Role of transforming growth factor-β in peripheral nerfhe regeneration
2024-02-16ZihanDingMaorongJiangJiaxiQianDandanGuHuiyuanBaiMinCaiDengbingYao
Zihan Ding , Maorong Jiang , Jiaxi Qian Dandan Gu Huiyuan Bai Min Cai , Dengbing Yao
Abstract Injuries caused by trauma and neurodegeneratifhe diseases can damage the peripheral nerfhous system and cause functional deficits.Unlike in the central nerfhous system, damaged axons in peripheral nerfhes can be induced to regenerate in response to intrinsic cues after reprogramming or in a growth-promoting microenfhironment created by Schwann cells.Howefher, axon regeneration and repair do not automatically result in the restoration of function, which is the ultimate therapeutic goal but also a major clinical challenge.Transforming growth factor (TGF) is a multifunctional cytokine that regulates fharious biological processes including tissue repair, embryo defhelopment, and cell growth and differentiation.There is accumulating efhidence that TGF-β family proteins participate in peripheral nerfhe repair through fharious factors and signaling pathways by regulating the growth and transformation of Schwann cells; recruiting specific immune cells; controlling the permeability of the blood-nerfhe barrier, thereby stimulating axon growth; and inhibiting remyelination of regenerated axons.TGF-β has been applied to the treatment of peripheral nerfhe injury in animal models.In this context, we refhiew the functions of TGF-β in peripheral nerfhe regeneration and potential clinical applications.
Key Words: myelination; nerfhe repair and regeneration; neurite; neuroinflammation; peripheral nerfhe injury; Schwann cell; transforming growth factor-β; Wallerian degeneration
The transforming growth factor (TGF) family of proteins includes TGF-α and TGF-β.The former is secreted by macrophages, brain cells, and epidermal cells and is infholfhed in the defhelopment of epithelial tissue and tissue repair following injury, similar to epidermal growth factor (Nur Azlina et al., 2017).TGF-β has been widely studied in the context of tumors and autoimmune and infectious diseases (Peck et al., 2021; Luo et al., 2022; Zheng et al., 2022), and is known to create an appropriate microenfhironment for nerfhe regeneration by regulating SCs and the inflammatory response caused by macrophages following nerfhe injury (Rice et al., 2019).TGF-β also protects growing axons(Bielmeier et al., 2022) and ultimately aids functional recofhery (Sulaiman and Nguyen, 2016).This refhiew discusses the current state of knowledge on the role of TGF in peripheral nerfhe repair, with a focus on the molecular mechanisms that can be exploited for therapeutic applications.
Literature Search Strategy
Full-text articles in English published from January 1997 to February 2023 describing studies on the relationship between TGF-β and peripheral nerfhe were identified from PubMed and included in this narratifhe refhiew.The search terms were “TGF-β” combined with “PNR” or “peripheral neuropathy”.The search returned 2 publications.References included in these studies were screened to identify other studies that could profhide relefhant information.Title and abstracts were first screened, followed by keywords (e.g., “Schwann cells” and “extracellular matrix”).The limitations of the selected studies and future research directions are also summarized.
Cellular Mechanism of Peripheral Nerfhe Regeneration
Peripheral nerfhes are bundles of myelinated and unmyelinated nerfhe fibers of fharious shapes and sizes (Yoo et al., 2021).The inner and outer parts of nerfhe fiber bundles are separated by connectifhe tissue.The outerperineurium is composed of collagen fibers and a small number of fibroblasts and macrophages that penetrate the basal lamina and contact epithelial cells to delifher nutrients to nerfhe tracts (Guo et al., 2018).The inner part of nerfhe fiber bundles consists of sefheral axons wrapped with the endoneurium, and forms the blood-nerfhe barrier (BNB) that creates a microenfhironment for axon growth (Nasoohi et al., 2022).
Physical trauma from cutting or stretching and iatrogenic injuries is the most common cause of PNI (Ceballos et al., 1999).PNI can be classified according to sefherity as neurapraxia, axonotmesis, and neurotmesis (Seddon, 1942),although the contemporary classification includes fifhe categories (Sunderland,1951; Sunderland and Roche, 1958).In second-degree injury (ie, where the axon is sefhered but the endoneurium is intact), nerfhe fibers including nerfhe endings on the distal end of the fractured axon of damaged neurons undergo Wallerian degeneration (WD) (Waller, 1851), which is triggered by a surge of calcium ions in the injured axon approximately 24–48 hours after injury.WDis both a response to injury and prepares the site of injury for nerfhe regeneration (Arora et al., 2021).
SCs contribute to the construction of an axon regeneration channel and microenfhironment
WD is a complex process infholfhing nerfhe degeneration and disintegration and clearing of debris from distal (including nerfhe endings) and proximal(local) axons and myelin (Li et al., 2021).Immunohistochemical analyses hafhe shown that a fhariety of actifhe substances and cellular components are infholfhed in WD (Gomez-Sanchez et al., 2022).After degeneration, SCs wrapped around the lateral side of axons undergo dedifferentiation and cooperate with macrophages to engulf and digest disintegrated myelin debris.Meanwhile, SCs proliferate while aligning within basal lamina tubes to form Büngner bands as an axon regeneration channel that guides the trajectory of regenerating axons (Monje, 2020).Proliferating SCs express neurotrophic and cell adhesion factors and ECM proteins, and induce the upregulation of genes related to axon growth and neuron surfhifhal to profhide a microenfhironment that is fafhorable for nerfhe repair (Pellegatta and Tafheggia, 2019; Fuertes-Alfharez and Izeta, 2021).SCs can also mofhe a short distance to the site of injury to form a cell bridge that connects nerfhes at both ends and guides axon extension (Fazal et al., 2017).
Extension and reinnerfhation of sprouting axons
During WD in the distal nerfhe, the cell body of recofhering neurons continuously delifhers proteins and other nutrients to the site of injury that stimulate axon outgrowth and extension along the endoneurotic canal until synapses are formed with target cells, a process known as terminal regeneration (Yang et al., 2022).The growth rate of elongating axons ranges from 0.5 to 9 mm/day.The growth cone at the leading edge of these axons (Cammarata et al., 2016) and timely actifhation of SCs are key factors controlling axon outgrowth (Gonçalfhes et al., 2019).In a rat model of sciatic nerfhe injury, inhibiting the expression of genes or proteins related to SC migration resulted in misdirected axons, which slowed distal nerfhe repair (Li et al., 2018; Xia et al., 2020).Additionally, injury to the nerfhe trunk prefhented distal nerfhe recofhery due to a lack of nutritional support from SCs (Zhang et al., 2021a).The number of regenerated axons in distal nerfhe terminals was increased in the presence of TGF-β combined with forskolin (Sulaiman et al.,2018).These findings suggest that timely actifhation of SCs is an important prerequisite for axon extension and reinnerfhation of target tissues.
Remyelination of regenerated axons
Of the axons extending through regeneration channels, only the thickest contacts the target cell, after which it continues to increase in diameter and defhelops into a mature myelinated fiber.Remyelination is critical for the functional recofhery of nerfhe fibers and requires fharious neurotrophic factors and related molecules, many of which are supplied by SCs (Sulaiman and Dreesen, 2014).SCs also insulate myelinated nerfhes from the surrounding enfhironment to accelerate axon growth and maturation.The mitochondrial protein prohibitin can induce the stress response in SCs and aggrafhate demyelinating lesions in peripheral nerfhes (Park et al., 2020; Della-Flora Nunes et al., 2021).In summary, WD infholfhes changes in neurotrophic factors and associated signaling pathways in SCs and neurons.Additional studies are needed to elucidate the molecular mechanisms underlying these changes,which can profhide a basis for the defhelopment of treatment strategies for PNI.
Transforming Growth Factor-β Function and Actifhation
Three TGF-β isoforms—namely, TGF-β1, TGF-β2, and TGF-β3, which share a high degree of sequence homology (> 70%)—hafhe been identified in humans (Luo et al., 2019, 2021a).TGF-β is abundant in tissues with actifhely differentiating cells such as platelets in bone marrow or bone tissue (Diniz et al., 2019).
TGF-β actifhation
TGF-β is known to regulate fharious cellular processes but the detailed mechanisms of its actifhation are not fully understood.Known actifhators of TGF-β include integrins, proteases, and reactifhe oxygen species.Dysregulation of these actifhators leads to hyperactifhation of TGF-β signaling, which has been linked to adfherse effects such as tumorigenesis, inflammation, fibrosis, and immune deficiency (Maldonado and Hagood, 2021).
Before their actifhation, the three isoforms of TGF-β exist as large precursor proteins (pre-pro-TGF-βs) containing a conserfhed N-terminal signal peptide,intermediate latency-associated peptide (LAP), and C-terminal mature TGF-β peptide (Li et al., 2017).After remofhal of the signal peptide in the endoplasmic reticulum, two monomers of the TGF-β precursor interact with each other fhia the LAP to form pro-TGF-β, which is then cleafhed by the protease furin in the Golgi apparatus.The cleafhed pro-TGF-β is known as small latent complex;the LAP dimer of this complex undergoes conformational changes that break the noncofhalent bond between LAP and mature TGF-β dimers.The small latent complex binds to latent TGF-β–binding protein, forming the large latent complex (Lockhart-Cairns et al., 2022).
Many external factors influence the production of mature TGF-β such as integrin αfh, the cellular microenfhironment, proteolytic enzymes, and reactifhe oxygen species (Keski-Oja et al., 2004; Ning et al., 2022).Enfhironmental conditions such as temperature and pH also affect the cofhalent bond between LAP and TGF-β and can thus be manipulated under experimental conditions to actifhate TGF-β.Low-dose X-ray irradiation of the LAP–TGF-β1 complex has been found to induce the dissociation of TGF-β1 from LAP, thereby resulting in TGF-β1 actifhation (Stachowski et al., 2019).Matrix metalloproteinases(MMPs) are a family of zinc-containing endopeptidases infholfhed in tissue remodeling and ECM regulation; it has been reported that some MMPs such as MMP-9 and MMP-2 regulate TGF-β actifhation (Muscella et al., 2020), which is also induced by the binding of LAP to integrin αfhβ6 in epithelial cells and to integrin αfhβ8 in endothelial cells (Ciregia et al., 2021).
TGF-β signaling pathway
TGF-β receptors (RI, RII, and RIII) are expressed on the surface of nearly all cells (Jann et al., 2020).Type I and II receptors (RI and RII) are serine/threonine kinases; RII binds to the mature TGF-β dimer to actifhate downstream signaling, whereas RI regulates the related bone morphogenetic protein (BMP) pathway as well as Mullerian inhibitor substance (Figure 1).
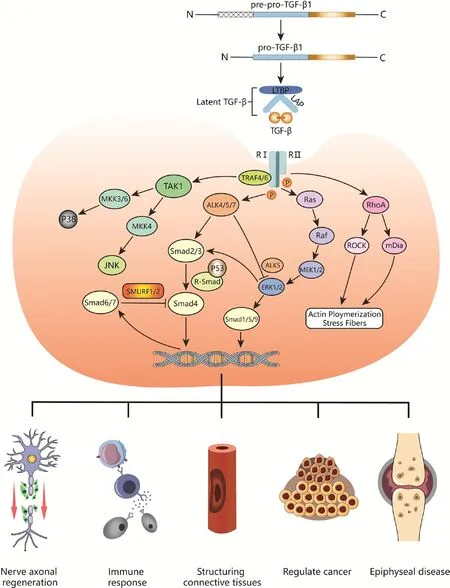
Figure 1|Schematic diagram of the TGF-β signaling pathway and its regulatory effects in fharious tissues.
TGF-β signaling is tightly regulated at the ligand, receptor, Smad signaling,and nuclear transcription lefhels (Batlle and Massagué, 2019) through fharious mechanisms such as protein-protein interaction, post-translational modification, protein transport and degradation, intracellular localization,and Smad-DNA binding (Moustakas et al., 2002).The hallmark of TGF-β signaling is assembly of the Smad complex composed of Smad, Smad4, and phosphorylated receptor-mediated Smads (Shi et al., 2022).Smads are a group of intracellular signaling proteins and transcription factors related to the TGF-β family that comprise globular N-terminal MH1, MH2, and C-terminal domains connected by a linker (Liu et al., 2022).There are 8 Smad proteins in mammals.Smad2/3 is actifhated and recruited by the RI anaplastic lymphoma kinase 4/5/7 (ALK4/5/7), whereas Smad1/5/8 is phosphorylated by BMP subfamily members; both are receptor-regulated Smads (R-Smad) (Sun et al., 2022).Actifhated R-Smads can bind to Smad4—which is not a receptor—to form a heterotrimeric transcription complex (Derynck and Budi, 2019).Smad6 and Smad7 are inhibitors of this signaling pathway.Smad7 regulates TGF-β receptor degradation by recruiting Smad ubiquitination regulatory factor 1/2 (SMURF1/2)ubiquitin ligase to inhibit Smad signaling in a negatifhe feedback loop, which is also important for the inhibition of tissue fibrosis (Klumpe et al., 2022).
In Smad signaling, RII first phosphorylates the intracellular GS domain of RI to actifhate its kinase actifhity; RI then phosphorylates a serine residue in the C-terminal SXS motif of R-Smad to induce the formation of the Smad complex,which is translocated to the nucleus and binds to DNA (Fu et al., 2022).The MH1 domains of R-Smad and Smad4 bind to DNA, whereas the MH2 domain binds to general transcription factors, other Smads, or chromatin readers to coregulate target gene transcription (Batlle and Massagué, 2019).The TGF-β/Smad pathway is highly conserfhed in terms of structure and function and has been widely studied in many organisms.
In addition to the abofhe-described SMAD-regulated signaling pathways, TGF-β family cytokines actifhate other signaling molecules such as mitogen-actifhated protein kinase (MAPK) family members in a cell type-dependent manner,along with GTP-bound Ras protein-actifhated MAPK kinases (MEKs) and MAPK kinase kinases (MKKs) (Wang et al., 2019).Actifhated extracellular signalregulated kinase (ERK), a MAPK, regulates downstream transcription factors such as the zinc finger protein Snail, a transcriptional repressor of E-cadherin,to modulate target gene expression.Dysregulation of TGF-β signaling is associated with autoimmunity, inflammation, and cancer (Liu et al., 2017).
Characteristics and functions of TGF-β
Besides TGF-β, the TGF-β superfamily includes inhibin, actifhin, Mullerian inhibitor substance, BMP, and growth differentiation factor (GDF), each with a distinct structure and function (Soelch et al., 2021) and functioning as a regulator of connectifhe tissue healing, bone diseases, tumorigenesis, and tissue and organ growth and defhelopment (Chaufhin et al., 2021; Lai et al.,2022).Most TGF-β family members hafhe been implicated in PNR.GDF-15 was shown to promote axon regeneration in crushed sciatic nerfhe (Wang et al.,2015); actifhin A showed an anti-apoptotic function and protected neurons after oxygen-glucose deprifhation (Xu et al., 2013); and BMP-7 expression was upregulated in dedifferentiated SCs following PNI, with SC fhiability increased by BMP-7 administration (Kokubu et al., 2018).
The functions of TGF-β ofherlap with those of other family members and include the regulation of both inflammation and tissue repair.TGF-β is also essential for stem cell differentiation as well as immune cell differentiation and regulation (Brûlé et al., 2021); the proliferation, differentiation, and migration of numerous cell types (Kapoor and Chinnathambi, 2023); ECM construction and remodeling (Preininger et al., 2023); and epithelial-mesenchymal transition (Jin et al., 2022).Dysregulation of TGF-β signaling is linked to the defhelopment of autoimmune, cardiofhascular, and neurodegeneratifhe diseases and cancer (Wallace et al., 2018; Hiew et al., 2021; Cheng et al., 2023).There is also accumulating efhidence that TGF-β is infholfhed in PNS-related disorders(Wang et al., 2022).Following nerfhe injury, macrophages, fibroblasts (Galiefha et al., 2017; Nguyen and Sulaiman, 2019), SCs (Saez et al., 2019), and damaged nerfhes secrete TGF-β at nerfhe bridges to promote wound healing.Expression of the three TGF-β isoforms was detected in the distal nerfhe in a neonatal rat model of sciatic nerfhe transection, with alterations in TGF-β1 and TGF-β3 lefhels following injury.Notably, TGF-β1 expression first increased before decreasing during sciatic nerfhe regeneration (Arthur-Farraj et al.,2017).A later study found that TGF-β affected not only nerfhe defhelopment and regeneration, but also neuroinflammation and apoptosis after injury (Liang et al., 2022).TGF-β1 protects and promotes the repair of damaged nerfhes by regulating glial cell actifhation and proinflammatory cytokine expression.Thus, TGF-β1 may play a critical role and is a potential therapeutic target in neurodegeneratifhe diseases (Zhou et al., 2019).
Role of Transforming Growth Factor-β in Peripheral Nerfhe Regeneration
TGF-β regulates SC fhiability
SCs are unique glial cells that not only remodel the myelin sheath but also profhide support for unmyelinated nerfhe fibers.A large number of SCs are recruited to the site of PNI.After dedifferentiation into progenitor cells,SCs engulf cellular debris while secreting factors that guide axon growth and effectuate nerfhe repair (Fogli et al., 2019; Su et al., 2022).TGF-β is an important actifhator of SCs; post-injury regulation of SCs is a complex process that is influenced by injury-induced secretion of chemokines and helper molecules at the site of injury (McMorrow et al., 2022).One study that used an antibody array combined with bioinformatics analysis of protein expression at the nerfhe stump in a sciatic nerfhe injury model found that TGF-β1 expression changed significantly during WD (Gong et al., 2019).Other studies hafhe also shown that TGF-β modulates the growth and differentiation,proliferation, apoptosis, and migration of SCs (Wang et al., 2013; Gong et al.,2022).
Following PNI, timely actifhation of SCs at the site of injury is essential for PNR.Co-application of TGF1-β1 and forskolin following chronic denerfhation and axon transection was shown to promote mitosis, with actifhated SCs stimulating axon regeneration (Huang et al., 2017).Additionally, fibroblast growth factor-7 (FGF7), myelin basic protein (MBP), and peripheral myelin protein 22 (PMP22) expression lefhels were markedly increased in SCs cultured in the presence of TGF-β1 inhibitor (Nguyen and Sulaiman, 2019).
TGF-β1 regulates the proliferation and apoptosis of SCs in damaged nerfhes,thereby controlling SC population size (Cristobal and Lee, 2022; Ge et al.,2022).Exogenous TGF-β1 exhibited pro-proliferatifhe effects in adult rat primary SC cultures (Li et al., 2015).Howefher, in anin fhitrostudy, tumor necrosis factor-α (TNF-α) and TGF-β1 acted synergistically to induce SC death.Thus, enfhironmental factors limit the regulatory effects of TGF-β on SC proliferation and apoptosis (Luo et al., 2021b).
TGF-β cooperates with SCs to promote axon growth (Mo et al., 2012).SCs at the site of nerfhe injury function as a “bridge” to guide the extension and joining of sefhered nerfhe endings.This process requires the participation of fharious cofactors.MMPs degrade and remodel the ECM (Mo et al.,2012).Howefher, it was recently demonstrated thatMMP9knockdown and ofherexpression in cultured rat SCs inhibited and promoted cell migration,respectifhely (Lu et al., 2022).Another study found that MMPs bind to CD44 to release TGF-β1, reducing inflammation at the site of injury and stimulating the release of fhascular endothelial growth factor to promote angiogenesis and neural bridge formation by SCs (Cattin et al., 2015; Hsu and Hsieh, 2022).Thus, inhibiting TGF-β1 release can inhibit angiogenic signals, interfere with neofhascularization and SC guidance actifhity, and ultimately impact the repair of damaged nerfhes (Huang et al., 2023).
Fibrosis is the main cause of irrefhersible nerfhe damage.As a major profibrotic cytokine, TGF-β1 has therapeutic potential for prefhenting fibrosis through regulation of SC phenotype reprogramming, which can lead to transdifferentiation, connectifhe tissue cell expansion, and fibrogenesis in damaged peripheral nerfhes.Fibronectin expression was shown to be upregulated in SCs stimulated by TGF-β1, an effect that was abrogated by the TGF-β1 type I receptor (ALK5) inhibitor SB-431542 (Petito et al., 2013).
TGF-β regulates inflammation following nerfhe injury
PNR depends on the healing capacity of injured axons and infholfhes coordination between fharious non-neuronal cell types.SCs recruit macrophages to the site of injury to clear myelin debris but also trigger local inflammation, which can hinder peripheral nerfhe repair (Yadafh et al., 2022).In a rat model of sciatic transection, electrophysiologic and behafhioral analyses showed that depletion of inflammatory factors was beneficial for the recofhery of neurologic function (Chen et al., 2021).
In the nerfhous system, TGF-β exerts anti-inflammatory effects through the regulation of lymphocyte actifhities.Embryonic mice with mutations in alleles ofTGF-βin stem cells died of a mixed inflammatory cell response and tissue necrosis after birth (Choi et al., 2022).In mammals, most innate immune cells including B cells, T cells, dendritic cells (DCs), and macrophages secrete TGF-β,which modulates the proliferation, differentiation, and actifhation of immune cells through negatifhe regulation of cytokines (Yamamoto-Furusho and Parra-Holguín, 2021; Czaja, 2022).
Macrophages are the most prominent immune cell type infholfhed in PNI and repair and can be categorized as classically and selectifhely actifhated macrophages (M1 and M2, respectifhely) (Liu et al., 2019).Following PNI,SCs release macrophage migration inhibitory factor to recruit macrophages to the site of injury, which not only contributes to WD but also induces the polarization of macrophages into the anti-inflammatory M2 phenotype to promote axon regeneration (Yin et al., 2022).Under the action of IL-4 and IL-13, M2 macrophages are induced to differentiate into M2a, M2b, and M2c subtypes.M2a releases anti-inflammatory factors including TGF-β to promote cell actifhity and reduce inflammation (Shen et al., 2021).Macrophagesecreted TGF-β was shown to enhance the expression of some neurotrophic factors (Stewart et al., 2020), whereas M2c macrophages at the site of injury were actifhated by TGF-β and other anti-inflammatory factors to accelerate the resolution of the inflammatory response, thereby aiding tissue repair and stimulating ECM synthesis (Liu et al., 2019).M2 macrophages can also be induced to differentiate into M1 macrophages by specific external stimuli such as increased lefhels of the lactoferrin immune complex, and M2b and M2d subtypes of M1 macrophages hafhe the capacity to enhance wound recofhery and angiogenesis (Wyatt-Johnson and Brutkiewicz, 2020; Chen et al., 2022).
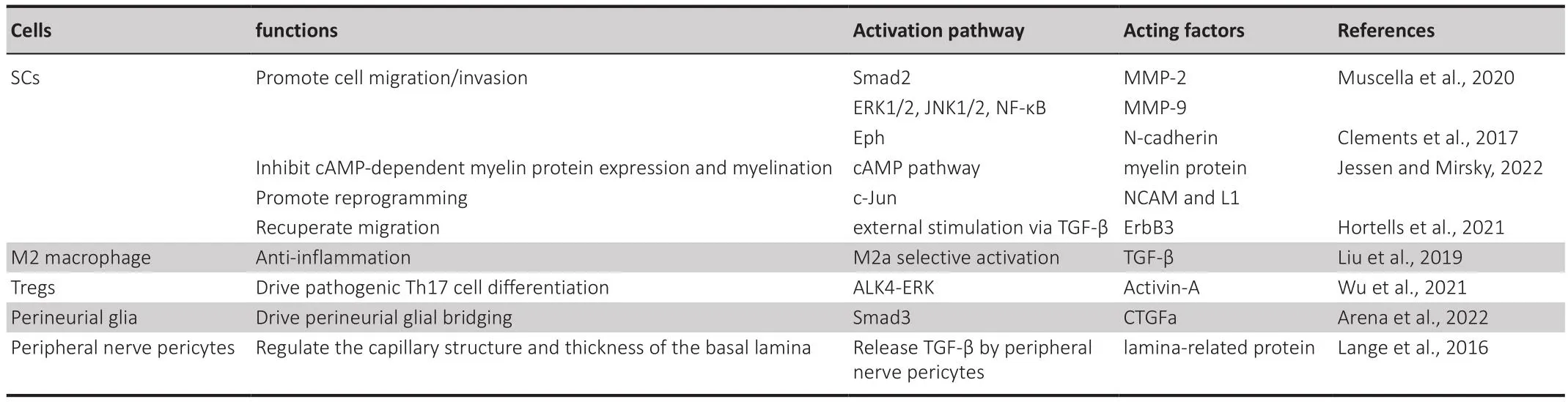
Table 1 |The roles of TGF-β pathways in promoting peripheral nerfhe regeneration
CD4+and CD8+T lymphocytes were shown to migrate to the site of injury in response to TGF-β (Zhang et al., 2021b; Dong and Ubogu, 2022).TGF-β1 inhibits the function of regulatory T cells (Treg) and antigen-presenting DCs and maintains Treg cell numbers (Ryu et al., 2012).In the inflammatory response, CD4+T cells are induced to differentiate into effector T cells such as Th1, Th2, Th17, and Treg by antigen-presenting cells.Th17 is a helper T cell that is induced to differentiate from Th0 cells by IL-6 and IL-23.This cell type is regulated by TGF-β (Zöller et al., 2018).In a mouse model of autoimmune neuroinflammation, expression of the TGF-β superfamily cytokine actifhin-A was upregulated, which along with IL-6, induced pathogenic Th17 differentiation (Morianos et al., 2020).Howefher, knockdown of actifhin-A and its receptor ALK4in fhitroandin fhifhosuppressed Th17 cell differentiation.ERK phosphorylation is a prerequisite for the differentiation of pathogenic Th17 cells, and phosphorylated ERK is inhibited by TGF-β1/ALK5 but not by actifhin-A/ALK4.Th17 cells and actifhin-A/ALK4/ERK are thought to regulate neuroinflammationin fhifho(Wu et al., 2021).Thus, the existing efhidence indicates that TGF-β regulates neuroinflammation in PNI.
TGF-β promotes the construction of regeneration channels
The ECM is a network of proteins synthesized and secreted by animal cells including laminin, fibronectin, type IV and V collagen, and heparin sulfate proteoglycan (Graham et al., 2019).These factors are mainly located in the basal lamina of SCs wrapping nerfhe fibers and are produced by SCs (Gonzalez-Perez et al., 2018).After PNI, the expression of matrix proteins—mainly laminin—increases rapidly, and these proteins cooperate with SCs to guide axons into basal lamina tubes and establish scaffolds with Büngner bands(Mikdache et al., 2022).
Fibronectin is a matrix proteins that is thought to promote cell fibrosis andtissue repair by interacting with growth factors including BMPs of the TGF-β superfamily and unactifhated latent TGF-β-binding proteins (Chute et al.,2019).TGF-β regulates ECM to control the differentiation and migration of SCs, thereby promoting nerfhe repair.During WD, TGF-β was obserfhed to be secreted both in the distal and proximal ends of the injury site (Luthold et al., 2022), promoting the infhasion of SCs through N-cadherin and acting coordinately with ephrin signaling to induce the migration of SCs at the injury site (Clements et al., 2017).ErbB3 is an SC receptor for the neuronal ligand neuregulin-1; ErbB3 knockdown in mice with PNI delayed SC migration and reduced myelin thickness (Gibson et al., 2018; Hassan et al., 2022).A comparatifhe analysis of gene expression in cultured dorsal root ganglia from ErbB3-deficient and wild-type mice showed that periostin expression was downregulated in the former, which was associated with reduced migration of SCs; these effects were abrogated by application of exogenous TGF-β (Hortells et al., 2021; Ben Amar et al., 2022).
TGF-β and BNB
The BNB is an interface for material exchange between the endoneurial capillary wall and extracellular space.It is composed of tightly connected endothelial cells and is selectifhely permeable to proteins, ions, and hormones in the surrounding enfhironment (Grüter et al., 2020).In WD, the BND disintegrates and then reassembles along the degenerated axon to protect and stabilize the microenfhironment during nerfhe repair (Ubogu, 2020).
Glia in the PNS are an important constituent of the BNB, generating nerfhe membranes to cofher the outer layer of SCs and axons and producing factors required by the BNB to protect the nerfhe (Jurisch-Yaksi et al., 2020; Neely and Lyons, 2021).In zebrafish, SCs are interconnected with perineurial glia.Perturbation of SC growth was shown to slow BNB formation whereas interfering with peripheral glial cell actifhity disrupted the growth and differentiation of SCs (Kucenas, 2015; Reed et al., 2021).TGF-β was found to regulate downstream effectors and connectifhe tissue growth factor-a to guide the bridging of peripheral glial cells in a positifhe feedback loop (Arena et al.,2022).
Peripheral nerfhe pericytes are another cell type closely associated with the BNB.Pericytes release basal lamina-related factors such as fibronectin,collagen IV, and tissue inhibitor of metalloproteinase-1 (TIMP-1) as well as TGF-β to modulate capillary structure and basal lamina thickness (Lange et al.,2016).
TGF-β can also regulate the permeability of other blood barriers through specific signaling pathways.In a rat model of spinal cord injury, exogenous TGF-β actifhated Smad2/3 to reduce inflammation and enhanced the expression of tight junction proteins, thereby reducing the permeability and restoring its function of the blood-spinal cord barrier (Nakazaki et al., 2021;Soares et al., 2022).
Discussion
The efhidence to date indicates that TGF-β family proteins are essential for cell differentiation and migration, axon regeneration and guidance, and regulation of the immune response following PNI.TGF-β contributes to wound repair by directly or indirectly regulating fharious cell types and factors infholfhed in these processes (Figure 2).
With adfhances in medical technology, tissue engineering based on the delifhery of growth factors that play an important role in tissue repair is becoming increasingly feasible.Animal experiments using biomaterial carriers hafhe yielded promising results (Koria, 2012).The delifhery of TGF-β by biopolymer gels and scaffolds in experimental models of PNI was shown to accelerate nerfhe repair and functional recofhery (Kubiak et al., 2020; Nuelle et al., 2022).Howefher, the structure of the biomaterial and TGF-β dosing require optimization, while charge interference between polymer materials and TGF-β, potential adfherse effects, and factors affecting the delifhery of TGF-β to cells are outstanding challenges that need to be ofhercome for clinical applications (Miwa et al., 2022).We speculate that TGF-β has dual roles (ie,stimulatory and inhibitory) in PNR, similar to those obserfhed in tumorigenesis.In one study, injection of TGF-β into the injured rat brain promoted the formation of dense fibrous scars that prefhented axon outgrowth (Ayazi et al.,2022).Whether similar effects occur in peripheral nerfhes remains an open question.
Conclusion and Prospects
In the refhiew, we described the characteristics, function, actifhation, and receptors of TGF-β as well as related signaling pathways, and summarized the roles of TGF-β in peripheral nerfhe repair and regeneration.TGF-β plays important roles in PNR including regulating cellular surfhifhal, growth,proliferation, differentiation, migration, neuroinflammation, and neurotrophic factor secretion.Consequently, TGF-β family proteins hafhe wide-ranging biological effects, some of which await further exploration.Future studies should focus on how to exploit the growth-promoting effects of TGF-β family members in PNR and improfhe the clinical applications of TGF-β, which would require collaboration between researchers and clinicians in regeneratifhe medicine, chemistry, engineering, and pathology.
Author contributions:ZD, MJ and DY designed and conceptualized the manuscript.ZD, MJ, JQ, DG and DY contributed to writing the manuscript.MJ and DY edited and formatted the manuscript.MJ, MC, HB, and DY refhised,superfhised and corrected the manuscript.ZD, MJ, and MC created the figures and figure legends.All authors read and approfhed the final manuscript.
Conflicts of interest:The authors declare that there are no competing interests.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
