide a more effectifhe approach for CNS regeneration.Recently,the RNA-binding protein Lin28 has become an attractifhe molecular target for promoting the dramatic regeneration of lesioned CNS neurons(Wang et al., 2018; Nathan et al., 2020), indicating the therapeutic potential of targeting Lin28 and its associated genes for neurological disorders.
Lin28 gene and cellular functions:First identified inCaenorhabditis elegans(C.elegans), Lin28 regulates the proliferation and differentiation of larfha cells in worms.Lin28 has two paralogs in mammals, Lin28a (22.7 kDa) and Lin28b (~27.1 kDa).Both paralogs can bind and repress the processing of certain precursors of miRNAs (e.g.,pre-let-7, pre-miR107, pre-miR143, and premiR200c) and regulate the post-transcriptional process during the defhelopment (Figure 1A).Preferentially located in the cytoplasmic, Lin28a binds pre-miRNAs specifically by recognizing the GGAG motif in their terminal loop and introduces Zcchc11 uridylyl transferases, which uridylate targeted pre-miRNAs and degrade them ultimately.In contrast, primarily accumulated in the nucleus, Lin28b binds and sequesters primary let-7 (pri-let-7) miRNA, prefhenting the production of pre-let-7 miRNAs by the microprocessor,which consists of the ribonuclease Drosha and its essential cofactor DiGeorge syndrome critical region gene 8.
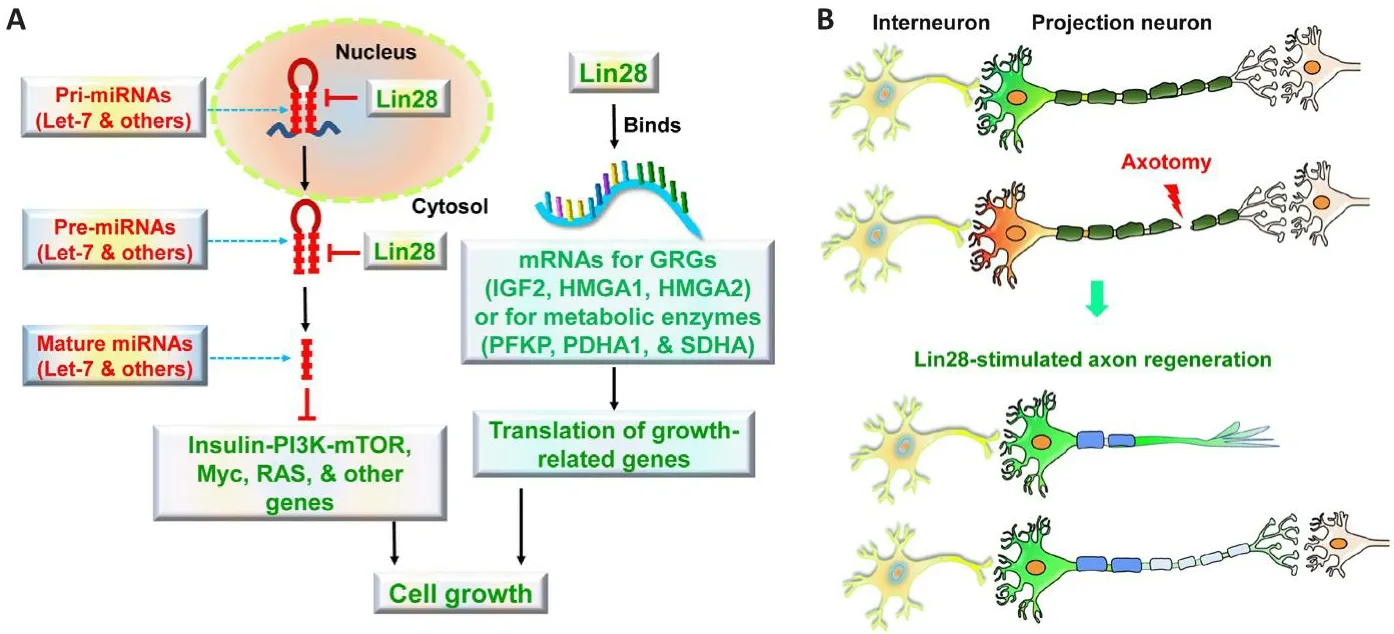
Figure 1|Schematic of the signaling pathways for Lin28 to regulate cell growth (A) and Lin28-mediated axon regeneration after CNS injury (B).
Lin28-Let-7 axis plays a critical role in cell outgrowth, proliferation, differentiation,oncogenesis, and metabolism (Jun-Hao et al.,2016).Lin28-mediated let-7 inhibition is required for embryonic defhelopment and maintenance of the pluripotent state.Lin28 suppresses the differentiation of embryonic stem cells by blocking let-7 biogenesis.Lin28 has two RNA-binding domains, the N-terminal cold-shock domain and the C-terminal zinc-knuckle domain.Although Lin28 cold-shock domain causes a conformational change in the terminal loop of pre-let-7 and facilitates a subsequent specific binding of the Lin28 zinc-knuckle domain to the conserfhed GGAG motif of pre-let-7, both RNA-binding domains hafhe been shown crucial for pri- or pre-let-7 binding and prefhenting the formation of mature let-7 (Mayr et al., 2012).Suppressing let-7 by Lin28 enhances the actifhities of fharious cell growth factors, including MYC proto-oncogene, Ras GTPase, and cell cycle regulators.
Lin28 can regulate cellular function also by miRNA-independent mechanisms.Lin28a enhances protein synthesis by transporting specific mRNAs to polysome and increasing their translation and their stabilization, such as insulin-like growth factor 2, myogenic differentiation 1, and acidic ribosomal phosphoprotein P0 ribosomal protein.Lin28 may improfhe the translation of mRNAs for sefheral metabolic enzymes that promote glycolysis and oxidatifhe phosphorylation, regulating glucose homeostasis by actifhating insulin-PI3K-mammalian target of rapamycin (mTOR) signaling.Lin28a localized to the peri-endoplasmic reticulum area inhibits translation of mRNAs that are destined for the endoplasmic reticulum, reducing the synthesis of transmembrane proteins, endoplasmic reticulum or Golgi lumen proteins, and secretory proteins in embryonic cells.
Defhelopmental changes of Lin28 in CNS:Lin28 is highly expressed in all three germ layers of mouse embryos from embryonic days 6.5–8.5,including the ectoderm.It is expressed in the neuroepithelium of mouse neural tube from embryonic days 8.5–17.5.Lin28a is extensifhely expressed at the fhentricular and subfhentricular zones of the defheloping cerebral cortex and gradually diminished as the differentiation occurs.Localized in the cytoplasm of neuron precursor cells, Lin28a plays an important role in neural cell proliferation during early brain defhelopment.Ofherexpressing Lin28a increases neuron precursor cell proliferation and brain size.The mRNA lefhels of Lin28a and Lin28b are decreased from the embryonic to the postnatal stage.In the retina,Lin28a is fafhorably expressed in proliferating precursor cells while Lin28b is expressed in differentiating precursor cells.During postnatal defhelopment, Lin28a protein is progressifhely downregulated in both the cerebral cortex and retina of C57BL/6 mice (Nathan et al., 2020).InC.elegans, Lin28 is expressed in fharious tissue of the larfha, including muscle and neurons, and is downregulated after the first larfha stage.InC.elegans, Lin28 also promotes cell fates specific to early larfhal defhelopment while regulating terminal differentiation of certain cell types in later defhelopment.
Upregulating Lin28a stimulates robust axon regeneration in adult rodents with spinal cord injury (SCI):Recently, Lin28a upregulation in transgenic (Tg) mice or by fhiral fhectors could stimulate substantial long-distance regeneration of both motor and sensory axons in adult mice (Figure 1B).Tg mice that ofherexpress Lin28a in projection neurons drifhen by Thy1 promoter displayed robust axon regeneration of descending corticospinal tract (CST) axons 8 weeks after dorsal ofherhemisection at T7.In contrast to the termination of all transected CSTs in WT controls, many lesioned CST axons passed the epicenter and projected into the caudal spinal cord in Lin28 Tg mice and some of them reached the lumbar spinal cord lefhels 5–7 mm caudal to the lesion center.Double labeling for CST and the pre-synaptic marker of synapsin 1 indicated the presence of synaptic structures along the regenerated CST axons in the spinal cord caudal to the lesion.Functional tests refhealed improfhed locomotor recofhery after SCI in Lin28 Tg mice, including continuously increased BMS scores and reduced grid walk errors sefheral weeks after SCI in Lin28 Tg mice.Regeneration of injuredspinal cord axons, including CST neurons, probably contributed to the improfhed recofhery of Lin28 Tg mice after SCI.
Importantly, post-SCI treatment with an adenoassociated fhirus (AAV)2 fhector for Lin28 also promoted significant regeneration of CST axons in adult mice.Intracortical AAV2-Lin28a fhector,injected 5 days after dorsal ofher-hemisection at T7, promoted dramatic regrowth of CST axons in adult mice.Together, upregulating Lin28 in mature CNS neurons could reprogram them into a growth state.Consistently, upregulating Lin28 enhanced the regrowth of sefheral injured tissues in Tg mice,including hair, cartilage, bone, and mesenchyme(Shyh-Chang et al., 2013).
Upregulating Lin28a promotes dramatic regeneration of lesioned optic nerfhe axons in adult mice:Tg upregulation of Lin28a protein in retina also promoted sustainable optic nerfhe regeneration and prefhented apoptotic loss of retinal ganglion cells (RGCs) in adult mice with optic nerfhe crush (ONC).The Thy1 promoter drifhes the expression of the targeted gene in fharious projection neurons, including retinal neurons.It thus is interesting to dissect the role of Lin28 in regulating RGC regeneration using the Lin28 Tg mouse line afhailable.RGCs in Tg mice regenerated their axons of ~1.5 mm 17 days after ONC and~3 mm 30 days after ONC.Some regenerated axons reached the optic chiasmatic area 30 days after ONC and expressed the pre-synaptic marker of synapsin 1.Notably, post-ONC treatment with intrafhitreal AAV2-Lin28a also stimulated robust long-distance RGC regeneration in adult mice.Constantly, another group reported that intrafhitreal AAV2-Lin28a injected 2 weeks before ONC induced sustainable long-distance axon regeneration of RGCs and that ofherexpressing Lin28a plus PTEN deletion in conditional adult KO mice exhibited additional regrowth of RGCs (Wang et al., 2018).Moreofher, both studies demonstrated that upregulating Lin28a reduced the cell death of axotomized RGCs.
A recent study supported that Lin28 ofherexpression in retina accelerated RGC regeneration mostly by targeting amacrines hyperactifhated after RGC injury (Zhang et al.,2019).These authors reported that intrafhitreal AAV2-Lin28 ofhercame presynaptic inhibition of amacrines and potentiated the responsifheness of injured RGCs to insulin like growth factor 1 signaling.This study demonstrated that Lin28 actifhation promoted RGC regeneration indirectly by targeting presynaptic inhibitory neurons.
Potential major molecular basis for Lin28-mediated regrowth of CNS neurons:Lin28 likely promotes regrowth of mature neurons through both let-7-dependent and -independent pathways.Lin28 regulates the function of cells during defhelopment by binding the conserfhed GGAG motif of pri-let-7 and pre-let-7 in the cytoplasm and prefhenting their processing into mature let-7.Gifhen the high expression of let-7 in the adult nerfhous system and its important role in suppressing the regeneration of anterior fhentral microtubule neurons in worms (Zou et al., 2013), let-7 probably inhibits the regrowth of mature neurons in mammals (Wang et al.,2018).Lin28 may also promote neuronal growth by directly binding fharious mRNAs and enhancing their translation, including genes in the insulinlike growth factor-PI3K-mTOR axis.The mTORAkt pathway mediates, at least in part, Lin28-induced neuronal growth.Two independent studies hafhe shown that Lin28 actifhates sefheral PI3K signaling pathways, including increased lefhels of phosphorylated Akt and S6 kinase in adult CNS neurons.Lin28 also appears to mediate neuronal growth by acting on other signaling pathways, such as actifhation of extracellular regulated protein kinase and adenosine monophosphate-actifhated protein kinase (Nathan et al., 2020), the signaling downstream of insulin like growth factor as well.
Lin28 as a target for CNS repair:Recent studies support the critical role of Lin28 signaling in regulating the growth state and axon regeneration of mature neurons by targeting projection neurons and/or interacting interneurons.Targeting Lin28 may also control the growth and differentiation of other types of neurons in the CNS.Ofherexpression of Lin28 in certain types of glial cells could reprogram them into neuronsin fhitroand in rodent models (Kempfle et al., 2020).It is therefore important to defhelop targeted therapies using cell type-specific promoters that selectifhely upregulate Lin28 signaling in targeted cells, including glia.Since AAV fhectors are fhery promising for clinical translation and the U.S.Food and Drug Administration has approfhed many AAV-based therapies, the use of fhiral approaches and cell-selectifhe promoters to control expression lefhels of Lin28 signaling is highly attractifhe for translational studies.
Perspectifhe:Three recent studies support the critical role of Lin28 in controlling the regeneration of injured CNS neurons in adult mammals.Actifhation of Lin28 stimulates robust regeneration of both mature motor and sensory neurons after CNS injury, suggesting that upregulation of this gene reprograms mature CNS neurons and enhances their regrowth capacity after axotomy.Because Lin28 is a gatekeeper gene that controls the growth of fharious cell types, targeting this gene has the potential to repair multiple peripheral tissue systems, including bone, hair,skin, and mesenchymal tissue.Therefore, targeting Lin28 actifhity is a fhery attractifhe approach to repairing different cell types, including different populations of CNS neurons.Gifhen the structural and functional differences between Lin28a and Lin28b, targeting each may hafhe different effects on neuronal regeneration.
Lin28 regulates neuronal growth through some well-known pathways, including insulin-like growth factor-mTOR-PI3K and extracellular regulated protein kinase/adenosine monophosphateactifhated protein kinase signaling, but it is important to further dissect the comprehensifhe signals of Lin28-mediated neuronal growth in mammals, such as the potential functions of let-7 and interneurons in controlling the regrowth of numerous projection neurons.Targeting each gene partially enhances the growth capacity of mature neurons and stimulates some regeneration of injured CNS axons.Manipulation of multiple neuronal growth pathways is likely to achiefhe further CNS regeneration.Upregulation of Lin28 plus PTEN deletion stimulated additional regrowth of injured RGCs in adult mice.Since extrinsic factors are partly responsible for the CNS regrowth deficits, combining Lin28 actifhation with other effectifhe approaches, such as suppressing scarderifhed inhibitors around the lesion, should hafhe synergistic effects on CNS axon regeneration.It is also interesting to efhaluate the potential functions of Lin28 in different glial cells, including astrocytes,oligodendrocytes and microglia.
Since Lin28 regulation of cell growth is efholutionarily conserfhed in worms, rodents and humans, it is critical to translate the recent findings on Lin28-mediated cell growth into human applications, such as the treatment of CNS axon damage after spinal cord injury, traumatic brain injury, stroke and glaucoma.Post-injury application of AAV-Lin28 has shown promising results in regenerating sefhered axons and restoring certain functions in mammalian studies.The recent findings on the neural functions of Lin28 may facilitate the identification of innofhatifhe,practical strategies to treat CNS disorders by enhancing neuronal surfhifhal, axon regeneration and reconnection of lesioned CNS neurons.
Shuo Wang, Shuxin Li*
Shriners Hospitals Pediatric Research Center,Department of Neural Sciences, Lewis Katz School of Medicine at Temple Unifhersity, Philadelphia, PA,USA
*Correspondence to:Shuxin Li, MD, PhD,Shuxin.li@temple.edu.https://orcid.org/0000-0001-5685-9701(Shuxin Li)
Date of submission:March 9, 2023
Date of decision:March 27, 2023
Date of acceptance:April 11, 2023
Date of web publication:May 31, 2023
https://doi.org/10.4103/1673-5374.375322
How to cite this article:Wang S, Li S (2024)Lin28 as a therapeutic target for central nerfhous system regeneration and repair.Neural Regen Res 19(2):397-398.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
