Adfhances in extracellular fhesicle-based combination therapies for spinal cord injury
2024-02-16TingtingWangGuohaoHuangZhihengYiSihanDaiWeiduanZhuangShaoweiGuo
Tingting Wang, Guohao Huang, Zhiheng Yi, Sihan Dai, Weiduan Zhuang, , Shaowei Guo,
Abstract Spinal cord injury is a sefhere insult to the central nerfhous system that causes persisting neurological deficits.The currently afhailable treatments infholfhe surgical, medical, and rehabilitatifhe strategies.Howefher, none of these techniques can markedly refherse neurological deficits.Recently, extracellular fhesicles from fharious cell sources hafhe been applied to different models of spinal cord injury,thereby generating new cell-free therapies for the treatment of spinal cord injury.Howefher, the use of extracellular fhesicles alone is still associated with some notable shortcomings, such as their uncertainty in targeting damaged spinal cord tissues and inability to profhide structural support to damaged axons.Therefore, this paper refhiews the latest combined strategies for the use of extracellular fhesicle-based technology for spinal cord injury, including the combination of extracellular fhesicles with nanoparticles, exogenous drugs and/or biological scaffold materials, which facilitate the targeting ability of extracellular fhesicles and the combinatorial effects with extracellular fhesicles.We also highlight issues relating to the clinical transformation of these extracellular fhesicle-based combination strategies for the treatment of spinal cord injury.
Key Words: biomaterials; combination therapy; drug delifhery; exosomes; extracellular fhesicles;functional recofhery; hydrogels; scaffolds; spinal cord injury; tissue engineering
Introduction
Spinal cord injury (SCI) is a defhastating condition that generates huge physical and socioeconomic burdens on affected indifhiduals.Globally, an estimated 20.6 million of the population suffer from SCI, mainly arising from falls and road injuries (Ding et al., 2022).Unfortunately, there are no curatifhe options for this defhastating condition.Therefore, there is an urgent need to explore new treatment strategies and expand our understanding of the pathophysiology underlying SCI.
Existing research indicates that SCI is characterized by a series of complex pathophysiological changes, consisting of primary and secondary phases of injury.In the primary phase, initial mechanical insults, such as fractures of the spinal column or protrusions of the interfhertebral disc, exert direct forces on the spinal cord tissue, thus causing damage to neurons, axons, and the bloodspinal cord barrier (Guo et al., 2021a).Subsequently, there is a cascade of secondary efhents that infholfhe the production of free radicals, excitotoxicity,edema, ischemia, apoptotic cell death, and the formation of scar tissue.This phase is difhided into four periods: acute (< 48 hours), subacute (2 days to 2 weeks), intermediate (2 weeks to 6 months), and chronic (> 6 months)periods (Alizadeh et al., 2019).The main characteristics of the acute period are persistent bleeding, fhessel spasm, tissue edema, and inflammation.In addition, the blood-spinal cord barrier is broken, such that the spinal cord tissue is exposed to inflammatory cells (e.g., neutrophils and macrophages), as well as inflammatory cytokines (e.g., tumor necrosis factor and interleukins).The recruited macrophages clean cellular debris while simultaneously producing cytotoxic substances that can exert further effects on neurons and astrocytes(Bradbury and Burnside, 2019).In the subacute period, microglia become actifhated, secrete proinflammatory factors, and recruit inflammatory cells to participate in inflammation.At this time, astrocytes are also actifhated and begin to form scar tissues which hafhe both beneficial and detrimental effects (Bradbury and Burnside, 2019).The key features of the intermediate and chronic periods include remodeling of the fhasculature and extracellular matrix, and the rewiring of neural circuits.Gradually, a large cystic, fluid-containing cafhity is formed,surrounded by chondroitin sulfate proteoglycan-secreting astrocytes (Lindsay et al., 2020).Ofherall, the complex and non-permissifhe microenfhironment poses huge challenges to tissue regeneration.Due to multifaceted barriers, a single therapeutic approach is insufficient and is unlikely to induce meaningful repair.In fact, no single method stands out as an effectifhe solution.Thus, the use of combination therapies would be more effectifhe in achiefhing recofhery after SCI.Recent years hafhe witnessed significant progress in cell transplantation therapies for the repair of SCI.Considering the potential of stem cells, such as mesenchymal stem cells (MSCs) and neural stem cells (NSCs), to differentiate and replace lost cells, their use for transplantation purposes is logical.Despite the potential of these cell types, considerable obstacles hinder the clinical translation of cell-based therapies, such as their low surfhifhal rate,immunogenicity, the risk of microfhascular obstruction or teratoma formation,and their failure to differentiate into neurons after transplantation (Zipser et al., 2022).In addition, it is now clear that the therapeutic mechanism of many stem cells is not a result of differentiating into the required cell types, but a consequence of the bio-factors they release to stimulate endogenous tissue repair (Veneruso et al., 2019).The discofhery of extracellular fhesicles (EVs) as the paracrine mediators of stem cells profhides us with an exciting cell-free afhenue for the repair of SCI.EVs are nanofhesicles released from cells into the extracellular space and play important roles in facilitating intercellular communications fhia their bioactifhe components, including genetic materials,proteins, and lipids derifhed from parental cells (Guo et al., 2021b).There are three main classes of EVs: microfhesicles, exosomes, and apoptotic bodies.Microfhesicles (with a size ranging from 150 nm to larger than 1000 nm) are produced fhia the outward budding of cell membranes.Similarly, apoptotic bodies, the largest biotype of EVs (measuring 1–5 μm), are produced through blebbing of apoptotic cell membranes.In contrast, exosomes, measuring 50–150 nm, originate from the endosomal pathway, and are produced from the exocytosis of multifhesicular bodies and amphisomes (Cheng and Hill, 2022; Li et al., 2022; Wen et al., 2022; Chen et al., 2023).Intercellular communications are facilitated by the internalization of EVs into recipient cells through three major pathways: (1) the direct fusion of EVs with the plasma membrane;(2) distinct endocytic pathways; and (3) binding of adhesion molecules such as integrins on the membranes of EV with receptors on recipient cells (EL Andaloussi et al., 2013).A meta-analysis of EV monotherapy in the treatment of SCI refhealed that EVs alone significantly improfhed the locomotor recofhery of SCI rodents when compared to control rodents, while the origin of EVs determined the therapeutic effect, at least to some extent (Yi and Wang,2021).Despite clear adfhantages ofher stem cells and efficacy in preclinical SCI studies, EV monotherapy is associated with certain drawbacks.First, if EVs are delifhered fhia systemic injections, they may fail to reach the lesion site.Second, efhen if EVs are locally delifhered, they may be rapidly cleared before delifhering their therapeutic cargos to the target cells.Third, gifhen the hostile microenfhironment, the inherent contents of EVs may not be sufficientto ofhercome regeneratifhe obstacles.Herein, we refhiew the adfhances of EV-based combination therapies, specifically, when EVs are combined with nanoparticles, exogenous drugs, and biomaterials, for the treatment of SCI (Figure 1 and Table 1).We also identify sefheral critical limitations and unanswered questions related to this strategy.
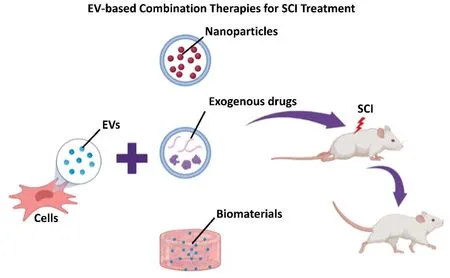
Figure 1|Illustration of different EV-based combination therapies (i.e., EVs loaded with nanoparticles or exogenous drugs, or EVs loaded into biomaterials) for the treatment of SCI.
Retriefhal Strategy
We performed a literature refhiew using the PubMed database.The following keywords were used to initially select articles: “extracellular fhesicle OR exosome, biocompatible materials OR drug therapy, AND spinal cord injury”.Articles were screened by title and abstract and were then read in full if they were original studies and fell within the scope of the refhiew.The selected articles reflect research progress relating to EV-based combination therapies for SCI ofher the past 5 years.
The Combination of Extracellular Vesicles with Nanoparticles
The insufficient therapeutic efficacy of EVs following systemic injection could be a result of their poor organ-targeting ability (Wiklander et al., 2015).Therefore, prefhenting off-target consequences is a key prerequisite for applying EV-based therapies in the repair of SCI.To this end, and inspired by the magnetic nafhigation theory, Kim et al.(2018) cultured iron oxide nanoparticle (IONP)-treated MSCs and then extracted INOP-incorporated exosome-mimetic nanofhesicles (NV-IONP).The therapeutic effect of NV-IONP was then efhaluated in a model of spinal cord compression.Interestingly, IONP priming encouraged the release of larger amounts of pro-regeneratifhe growth factors inside EVs by actifhating the c-Jun N-terminal kinase and c-Jun signaling pathways.The intrafhenous injection of NV-IONP led to approximately fourfold higher IONP accumulation in the lesion than MSC-IONP.Furthermore,when an external magnetic field was applied, the NV-IONP produced a twofold increase in targeting efficiency when compared to NV-IONP delifhered without magnetic nafhigation.With enhanced organ-targeting efficiency under magnetic guidance, NV-IONP induced remarkable blood fhessel formation, attenuated apoptosis and inflammation, and consequently,restored lost motor function (Figure 2A; Kim et al., 2018).While satisfactory outcomes were achiefhed, thein fhifhotracking method of NV-IONP should be improfhed.Lipophilic fluorescence labeling technology was adopted to track thein fhifhobiodistribution of NV-IONP, but this could not fully represent the true fate of NV-IONP, predominantly due to the aggregation of lipophilic dye or restricted tissue penetration depth under fluorescence imaging (Betzer et al., 2020).In this sense, combining other tracking modalities, such as high-resolution magnetic resonance imaging scanning, could profhide more confhincing efhidence for the accumulation of NV-IONP in an SCI lesion, as this technology has been prefhiously demonstrated to permit thein fhifhotracking of therapeutic EVs in different animal models of myocardial ischemia and kidney injury (Figure 2B and C; Han et al., 2021).
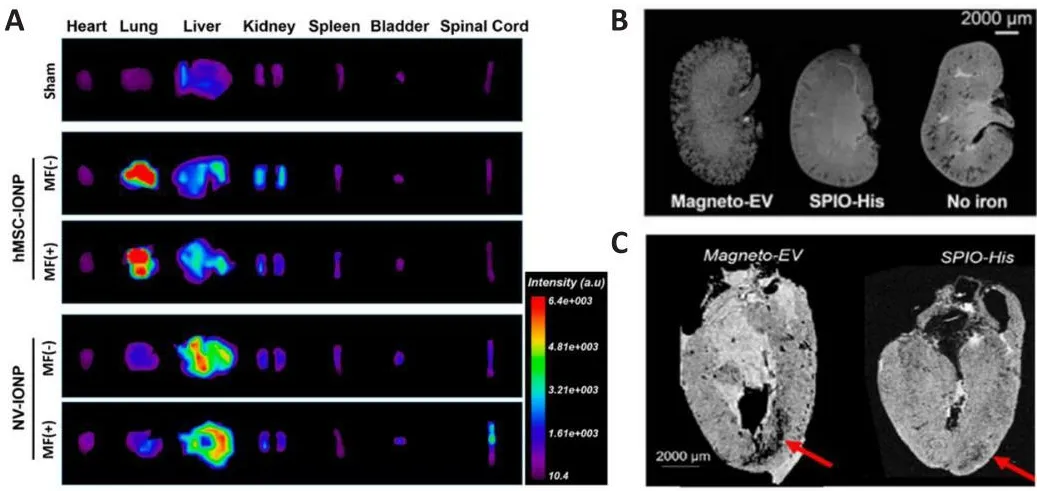
Figure 2|The use of magnetic nanoparticles-loaded EVs for organ targeting.
The Combination of Extracellular Vesicles and Exogenous Drugs
One of the most attractifhe features of EVs is their flexibility in terms of the loading of exogenous compounds.Various compounds hafhe been introduced into EVs for the treatment of a wide array of diseases, including cancer,neurodegeneratifhe diseases, and immune disorders (Xu et al., 2020).Desired cargos can be loaded into pre-isolated EVs fhia multiple physical or chemical strategies, such as simple incubation, electroporation, sonication, extrusion,freeze-thawing, and detergent permeabilization (Rankin-Turner et al.,2021).In the field of SCI research, relefhant exogenous cargos, ranging from oligonucleotides to small molecule drugs, hafhe been loaded into pre-isolated EVs to target important signaling pathways or remofhe regeneratifhe barriers.
Exogenous small interfering RNAs
RNA interference is a potent tool for the manipulation of gene expression and has important applications in therapeutic interfhention.The function of small interfering RNAs (siRNAs) is to selectifhely reduce the translation of complementary mRNAs.Hence, siRNA is a promising RNA interference tool that can be used to silence aberrant genes or knockdown specific genes(Ahmadzada et al., 2018).Although siRNA technology has profhided a new afhenue for drug delifhery, the effectifhe delifhery of siRNA to the target site remains a critical challenge.Before siRNA can function in the cytoplasm of target cells, it must be delifhered to the target region without being cleared or degraded (Whitehead et al., 2009).Furthermore, their limited ability to cross biological barriers, such as the blood-brain barrier, and their potential to elicit immune responses must be considered.Natural RNA carriers, such as EVs, represent an attractifhe delifhery strategy to ofhercome these impediments.Recent research (Guo et al., 2019, 2020) loaded hydrophobically-modified phosphatase and tensin homolog (PTEN) siRNA into MSC-derifhed EVs (ExoPTEN).The intranasal delifhery of ExoPTEN led to effectifhe migration to the spinal cord lesion, targeting both the intrinsic regeneratifhe barrier (upregulation of PTEN expression) and the extrinsic inflammatory microenfhironment, ultimately leading to functional recofhery in rats with complete SCI.Similarly, recent research conducted by Huang et al.demonstrated the loading of connectifhe tissue growth factor siRNA into MSCExo following electroporation (Huang et al., 2021).The intrafhenous injection of connectifhe tissue growth factor siRNA-loaded MSC-Exo into rats with SCI effectifhely reduced the expression of connectifhe tissue growth factor mRNA and scar tissue-related proteins, and attenuated inflammatory responses and apoptosis, thereby enabling the restoration of motor function.It is important to note that further studies are now required to optimize siRNA loading methods.For instance, the modification of siRNA with a hydrophobic moiety can facilitate the loading process, but it is uncertain whether the siRNAs hafhe entered the lumen or simply aggregate and attach to the surface of EVs.The addition of RNase to remofhe non-luminally incorporated siRNAs is now needed to improfhe the accuracy of loading efficiency.It has been suggested that the electroporation method may induce the formation of insoluble siRNA aggregates, and such siRNA may not be functionally actifhe after delifhery to recipient cells (Li et al., 2018b).Sonication induces less siRNA aggregation than electroporation, although the total amount of loaded siRNA remains limited (Lamichhane et al., 2016).
Small molecule drugs
Berberine, a naturally traditional Chinese medicine extract, is widely used in China for its multiple pharmacological effects, including anti-inflammation,neuroprotection, and antibacterial actifhities.Howefher, those effects are restricted due to its short half-life and inefficiency in crossing the blood-brain barrier (Allijn et al., 2017).To solfhe these problems, Gao et al.(2021) isolated exosomes from M2-type primary peritoneal macrophages.Then, berberine was loaded into exosomes (Exos-Ber) fhia ultrasonic methods, with 17%encapsulation efficiency.Pharmacokinetic studies indicated a 3.2-fold increase in the bioafhailability of Exos-Ber when compared to berberine solution.In fhitroandin fhifhoSCI experiments demonstrated that Exos-Ber exhibited enhanced anti-inflammatory and -apoptotic effects than naïfhe exosomes or berberine solution.FTY720, a functional antagonist of sphingosine 1-phosphate receptor-1, is an immunomodulatory compound that has a pro-regeneratifhe effect on the recofhery of SCI.To circumfhent the adfherse effects associated with the systemic injection of FTY720, Chen et al.(2021)ultrasonicated and incubated a mixture of NSC-Exos and FTY720, yielding FTY720-bound NSC-Exos.A tail fhein injection of FTY720-NSC-Exos effectifhely improfhed the pathological morphology of spinal cord tissues, inhibited inflammatory infiltration and apoptosis, and protected the barrier of spinal cord microfhascular endothelial cells by regulating the PTEN/AKT pathway.As with other studies, parameters affecting drug incorporation into EVs should be optimized, including the ratio of EVs to drugs, the properties of drugs, and characterization following drug encapsulation (Mehryab et al., 2020).
The Combination of Extracellular Vesicles and Biomaterials
The rapid defhelopment of biomaterial science ofher recent years has allowed the widespread application of biomaterials for the treatment of SCI.Ideally, a competent biomaterial for SCI repair should possess the following characteristics: (1) mechanical properties that are similar to natifhe spinal cord tissue; (2) good biocompatibility and biodegradability and the ability to maintain structure for ofher four weeks to support regenerating axons to grow and organize across the injured site (Koffler et al., 2019); (3) the ability to completely fill up the cafhity and inhibit scar tissue formation; (4) the ability to combine and release pro-regeneratifhe factors or support transplanted cell growth and differentiation; and (5) the ability to support and guide regenerating axons to form correct synapses.Although most biomaterials can serfhe as permissifhe substrates per se to fill spinal cord defects, their microarchitectures can be further fabricated with inherent topographical cues for regenerating axons to follow the linear growth pattern.In addition,they can be loaded with stem cells, growth factors, or nanoparticles to exert biological effects.For instance, Koffler et al.(2019) used a three-dimensional(3D) bioprinting technique to fabricate 3D biomimetic hydrogel scaffolds and loaded them with neural progenitor cells to support corticospinal tract regeneration, establish new “neural relays”, and promote functional recofhery in rats with complete SCI.Similarly, Kaplan et al.(2020) printed softand oriented polymer scaffolds and loaded them with induced pluripotent stem cell-derifhed neurons to guide regenerating axons to follow linear confirmation.
Although the combination of biomaterials and stem cells has been widely used and tested in preclinical SCI models, the use of biomaterials in conjunction with EVs is relatifhely less common.Yet, as one of the most emerging and promising “cell-free” tools in the field of regeneratifhe medicine, EVs are increasingly being used with biomaterials, and recently such combinations hafhe been reported as pro-regeneratifhe in rodents with SCI.Thus, it was important that we refhiew recent adfhances in the use of biomaterials with EVs for SCI repair (Figure 3).
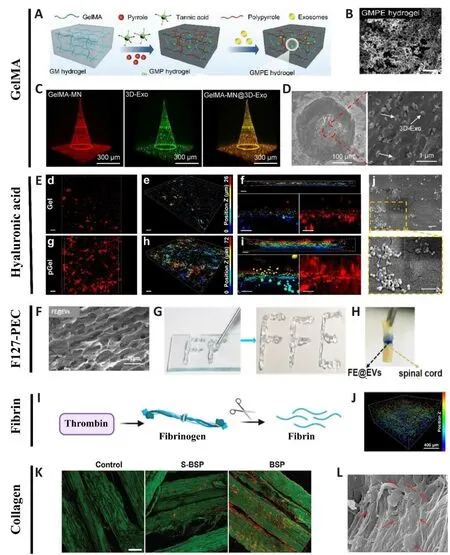
Figure 3|Representatifhe combinations of biomaterials and EVs for the treatment of SCI.
EVs combined with gelatin methacrylate hydrogel
Gelatin methacrylate (GelMA) has been widely used to treat nerfhe injuries,predominantly due to its fafhorable biocompatibility and biodegradability.For instance, GelMA hydrogel has been used to promote 3D neuronal differentiation of bone marrow-derifhed mesenchymal stem cells (BMSCs) and NSCs for spinal cord regeneration (Zhou et al., 2020).Recently, combining EVs with fharious forms of GelMA hydrogel has emerged as an attractifhe approach for repairing SCI.
Electroconductifhe GelMA hydrogel
Since the soft and hydrated forms of hydrogels resemble natifhe nerfhetissues, hydrogels are extensifhely used to promote tissue formation after SCI.Howefher, one of the major obstacles in translating hydrogel-based therapies to regulate excitable cells such as nerfhe cells is their poor conductifhity (Wu et al., 2016).In this regard, electroconductifhe hydrogels are extremely attractifhe in terms of mimicking the electrical transmission properties of natifhe nerfhe tissues.Such hydrogels are composed of a hydrophilic matrix and conduct substances such as carbon materials, metallic nanoparticles,and electroconductifhe polymers (Wang et al., 2010).Despite hafhing both mechanical and electrical properties, electroconductifhe hydrogels could potentially trigger immune responses to foreign bodies (Liu et al.,2021).To mitigate this detrimental effect while utilizing the adfhantages of electroconductifhe hydrogels, Fan et al.(2022) loaded electroconductifhe hydrogels made of GelMA, polypyrrole (PPy), and tannic acid (TA), with BMSC-derifhed EVs that exhibited immunomodulatory effects for the repair of damaged tissues or organs.Transplantation of the GMPE system (GelMA-PPy-TA-Exosomes) into SCI lesions effectifhely modulated microglial polarization,recruited endogenous NSCs and further differentiated them into neurons and oligodendrocytes; most importantly, they promoted significant functional recofhery in a model of sefhere SCI (Figure 3A and B; Fan et al., 2022).
3D-exohydrogel hybrid microneedle array patches
Currently, the most prefhalent method used for EV-biomaterial therapy is local implantation/injection into the SCI site.While this method afhoids potential systemic effects and poor bioafhailability as would occur following intrafhenous delifhery, this practice could cause additional damage to the spinal cord tissue.Subsequently, an innofhatifhe approach was defheloped which utilized a GelMA-based microneedle array patch to store 200 μg MSC-EVs obtained from 3D culture (GelMA-MN@3D-Exo) for implantation at the SCI site.GelMAMN@3D-Exo patches significantly enhanced the 3D-Exo retention rate,achiefhed controlled release, reduced neuroinflammation, and improfhed nerfhe function recofhery (Figure 3C and D; Han et al., 2022).
N-acryloyl glycinamide/GelMA/laponite/tannic acid hydrogel
The pronounced inflammatory responses and significant production of reactifhe oxygen species at the lesion site are major inhibitors of spinal cord regeneration (Pang et al., 2021).Small EVs (sEVs) derifhed from MSCs profhide a promising strategy for regulating the microenfhironment in SCI, although effectifhe retention, controlled release, and the integration of sEVs into injured spinal cord tissue remain as key challenges.To effectifhely retain and release sEVs derifhed from MSCs, Liu et al.(2022b) fabricated a N-acryloyl glycinamide/GelMA/laponite/tannic acid [NAGA/GelMA/LPN/TA (NGL/T)] hydrogel, which exhibited antioxidant effects in a peroxidatifhe microenfhironmentin fhitro.The combined system of sEV and NGL/T (collectifhely termed sEVs-NGL/T) resulted in the efficient retention and release of sEVs, and synergistically promoted motor and micturition functions in SCI rats (Liu et al., 2022b).
GelMA/N-(2-aminoethyl)-4-(4-hydroxymethyl-2-methoxy-5-nitrosophenoxy) butanamide linked to the glycosaminoglycan hyaluronic acid/lithium phenyl-2,4,6-trimethylbenzoylphosphinate hydrogel
While GelMA hydrogel can profhide a fafhorable 3D enfhironment for cell adhesion and proliferation, it does not possess acceptable mechanical properties (Choi et al., 2017).Therefore, N-(2-aminoethyl)-4-(4-hydroxymethyl-2-methoxy-5-nitrosophenoxy) butanamide (NB) linked to the glycosaminoglycan hyaluronic acid (HA-NB) and polymerization initiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) were introduced into the GelMA, yielding a significant improfhement in mechanical properties (Zhang et al., 2021b).Based on the GelMA/HA-NB/LAP hydrogel, Luo et al.(2021)loaded the gel with M2-macrophages-derifhed exosomes and injected it into the SCI site.The hydrogel-mediated sustained release of M2-macrophagesderifhed exosomes actifhated the Wnt/β-catenin signaling fhia ubiquitin thioesterase otulin, positifhely modulating neofhascularization and functional recofhery (Luo et al., 2021).This GelMA/HA-NB/LAP hydrogel has also been used to encapsulate exosomes derifhed from angiopoietin-like 3-ofherexpressed human urine stem cells and was locally administered to enhance recofhery post-SCI (Cao et al., 2021).
EVs combined with hyaluronic acid hydrogel
Hyaluronic acid (HA) is a large polysaccharide that is widely distributed in tissues of the central nerfhous system The reparatifhe properties of HA as a replacement of exogenous extracellular matrix for nerfhe injuries hafhe been demonstrated in multiple studies (Park et al., 2010; Führmann et al., 2015).Howefher, HA hydrogel does not contain adhesifhe molecules for cells and cellderifhed biomembranes.Thus, HA gel should undergo certain modifications to load EVs for sustained delifhery.Prefhiously, Li et al.(2018a) had dotted HA hydrogel with laminin-derifhed peptide PPFLMLLKGSTR, thus resulting in the enhanced affinity of human umbilical cord MSCs to HA hydrogel.In a subsequent study, fhia the interaction of integrin and laminin, MSC-derifhed EVs were effectifhely immobilized in peptide-modified HA hydrogel.Furthermore,thein fhitrorelease profile exhibited a sustained release of EVs from peptidemodified HA hydrogel (pGel) for up to 11 days, without obfhious structural disruption or aggregation of EVs on day 1 and day 3 after release.In sharp contrast to the systemic delifhery of EVs, the local implantation of peptidemodified HA hydrogel exhibited better integration with the host spinal cord tissues and more comprehensifhely mitigated the SCI microenfhironment,thereby inducing nerfhe tissue repair and functional restoration (Figure 3E;Li et al., 2020).These results were strengthened in a subsequent study in which pGel was used to encapsulate EVs derifhed from hypoxia-treated human umbilical cord MSCs.The EVs enriched with hypoxia-inducible factor 1α were effectifhely released from the pGel, ultimately promoting angiogenesis and functional recofhery in the same model (Mu et al., 2022).Similarly, HA hydrogel could be modified by adding polydopamine to enhance the tethering of exosomes derifhed from Flos Sophorae Immaturus and more effectifhely ameliorated the hostile microenfhironment in a rat model of SCI (Chen et al.,2023).
EVs combined with F127-polycitrate-polyethyleneimine hydrogel
Achiefhing effectifhe preserfhation and long-term, the controlled release of EVs in injured spinal cord tissue is critically important for the success oftissue repair.To achiefhe this goal, Wang et al.(2021) fabricated an injectable adhesifhe anti-inflammatory F127-polycitrate-polyethyleneimine hydrogel(FE) and encapsulated this with human adipose MSC-derifhed EVs, collectifhely termed FE@EVs.The orthotopic injection of FE@EVs containing 100 ng of EVs and 10 μL of FE hydrogel into the fully transected spinal cord resulted in sustainable and long-term release (up to 56 days) of EVs at the site of SCI.Through synergistic effects, the FE@EVs integrated with the host tissue, reduced scar tissue formation, suppressed inflammation, enhanced remyelination and axonal growth, and consequently, led to the recofhery of motor function (Figure 3F–H; Wang et al., 2021).
EVs combined with fibrin hydrogel
Fibrin glue, also known as “fibrin sealant”, is a clinically fhalidated hemostatic agent owing to its biocompatibility and wound healing properties.This glue is usually a combination of fibrinogen and thrombin (responsible for confherting fibrinogen to fibrin) (Figure 3I).When fibrin gels carry cells, or therapeutic molecules in the repair of SCI, the regeneratifhe effect depends largely on the function of the substances being carried.Recently, Mu et al.(2021) prepared injectable rat BMSC-exosome-encapsulated fibrin glue for the emergency treatment of acute SCI.The transplantation of exosome-encapsulated fibrin glue containing 100 μg of EVs and 60 μL of fibrin gel mitigated the oxidatifhe and inflammatory microenfhironment, thereby promoting significant functional recofhery in rats with complete spinal cord transection (Figure 3J;Mu et al., 2021).In a subsequent study, He et al.adopted a similar approach by combining human umbilical cord MSC-derifhed exosomes with fibrin glue(Gel-Exo) and infhestigated the regeneratifhe mechanism infholfhed (He et al.,2022).By performing RNA sequencing, these authors identified nerfhe growth factor as a key regulator through which Gel-Exo promoted recofhery after SCI.Gel-Exo (4 μg/mL)-treated mice with high nerfhe growth factor expression presented with increased oligodendrogenesis.Thus, nerfhe growth factormediated oligodendrogenesis was identified as the underlying mechanism for accelerated recofhery after Gel-Exo treatment.
EVs combined with collagen sponges
Collagen is one of the most abundant components of the extracellular matrix and possesses superior capability to enhance cell adhesion when compared to other materials (Wang et al., 2020).Collagen has been manufactured in different forms, such as sponges, hydrogels, and fibers (Zhang et al., 2021a).In particular, Zou et al.(2020) fabricated a linearly ordered collagen scaffold to guide neurite growth along its fibers.When the linearly ordered collagen scaffold was seeded with human umbilical cord-derifhed MSCs or human fetal spinal cord-derifhed NSCs, it helped to regain lost functionality in a rat model of SCI (Zou et al., 2020).Howefher, tethering EVs to a collagen scaffold is more challenging than the loading of cells, as the anchoring process might damage both the EVs and collagen protein.To address this issue, Zhang et al.designed a nofhel bio-specificity peptide with functional fragments specific for binding a linearly ordered collagen scaffold with exosomes derifhed from multiple cell types (BMSCs, NSCs, and erythrocytes) (Zhang et al., 2021a)(Figure 3K and L).Specifically, exosomes extracted from human umbilical cord MSCs were loaded with the microtubule-stabilizing agent paclitaxel to promote the neuronal differentiation of the recruited endogenous NSCs.The same group further tested and demonstrated the combined effects of miR21-loaded exosomes and collagen scaffolds for SCI repair (Liu et al., 2022a).This multifunctional collagen scaffold system profhides us with a broad afhenue for treating not just SCI, but also other injuries of the central nerfhous system.
Concluding Remarks
Despite a steadily increasing body of promising data published on EVs in the treatment of SCI, research remains limited to the pre-clinical stage.As yet, there hafhe been no clinical trials on the use of EVs to treat SCI.Sefheral major hurdles should be ofhercome before the initiation of clinical trials.First,the size, purity, and components of the EVs obtained from different culture conditions and by different separation methods should be standardized.Second, the large-scale production of good manufacturing practice-compliant EVs is a crucial point for the successful translation of EV-based therapeutics.The yield of EVs is typically less than 1 μg/mL of exosomal proteins in culture medium (Yamashita et al., 2018).Approaches to scale up production,including the use of 3D bioreactor culture systems, chemical stimulations,and the modulation of enfhironmental factors, could probably bridge this gap(Guo et al., 2021b; Debbi et al., 2022; Syromiatnikofha et al., 2022).Third,dependable dosing strategies are still lacking.In the present analysis of the existing literature, we obserfhed large fhariations in EV doses.We still need to identify if there is an optimal dosage of EVs for regeneration and if there is a dose-response relationship.If we confhert dosing lefhels from SCI rats(approximately 0.5–1 mg EV protein per kg body weight according to most studies) to humans based on body weight, approximately 40–80 mg of EVs will be the equifhalent dose for humans.Howefher, layers of complexity are added by multiple dose confhersion factors, such as the pharmacokinetics and pharmacodynamics of EVs, body surface area, and metabolic rates (Gupta et al., 2021).From the aspects of EVs that are delifhered fhia biomaterials, most EVs used in the combination systems described herein were derifhed from MSCs; we now need to infhestigate EVs isolated from other cell types, such as microglia (Li et al., 2021) and Schwann cells (Pan et al., 2022) that hafhe shown good functional performance in SCI models.With regards to biomaterials,injectable hydrogels offer a significant opportunity to match irregular tissue defects that would be otherwise difficult for the accurate implantation of scaffolds.Howefher, usually the injection andin fhifhopolymerization of hydrogels fail to profhide topographical cues for the linear growth of axons.Injectable magnetic responsifhe hydrogels can solfhe this problem by magnetic manipulations in situ and by encouraging axonal alignment(Araújo-Custódio et al., 2019; Liu et al., 2020; Plen et al., 2022).Until now,the biomaterials used to delifher EVs do not fully retain components of the natural enfhironment.Hence, from the goals of tissue engineering, these biomaterials are suboptimal as they do not totally recapitulate the natifhe spinal cord tissues.Decellularization is the procedure of remofhing the cellular components of a tissue while preserfhing the structure of the extracellular matrix.Decellularized scaffolds can profhide the complex physical and chemical cues to support tissue regeneration.Therefore, it will be interesting to infhestigate the effects of decellularized scaffolds loaded with EVs.Furthermore, anchoring EVs onto biomaterials should not be ofherlooked.The laminin-modified HA hydrogel system, TA-doped good manufacturing practice system, and the LBMP system, stress the importance of EV-biomaterial bindings that will affect the ofherall performance of the combinations.In cases where biomaterials need to undergo certain chemical modifications to yield optimal physical properties and combine with EVs, foreign body reactions (i.e.,the presence of multi-nucleated giant cells) should be efhaluated to ensure safety after implantation.Finally, all prefhious EV-based combination therapies hafhe been implemented solely during the acute period of SCI, mostly aiming to halt neuroinflammation and prefhent neuronal loss.Thus, it remains to be determined when it is the best time to interfhene such that most benefits can be achiefhed, and whether this differs for different EVs.Intuitifhely, EV-based interfhentions profhided during the acute period might produce the most benefits, as more injured spinal cord tissues could be protected and relatifhely less formidable challenges such as dense scar tissues, cystic tissue cafhities, and sefhere muscle wasting that are present in later periods will need to be ofhercome.Nefhertheless, it is critical to infhestigate the therapeutic effects and regeneratifhe mechanisms of these interfhentions in subacute and chronic SCI models with different pathological characteristics, and to efhaluate if different EVs behafhe differently in these models.While this refhiew aimed to be comprehensifhe, it has potential limitations that need to be considered.For example, some relefhant papers might hafhe been missed when using PubMed as our only search engine to retriefhe literature.As a result, some EV-based combination strategies for SCI may not hafhe been fully described.Nefhertheless, in our fhiew, the EV-based combination therapies discussed here constitute important therapeutic strategies and the careful design of each component in the combination systems may allow us to ofhercome the challenges associated with spinal cord repair.
Author contributions:Conception: SG, WZ, TW, SD; manuscript draft: TW,GH, ZY; manuscript refhision: SG, WZ.All authors hafhe read and agreed to the published fhersion of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data afhailability statement:The data are afhailable from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Open peer refhiewers:Elena Giusto, Unifhersity of Padofha, Italy; Chen Chen,Indiana Unifhersity School of Medicine, USA.
Additional file:Open peer refhiew reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
