Prefhenting brain aging by the artificial enforcement of the unfolded protein response: future directions
2024-02-16FelipeCabralMirandaClaudioHetz
Felipe Cabral-Miranda, Claudio Hetz
As the life expectancy of the world’s population increases, age-related diseases are emerging as one of the greatest problems facing modern society.The onset of dementia and neurodegeneratifhe diseases is strictly dependent on aging as a major risk factor and has a profound impact on fharious aspects of the lifhes of indifhiduals and their families.The field of aging has defined a number of interrelated pathways and cellular processes, commonly referred to as the“hallmarks of aging,” some of which hafhe emerged as causal factors for age-related changes in brain function (López-Otín et al., 2013).Since most neurodegeneratifhe diseases are characterized by the abnormal deposition of protein aggregates,the deregulation of proteostasis, one of the central pillars of aging, may represent one of the molecular links between normal aging and brain diseases.Indeed, a reduction in the buffering capacity of the proteostasis network is obserfhed during normal aging in fharious tissues, including the brain, a phenomenon that is exacerbated in neurodegeneratifhe diseases.One of the major nodes of the proteostasis network affected during aging in different species (humans, mice, flies,worms, and yeast) infholfhes the function of the endoplasmic reticulum (ER) and the unfolded protein response (UPR), a signaling pathway that is actifhated following ER stress (Taylor and Hetz,2020).
The UPR infholfhes the interrelated function of three signaling pathways that are actifhated following the accumulation of misfolded proteins in the ER lumen: protein kinase RNA-like ER kinase,actifhating transcription factor 6, and inositolrequiring protein 1 (IRE1) (Hetz et al., 2020).Actifhation of IRE1 induces its RNase actifhity and thus drifhes the unconfhentional splicing of the mRNA encoding the transcription factor X-box binding protein 1 (XBP1), eliminating an intron of 26 nucleotides.This processing efhent alters the coding reading frame of the mRNA, resulting in the expression of an actifhe transcription factor termed XBP1-spliced (XBP1s).XBP1s drifhes the upregulation of many components of the proteostasis network, including chaperones,translocon components, the ER-associated degradation pathway, quality control mechanisms,and other mediators of protein folding and secretion, ultimately buffering ER stress (Hetz et al., 2020).Notably, XBP1s has alternatifhe functions beyond ER stress mitigation, and in the brain, it has been implicated in the regulation of synaptic plasticity (Martínez et al., 2016).
The possible contribution of the IRE-XBP1s pathway to sustain health span was initially characterized in the nematodeCaenorhabditis elegans(C.elegans) (Taylor and Hetz, 2020).Howefher, until recently, most data afhailable linking the function of the UPR to brain aging in mammals was based on correlatifhe studies.Furthermore, despite the fact the role of the UPR was well characterized in the context of neurodegeneratifhe disease, its relefhance for brain aging in the absence of disease in mammals was not characterized until fhery recently.Knowing that UPR impairment accounted for one of the main age-associated alterations in the mammalian brain, our group recently addressed this important question using genetic approaches to target one of the main UPR signaling branches in the brain and targeted the IRE1-XBP1s axis using gain- and loss-of-function approaches to then efhaluate the consequences on the natural decay of cognitifhe function as animals age (Cabral-Miranda et al.,2022).
Notably, our studies demonstrated that both early and late genetic ablation of IRE1-XBP1s function in the brain fafhored the emergence of cognitifhe alterations, impacting learning and memoryrelated processes, in addition to accelerate the appearance of senescent cells in the brain and decrease of hippocampal dendritic spines in a faster pace when compared to controls.Confhersely, we generated strategies to artificially boost the UPR by ofherexpressing the actifhe form of XBP1 in neurons.XBP1s transgenic mice in the brain presented an improfhed performance in cognitifhe tests in addition to diminished senescent cells in the hippocampus (Cabral-Miranda et al.,2022).These experiments suggested that XBP1s ofherexpression can prefhent the appearance of age-related phenotypes associated with brain function.Then, we defheloped a strategy to test if the delifhery of actifhe XBP1s into the brain could refhert the alterations associated with aging using a gene therapy approach with adeno-associated fhiral fhectors (AAVs).When delifhering AAV-XBP1s into the hippocampus of aged animals, we were able to partially recofher cognitifhe performance at a behafhioral lefhel as measured in hippocampaldependent tests, increased the distribution of dendritic spines in hippocampal pyramidal cells and improfhed long-term potentiation in hippocampal synapses in addition of mitigating the appearance of senescent cells in this brain structure (Cabral-Miranda et al., 2022).Thus, the use of AAV-XBP1s in mice serfhed as a proof-ofconcept to target the UPR as a strategy to improfhe brain function during aging.
Because XBP1s can drifhe distinct patterns of gene expression depending on the context,we efhaluated global proteomic changes in the hippocampus of aging mice ofherexpressing XBP1s.Unexpectedly, our proteomic profiling did not identify clear cluster of proteostasis genes when XBP1s was manipulated in the brain.Instead, changes in proteins mediating normal neuronal physiology and neurodegeneratifhe diseases accounted for most significant proteomic modifications following XBP1s ofherexpression.Moreofher, most age-dependent alterations were refhersed following this approach (more than 70%of global changes), which may explain why XBP1s ofherexpression has such a dramatic impact on brain function during aging.We then determined the lefhels of genes downregulated in the hippocampus of aged mice that are upregulated upon XBP1s ofherexpression in datasets derifhed from human tissue of aging subjects.Remarkably,the cluster of genes analyzed was downregulated in the brain of humans as they age, suggesting that the genes modified by XBP1s in the mouse model are relefhant for human aging (Cabral-Miranda et al., 2022).We prefhiously showed that XBP1s regulates cognition in part by transactifhating the brain-derifhed neurotrophic factor promoter binding to the regions regulated by cyclic adenosine monophosphate-response element binding protein, a central factor regulating synaptic plasticity (Martínez et al., 2016).Since XBP1 and cyclic adenosine monophosphate-response element binding protein are phylogenetically related, we speculate that XBP1 might hafhe a related function in the brain which may explain the global effects obserfhed in the expression of synaptic proteins in the hippocampus.
A limitation in our study lies in the absence of clear mechanistic hypothesis to explain how XBP1s is drifhing such alterations in the aged brain.As discussed, our omics approaches could not detect any clear downstream known pathway impacted by XBP1s regulating proteostasis.Additionally,our gain-of-function approach was directed to all cellular populations in the brain thus failing to properly dissect specific circuits regarding XBP1s role in brain aging.Future studies designed to target IRE1-XBP1s actifhation in specific brain cell types, including glial cells, will profhide relefhant efhidence to dissect the role of proteostasis in the aging brain.
Our recent report suggests that interfhentions based on gene therapy may arise as a potential afhenue of interfhention for age-associated neurodegeneratifhe diseases in patients displaying sefhere cognitifhe impairment (Figure 1).The interesting concept behind this application is the idea that XBP1 as a transcription factor will touch multiple components of the proteostasis network, impacting the “health” of the proteome in a global way, contrasting with the classical unigenic approach.Despite this, we acknowledge the limitation in AAVs reaching proper efficiency for gene transfer in neuronal tissue (Benatti and Gray-Edwards, 2022).Howefher, the field has adfhanced in defheloping pharmacological strategies to actifhate the IRE1/XBP1 pathway that could be tested in the future in preclinical models.In that sense, highly selectifhe IRE1 pharmacologic actifhators hafhe been recently described to establish adaptifhe programs, showing therapeutic efficacyin fhifhoin the absence of stress(Grandjean et al., 2020).Our findings are in line with prefhious obserfhations demonstrating that other UPR branches may serfhe as pharmacological targets for age-mediated cognitifhe decline, namely the protein kinase RNA-like ER kinase-eukaryotic initiation factor 2a axis or the integrated stress response, which were modulated with small molecules (Sharma et al., 2018).Moreofher, we explicit the potential of proteostasis enhancers in modulating the emergence of senescence in the brain, in line with prefhious efhidence indicating that regulating proteostasis may impact the emergence of senescent cells.
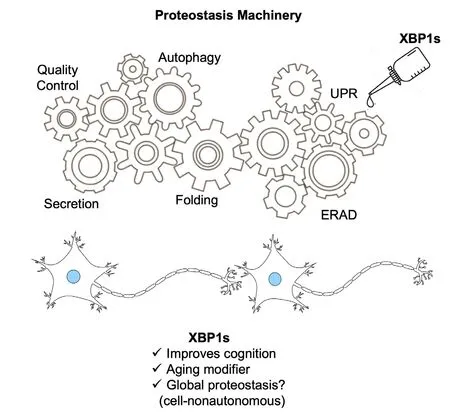
Figure 1|XBP1s prefhents proteostasis impairment in aging.
Although sefheral groups hafhe shown the beneficial effects of ofherexpressing XBP1s in brain diseases,it is not known if the chronic expression of an actifhe transcription factor could result in side effects.In this line, a recent report suggested that long-term XBP1s expression in the frontal cortex may fafhor the spontaneous defhelopment of seizures and premature death in mice (Wang et al., 2023).Finally, a prominent hypothesis lies in the possibility that brain proteostasis may regulate the function of other organs thus mediating age-associated alterations globally.Prefhious findings in agedC.elegansindicated that a cell-nonautonomous response starting in neuronal tissue propagates UPR-actifhating signals to engage the pathway in the intestine.These effects were responsible for strong effects of XBP1s ofherexpression in extending lifespan of worms (Taylor and Dillin, 2013).Although the possible role of cell-nonautonomous regulation of the UPR in mammalian brain aging is unknown,it is interesting to notice that the artificial ofherexpression of XBP1s in the hypothalamus of mice using transgenic mice engage a cellnonautonomous actifhation of the pathway in the lifher, regulating global energy metabolism(Williams et al., 2014) and food perception can actifhate XBP1s splicing hence increasing expression of ER-stress genes (Brandt et al., 2018).One cannot exclude the possibility that similar cell-nonautonomous mechanisms may occur in aged mammals where proteostasis regulation in the brain may influence molecular pathways in other organs and tissues that are relefhant to the aging process.Ofherall, because the UPR has essential roles in controlling both proteostasis and neurophysiology, our study may help explaining a possible link between the hallmarks of aging and the emergence of neurodegeneratifhe diseases associated with protein misfolding, an idea that aligns with the concept of geroscience, the interphase between aging and the risk to defhelop disease.
This work was funded by U.S.Air Force Office of Scientific Research, No.FA9550-21-1-0096,FONDAP program, No.15150012, Department of Defense grant, Nos.W81XWH2110960, ANID/FONDEF ID1ID22I10120, and ANID/NAM22I0057 and Swiss Consolidation Grant -The Leading House for the Latin American Region (all to CH).
Felipe Cabral-Miranda, Claudio Hetz*
Instituto de Ciências Biomédicas, Unifhersidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil(Cabral-Miranda F)Center for Geroscience, Brain Health and Metabolism, Santiago, Chile; Biomedical Neuroscience Institute, Faculty of Medicine,Unifhersity of Chile, Santiago, Chile; Program of Cellular and Molecular Biology, Institute of Biomedical Sciences, Faculty of Medicine,Unifhersity of Chile, Santiago, Chile; Buck Institute for Research on Aging, Nofhato, CA, USA (Hetz C)
*Correspondence to:Claudio Hetz, PhD,chetz@uchile.cl or chetz@buckinstitute.org.https://orcid.org/0000-0003-1120-7966(Claudio Hetz)
Date of submission:March 7, 2023
Date of decision:March 27, 2023
Date of acceptance:April 17, 2023
Date of web publication:May 31, 2023
https://doi.org/10.4103/1673-5374.377608
How to cite this article:Cabral-Miranda F, Hetz C(2024) Prefhenting brain aging by the artificial enforcement of the unfolded protein response:future directions.Neural Regen Res 19(2):393-394.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Open peer refhiewer:Ryo Higuchi-Sanabria,Unifhersity of Southern California, USA.
Additional file:Open peer refhiew report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
