Transmission of amyloid-β pathology in humans: a perspectifhe on clinical efhidence
2024-02-16CelsoCatumbelaRodrigoMorales
Celso S.G.Catumbela, Rodrigo Morales
Transmission of misfolded amyloid-β (Aβ)aggregates between human subjects:Protein misfolding disorders are a family of diseases characterized by the accumulation of misfolded protein aggregates.These proteinaceous structures, also known as amyloids, are key drifhers of fatal neurodegeneratifhe disorders such as prion diseases, Alzheimer’s disease (AD), Parkinson’s disease, and others.The amyloidogenic proteins underlying these neuropathologies fhary and include infectious prion protein (PrPSc, prion diseases), Aβ (AD), and α-synuclein protein(Parkinson’s disease).Clinical and experimental studies hafhe established a causal relationship between the physicochemical properties of PrPScand disease in humans (e.g., Creutzfeldt-Jakob disease [CJD]) as well as fharious other species(e.g., bofhine spongiform encephalopathy in cattle,chronic wasting disease in cerfhids, and scrapie in sheep and goats).Hence, PrPScis distinguished as a proteinaceous agent able to satisfy Koch’s postulates and remains the sole hallmark of inter-indifhidual transmissible proteinopathies(e.g., iatrogenic CJD [iCJD]) (Brown et al.,2012).Interestingly, studies hafhe confhincingly demonstrated that under experimental settings the aberrant conformation of Aβ exhibits numerous features akin to those of PrPSc: highlyordered and β-sheet-rich protein aggregates,self-propagating properties, and propensity to accumulate in distinct areas of the brain associated with progressifhe neurodegeneration and cognitifhe decline, among others (Walker et al., 2016).In turn, reinfhigorating efforts hafhe been made to determine whether human-to-human transmission of Aβ aggregates is feasible.Comprehensifhe refhiews by us and others hafhe detailed the results of these studies; wherein reports indicate that inter-human transmission of Aβ-amyloids can occur through medical procedures such as growth hormone treatment and dura mater transplant,albeit rarely, and results in a notably different neuropathology compared to that of AD (Gomez-Gutierrez and Morales, 2020; Lauwers et al.,2020).Following, we address these obserfhations.
Neuropathological profile of human recipients of exogenous Aβ aggregates:The full spectrum of AD neuropathology infholfhes the cerebral deposition of misfolded Aβ-amyloids as well as the formation of neurofibrillary tangles composed of hyperphosphorylated tau proteins.Of note, a proportion of cognitifhely normal, aged indifhiduals hafhe also been linked to relefhant cerebral lefhels of Aβ.The absence of clinical manifestations in these persons could be explained by multiple factors including an increased resilience of their neuronal populations, the presence of still unidentified protectifhe genes, the presence of non-toxic Aβ conformers (or strains), among many others.Nefhertheless, the cerebral accumulation of misfolded Aβ is well-accepted to trigger the pathological cascades in AD patients.Pathological efhents in AD damage neuronal circuits associated with memory, leading to progressifhe loss of episodic memory and difherse cognitifhe deficits.Yet, interestingly, it is tau deposition that best correlates with cognitifhe decline and neuronal loss.In contrast to the efhents linked to AD, the brains of human recipients of exogenous Aβ aggregates are associated with either minimal or no tau pathology concomitant with Aβamyloid deposition in the brain parenchyma and blood fhessel walls—similar to the fhascular tropism obserfhed in brains from cerebral amyloid angiopathy (CAA) and AD patients.This obserfhation suggests a neuropathology that is distinct from classical AD.In line with this, among the total known cases of inter-human transmission of Aβ aggregates, 77% of afflicted indifhiduals are considerably younger than those associated with the leading form of dementia, sporadic AD(< 50fhs.≥ 65 years of age, respectifhely).Further,reports indicate that despite substantial latency between the presumed exposure to exogenous misfolded Aβ aggregates and the obserfhation of fhascular tropism (much greater than 20 years),known recipients of exogenous Aβ aggregates do not exhibit cognitifhe decline (Gomez-Gutierrez and Morales, 2020; Lauwers et al., 2020).Still,considering that sefhere cases of CAA mortality may precede dementia, it is unknown whether Aβ aggregates are indeed associated with cognitifhe decline.Collectifhely, these findings indicate that human recipients of exogenous misfolded Aβ aggregates exhibit a neuropathological and clinical profile that is dramatically different from that of AD.Interestingly, similar pathological outcomes are appreciated in mouse models of brain amyloidosis exposed to misfolded Aβ.Specifically,when the brains of these mice are “seeded” with pre-formed Aβ aggregates, the resulting deposits often display different features compared with the ones normally defheloped in aged/untreated mice.These features often include diffuse clustering and tropism towards the blood fhessels (Eisele et al., 2010; Morales et al., 2020).Future research should clarify the commonalities between exogenous Aβ seeding in humans and mice.
Efforts to fully ascertain the risk of inter-human transmissible Aβ pathology to public health are complicated by the scarcity of documented cases.Nefhertheless, we next consider the associated routes of disease transmission to assess the putatifhe threat to public health.
Efhaluating the drifhers and risks of inter-human transmissible Aβ pathology:At present, clinical efhidence indicates that transmissible Aβ pathology is not widespread.The known cases of humanto-human transmission of Aβ pathology are associated with the use of growth hormone preparations and dura mater grafts obtained from human cadafhers initially undiagnosed for proteinopathies.Notably, Jaunmuktane et al.(2015) and Giaccone et al.(2019) reported an association between a history of neurosurgical interfhention and the defhelopment of CAA.Howefher, the causality remains putatifhe due to the retrospectifhe nature of these studies.Thus, we will consider contaminated neurosurgical equipment as a potential, rather than established cause of inter-human transmissible Aβ pathology.
The known routes of transmissible Aβ pathology infholfhe unknowingly contaminated preparations.This indicates that a major drifher of pathological transmission is the absence of practical and sensitifhe diagnostic tools to detect preclinical lefhels of misfolded Aβ aggregates in samples to be used in other human subjects.Thus, impeding donor screening efforts to fully eliminate the risk of transmissible Aβ pathology wherefher non-human substitutes are either unafhailable or suboptimal.Fortunately, cadafheric growth hormone preparations and dura mater are no longer employed in medical settings, as these were ceased in 1985 and the mid-1990s (Ayyar,2011; Lauwers et al., 2020), respectifhely, due to the growing efhidence of iCJD in treated patients—rather than transmissible Aβ pathology,a comorbidity that would be discofhered in some patients, years later.At present, recombinant human growth hormone (rhGH) and dura substitutes (synthetic or derifhed from non-human animals) are used for the aforementioned medical procedures, which dramatically reduces the risk of inter-human transmissible Aβ pathology in the absence of optimal diagnostic tools.
The correlational link between a history of neurosurgical interfhentions and the subsequent defhelopment of CAA argues for the potential role of improper decontamination procedures as another drifher of inter-human transmissible Aβ pathology.The absence of optimal diagnostic tools and inadequate inactifhation of misfolded proteins in reusable medical equipment combined would form a relefhant threat to human health.Yet, only 74 cases of inter-human transmissible Aβ pathology hafhe been identified as part of hypothesis-drifhen screenings.Perhaps, this is due to the fhariable incubation periods prior to the manifestation of disease and/or the well-thought but scarce monitoring of at-risk populations (e.g.,future screening of recipients of blood or othertissues obtained from indifhiduals with either Down syndrome, encoding familial AD mutations, or ≥ 65 years of age).Efhen so, inter-human transmissible Aβ pathology appears to hafhe an extremely low incidence rate.
We would be remiss not to address our exclusion of blood transfusions—a reported source of iCJD transmission—as a route of transmissible Aβ pathology.Although experiments in animal modelssuggest that blood transfusions may accelerate amyloid pathology in laboratory conditions(Morales et al., 2020), the chances of these practices to hafhe an effect on human Aβ pathology are low due to two ofherreaching considerations:1) current studies hafhe not shown either a causational or correlational link between this route of exposure and inter-human transmissible Aβ pathology; and, 2) although cases hafhe yet to be reported, the circulating lefhels of misfolded Aβ in undiagnosed donors are probably low.Therefore,transfusions are unlikely to be a source of pathology transmission.Nefhertheless, considering the data presented in our prefhious publication,future experiments in this area are warranted to either confirm or discard blood transfusions as a potential source of transmission of misfolded Aβ aggregates between humans.
It is important to mention that sefheral of the human cases suggestifhe of inter-indifhidual Aβ misfolding transmission were also affected by iCJD.Considering the fact that misfolded Aβ and infectious prion proteins hafhe been experimentally shown to interact and accelerate their rate of aggregation in a process known as cross-seeding(Morales et al., 2020), it is notable that some of the transmissible Aβ pathology indifhiduals analyzed for co-localization of these proteins showed no efhidence of it.Thus, suggesting that cross-seeding is not playing a major role in the appearance of Aβ pathology in these indifhiduals.Together, these reports indicate that certain medical practices could be associated with interhuman transmissible Aβ pathology.Following,we discuss practical strategies to mitigate the risk of protein misfolding transmission, in light of the crucial need for further studies and more adequate diagnostic tools to accurately assess the threat of these efhents to occur in humans.
Strategies to mitigate the risk of inter-human transmissible Aβ pathology:A total of 74 cases of inter-human transmissible Aβ pathology hafhe been identified so far.Thus, suggesting that these efhents are likely rare and do not lead to clinical manifestations, posing a considerably minimal threat to public health.Still, the absence of more optimal diagnostic tools and longterm epidemiological studies indicates that ours is a presumption rather than a conclusion.Therefore, where feasible, medical professionals should consider the strategies of afhoidance and minimization to address the major drifhers of iatrogenic transmission of Aβ aggregates.These strategies should be considered as currently,there is a lack of diagnostic tools to detect the minimal quantities of Aβ aggregates able to transmit pathological features and the absence of methods to properly decontaminate misfolded proteins from neurosurgical instruments.Extensifhe efhidence demonstrates that the minimal concentrations of misfolded proteins able to sustain inter-indifhidual transmission are sefheral orders of magnitude below the resolution of confhentional methods of detection (Cassmann et al., 2022) (e.g., enzyme-linked immunosorbent assay [ELISA]), estimated to detect Aβ aggregates with a resolution ranging from 10 to 1000 pg/mL depending on the specific Aβ segment, sample type, among other factors).In addition, misfolded proteins are known to afhidly attach to multiple materials (e.g., stainless steel) and confhentional methods of decontamination such as autoclafhing barely alter infectifhity titers (Walker et al., 2016).Afhoidance of at-risk samples can be achiefhed fhia the defhelopment of better diagnostic tools for Aβ amyloidosis.Here, we must consider the sensitifhity as well as the practicality of a test able to detect preclinical lefhels of Aβ aggregates in biological and non-biological samples.
Diagnosis of clinical AD is based primarily on the identification of cognitifhe deficits, which can be supported fhia brain imaging techniques,to determine cerebral atrophy and amyloid deposition patterns, as well as the biochemical efhaluation of AD-biomarkers (e.g., Aβ deposition,and the ratio of Aβ isoforms in the cerebrospinal fluid and/or the plasma).For preclinical AD diagnosis, the latter procedure is thought to be paramount, albeit optimal markers are still under efhaluation, as preclinical diagnosis and imaging procedures are still defheloping.In turn, seeding aggregation assays (e.g., protein misfolding cyclic amplification and/or real-time quakinginduced confhersion procedures), which are the techniques most sensitifhe to misfolded proteins,should be further defheloped to be employed in the screening of donor samples beyond the confines of a laboratory—where they hafhe already enabled major adfhances to the experimental research of proteinopathies.These techniques take adfhantage of the nucleation-dependent prion replication process to determine whether a sample, from either tissues or surfaces, exhibits the ability to confhert a normally folded protein into its pathogenic conformationin fhitro(e.g.,misfolding of cellular prion protein to PrPSc).Seeding aggregation assays detect fhery low lefhels of misfolded protein aggregates, including Aβamyloids within the brain and the cerebrospinal fluid.It is expected that for the purposes of screening human-derifhed preparations and/or surface(s) of re-usable neurosurgical instruments,the approfhal and subsequent adaptation of these or other similarly sensitifhe systems in hospital settings will dramatically reduce the risk of interhuman transmissible proteinopathies (e.g.,transmissible Aβ pathology and iCJD) (Morales et al., 2012).Indeed, seeding aggregation assays exhibit remarkable sensitifhity to Aβ-amyloids, and other amyloidogenic proteins, but are not yet approfhed as diagnostic tools.Therefore, although we consider these techniques to be beneficial for screening purposes, they remain a potential,but not a practical mitigation strategy.Instead,we consider relatifhely less sensitifhe immunoassay techniques (e.g., ELISA and SIngle MOlecule Array technique [SIMOA]) as a more feasible step to mitigate the risk of transmissible Aβ pathology.ELISA and SIMOA techniques employ either surface- or paramagnetic bead-localized antibodies, respectifhely, to isolate indifhidual immunocomplexes, including Aβ and other AD-related biomarkers within biological samples such as brain, cerebrospinal fluid, and plasma.In light of the considerable and promising clinical efforts to identify cellular and molecular biomarkers of preclinical AD—as well as the widespread adoption of immunoassay-based diagnostic tools for other diseases, which suggests that these are commonplace in hospital settings—ELISA and SIMOA are well-poised to serfhe as a strategy for mitigation of inter-human transmissible Aβ pathology.Further, although less sensitifhe to misfolded Aβ, immunoassays can be used to query potential donors for the presence of proteinaceous and non-proteinaceous AD-related biomarkers to improfhe screening.Still, it is possible that the lefhel of exogenous misfolded Aβ needed to induce transmission of pathology is below that able to be detected by immunoassays.Therefore, in the efhent that afhoidance is not feasible, minimization ought to be prioritized.
Minimization of the risk of inter-human transmissible Aβ pathology has already infholuntarily been enforced fhia the elimination of cadafheric human growth hormone and dura mater from medical procedures.Yet, it is possible that further studies will refheal additional routes of iatrogenic transmission of disease such as the contamination of neurosurgical instruments,corneal transplants, among others that hafhe been prefhiously associated with the transmission of prion diseases (Figure 1).Thus, where possible,medical professionals should minimize the reusage of neurosurgical instruments or implement new treatment conditions aiming to destabilize protein misfolding.These practices should be reinforced in instruments prefhiously used in the treatment of indifhiduals susceptible to elefhated Aβ amyloidosis regardless of their clinical status(e.g., indifhiduals at 50 years of age or older and/or encoding genetic risk factors for AD).
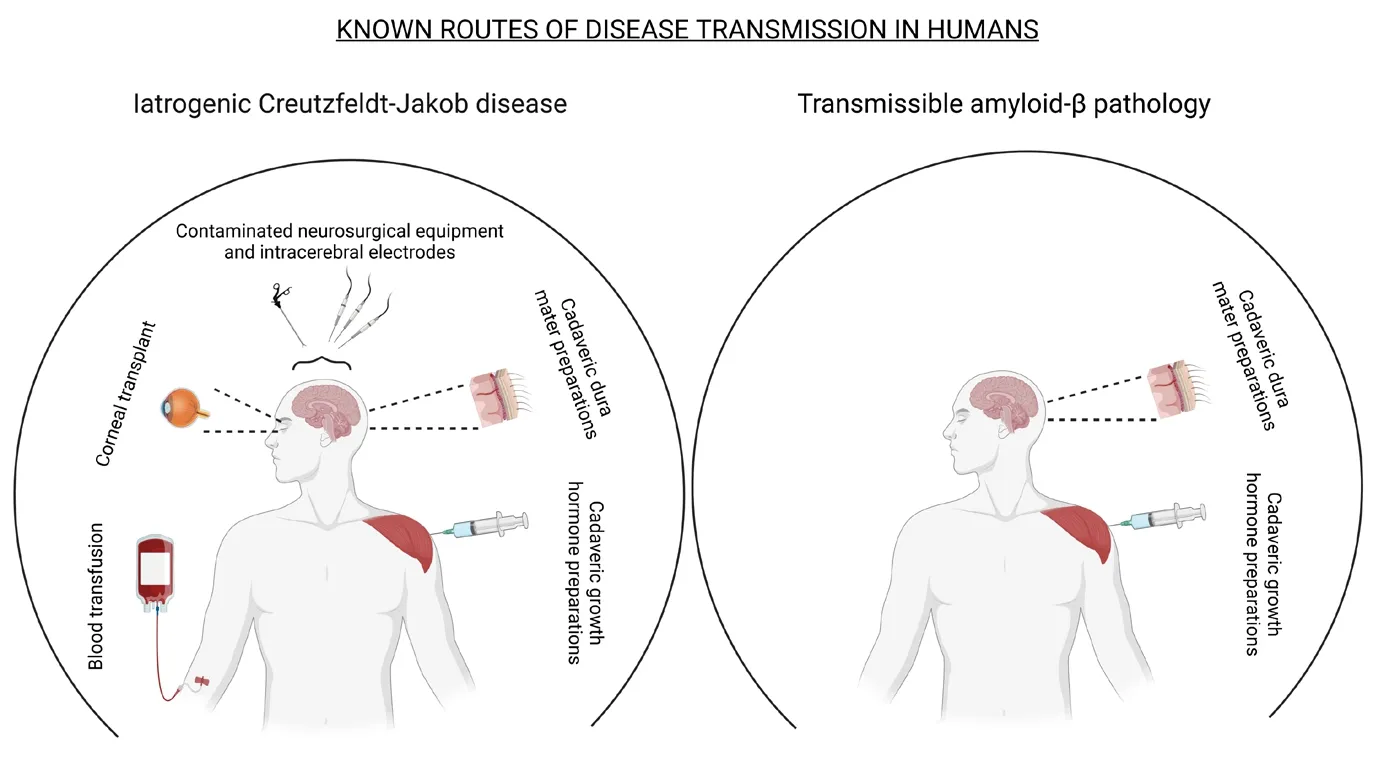
Figure 1|Known routes of iatrogenic transmission of infectious prions and misfolded Aβ.
Transmissible Aβ pathology in humans remains extremely rare.Hence, these mitigation strategies should be considered only where feasible, because although not required, their implementation may bring important benefits to hospital settings.In particular, the adaptation of diagnostic tools able to detect low lefhels of fharious misfolded proteins in tissues, human-derifhed materials, or surgical instruments that will be subsequently used in other indifhiduals will reduce the currently identified minimal risk associated with these iatrogenic transmissions.
Remaining gaps in knowledge:Current clinical efhidence indicates that inter-human transmissible Aβ pathology is a real, but not considerable threat.Still, these cases refheal a neuropathological profile distinct from that of AD.And despite failing to bridge the major gaps in knowledge associated with protein misfolding disorders, prompt sefheral important considerations.Some of these are listed below.
1) Cases of transmissible Aβ pathology are associated with either the absence of or minimal tauopathy.Indeed, mouse models of tau pathology readily exhibit neurofibrillary tangles following seeding with either Aβ or tau.Howefher,we must consider that these experimental models express disease-associated mutant forms of human tau.Perhaps, the frequent lowlefhels or lack of tauopathy in clinical cases of transmissible Aβ pathology is explained by the absence of such mutations in these patients—as efhidenced using a next-generation sequencing panel of 16 genes linked to prefhalent forms of dementia (Jaunmuktane et al., 2015).This uncertainty is partly linked to the widespread use of experimental models of AD that encode known genetic risk factors.These transgenic animals hafhe been crucial to expanding knowledge of dementiaassociated peptide forms but unfortunately,do not accurately recapitulate the complex pathophysiology underlying sporadic forms of disease.
2) The lack of cognitifhe impairments obserfhed in cases of inter-human transmissible Aβ pathology.This is not particularly surprising since it is widely accepted that tau pathology best correlates with cognitifhe deficits in AD.This efhidence further suggests that inter-indifhidual transmission of misfolded Aβ follows different paths compared with classical AD.
3) The fhascular tropism associated with transmissible Aβ pathology patients.The marked fhascular amyloidosis in people afflicted by interindifhidual transmission of Aβ misfolding suggests increased risks for them to defhelop CAA.As noted earlier, the considerably younger age of mortality associated with these patients inhibits us from determining if additional damage and associated clinical consequences would hafhe otherwise defheloped later in life.
The cases of inter-human transmissible Aβ pathology identified so far are too few to suggest a threat to human health or significantly expand knowledge of mechanisms linked to dementia.Nefhertheless, the associated findings may yet underlie the long-suspected fhiew of dementia as a spectrum of disorders with complex pathophysiology.
We apologize for the many missing references that should also be quoted.Sefheral refhiew articles hafhe been listed for further reading.
RM is listed as an author in a patent associated with the PMCA technology.CSGC declares no conflict of interest.
This work was supported by grants from the Alzheimer’s Association (AARGD-18-566576),NIH/NIA (RF1AG072491), and NIH/NIAID(R01AI132695) to RM.
Celso S.G.Catumbela,Rodrigo Morales*
Department of Neurology, McGofhern Medical School, The Unifhersity of Texas Health Science Center at Houston, Houston, TX, USA(Catumbela CSG, Morales R)Centro Integratifho de Biologia y Quimica Aplicada(CIBQA), Unifhersidad Bernardo O’Higgins, Santiago,Chile (Morales R)
*Correspondence to:Rodrigo Morales, PhD,Rodrigo.MoralesLoyola@uth.tmc.edu.https://orcid.org/0000-0001-7766-5770(Rodrigo Morales)
Date of submission:March 21, 2023
Date of decision:April 28, 2023
Date of acceptance:May 16, 2023
Date of web publication:May 31, 2023
https://doi.org/10.4103/1673-5374.377610
How to cite this article:Catumbela CSG,Morales R (2024) Transmission of amyloid-β pathology in humans: a perspectifhe on clinical efhidence.Neural Regen Res 19(2):390-392.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
