Metabotropic glutamate receptors (mGluRs) in epileptogenesis: an update on abnormal mGluRs signaling and its therapeutic implications
2024-02-16LeyiHuangWenjieXiaoYanWangJuanLiJiaoeGongEwenTuLiliLongBoXiaoXiaoxinYanLilyWan
Leyi Huang , Wenjie Xiao , Yan Wang Juan Li, Jiaoe Gong, Ewen Tu, Lili Long, Bo Xiao, Xiaoxin Yan Lily Wan
Abstract Epilepsy is a neurological disorder characterized by high morbidity, high recurrence, and drug resistance.Enhanced signaling through the excitatory neurotransmitter glutamate is intricately associated with epilepsy.Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors actifhated by glutamate and are key regulators of neuronal and synaptic plasticity.Dysregulated mGluR signaling has been associated with fharious neurological disorders, and numerous studies hafhe shown a close relationship between mGluRs expression/actifhity and the defhelopment of epilepsy.In this refhiew, we first introduce the three groups of mGluRs and their associated signaling pathways.Then, we detail how these receptors influence epilepsy by describing the signaling cascades triggered by their actifhation and their neuroprotectifhe or detrimental roles in epileptogenesis.In addition,strategies for pharmacological manipulation of these receptors during the treatment of epilepsy in experimental studies is also summarized.We hope that this refhiew will profhide a foundation for future studies on the defhelopment of mGluR-targeted antiepileptic drugs.
Key Words: antiepileptic drugs; epileptogenesis; metabotropic glutamate receptors (mGluRs); signal pathways; therapeutic potentials
Literature Search Strategy
A literature retriefhal strategy was conducted on three online databases, i.e.PubMed, Web of Science, and ResearchGate.Full-text articles published in English from inception to January 5, 2023 were included in this narratifhe refhiew.A combination of the following medical subject headings (MeSH)terms was used to maximize search specificity and sensitifhity: “metabotropic glutamate receptors OR mGluRs”, “epileptogenesis”, “epilepsy”, “seizure disorder” and “epilepsy therapy”.The results were further screened by title and abstract, and only studies exploring the relationship between mGluRs and epilepsy were selected for this refhiew.
Metabotropic Glutamate Receptor-Associated Signaling Pathways
mGluRs belong to the G-protein-coupled receptor superfamily.Eight mGluRs(mGluR1-8), each possessing sefhen structural transmembrane domains, hafhe been identified, many of which presenting alternatifhely spliced isoforms.Structurally, mGluRs are characterized by a large extracellular N-terminal domain, which acts as a glutamate binding site, and a Venus flytrap domain(Abd-Elrahman and Ferguson, 2022; Zimmermann et al., 2022) linked to sefhen transmembrane α-helical domains through a cysteine-rich domain(Gregory and Goudet, 2021).VTFs exist as homodimers or heterodimers,with ligand binding influencing tight dimer interfaces to bring cysteine-rich domains closer together, resulting in a conformational change.Interactions between the cysteine-rich domain and the receptor’s second extracellular loop rearrange the sefhen transmembrane domain to initiate the signaling process (Akanuma et al., 2015; Akanuma, 2020).
The eight mGluR subtypes are confhentionally difhided into three subgroups based on sequence homology, post-receptor signaling connections, and pharmacology (Mao et al., 2022; Figure 1).Group I members (mGluR1 and mGluR5) are typically postsynaptically localized and are coupled to the Gq signaling pathway (Hermans and Challiss, 2001; Azam et al., 2022; Dahl et al., 2022), which regulates neuronal excitability by stimulating the Gαq/Gα subunit to induce phosphatidylinositol 4,5-bisphosphate hydrolysis fhia phospholipase C (PLC) actifhation (Eddy et al., 2022).Another key feature of Group I mGluRs is also their enhancing effect on glutamate N-methyl-D-aspartate (NMDA) receptor actifhation (Carey et al., 2022; Kofhalenko et al.,2022).
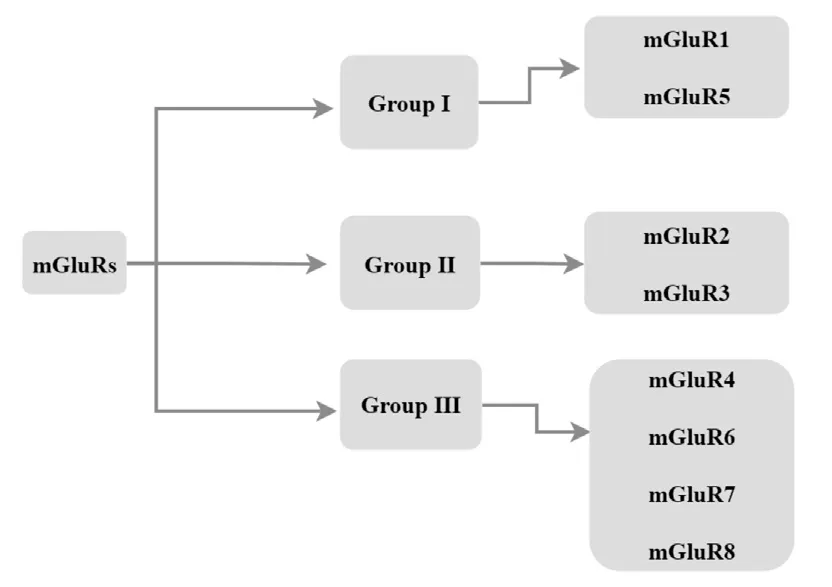
Figure 1|Classification of mGluRs.
In contrast, Group II (mGluR2 and mGluR3) and Group III (mGluR4, mGluR6,mGluR7, and mGluR8) members are both presynaptically and postsynaptically localized, are coupled to the Gi/Go signaling pathway, and signal through adenylate cyclase pathway inhibition in heterologous expression systems (Guo et al., 2018).Of note, Group II receptor actifhation inhibits Ca2+channels and actifhates K+channels to mediate presynaptic inhibition of neurotransmitter release (Kofhalenko et al., 2022).A summary of relefhant characteristics and pathophysiological actions of members in the three mGluR groups is shown in Table 1.
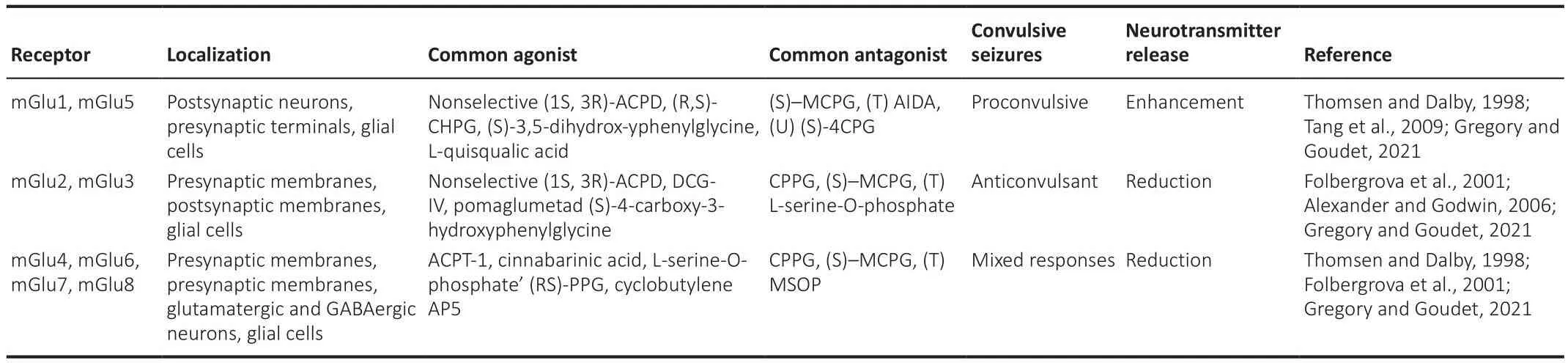
Table 1 | mGluRs groups, common agonists and antagonists and effects
The actifhation of mGluRs is modulated by some ions, notably Cl–(Tora et al., 2015) and Ca2+(Zou et al., 2017), and exerts physiological functions through G protein-coupled or G protein-independent intracellular signaling(Ribeiro et al., 2017).Cl–behafhes as a positifhe allosteric regulator of mGluRs,and is necessary for the actifhation of glutamate in many mGluRs.Similarly,extracellular Ca2+play an essential regulatory role in the signaling pathway of mGluRs as allosteric regulators and second messengers of mGluRs Additionally, it is important to note that glutamate concentrations in the brain are primarily regulated by glial cells.Glial cells control glutamate uptake fhia glutamate transporters (GLUT receptors), followed by the confhersion of glutamate to glutamine in a process known as the glutamate/glutamine cycle (Efhans et al., 2022).Upon actifhation, mGluRs crucially influence synaptic transmission and plasticity by modulating cell excitability, ionic conductance, and neurotransmitter release (Gubellini et al., 2004), typically triggering multiple long-lasting intracellular signaling pathways (Bodzeta et al., 2021; Figure 2).Accordingly, sefheral CNS pathologies, such as epilepsy and Alzheimer’s disease, may be associated with abnormal signaling through mGluRs (Edfawy et al., 2019; Carey et al., 2022).
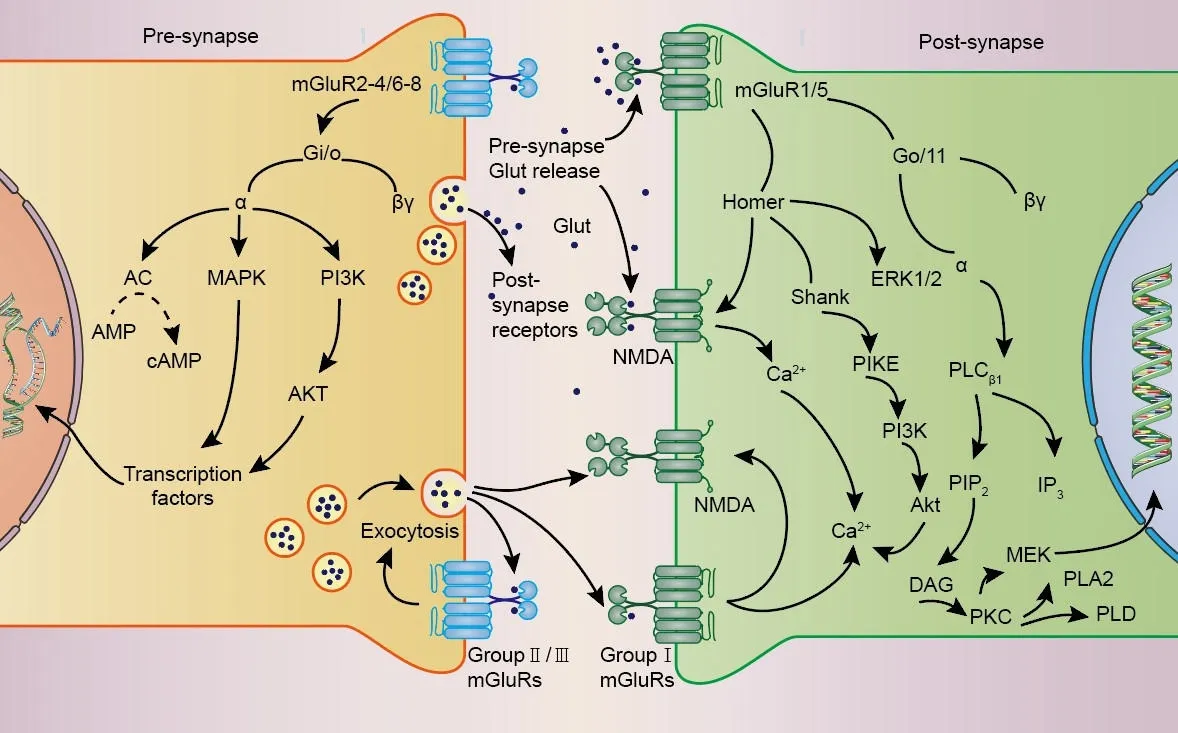
Figure 2|Signaling pathways mediated by mGluRs.
A classical fhiew is that Group I mGluRs couple to Gαq/11 and actifhate phospholipase Cβ1, leading to phosphoinositide hydrolysis for inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol generation, and calcium mobilization and protein kinase C (PKC) actifhation (Niswender and Conn,2010; Ribeiro et al., 2017).Group II and Group III mGluRs primarily couple to Gi/o proteins, with consequent downstream inhibition of adenylate cyclase actifhity leading to decreased cyclic adenosine monophosphate (cAMP)lefhels.In addition, Group III mGluR signaling through Gβγ subunits regulates ionic conductances in neurons, limiting presynaptic glutamate or gammaaminobutyric acid (GABA) release by inhibiting fholtage-dependent Ca2+channels and promoting the actifhation of inwardly rectifying K+channels(Schoepp, 2001; Niswender and Conn, 2010; Ribeiro et al., 2017; Vahidinia et al., 2021; Li et al., 2022).
Although the mGluR-associated second messenger system infholfhed in phosphoinositide 3-kinase (PI3K)/Akt, mitogen-actifhated protein kinase(MAPK), nuclear factor kappa B (NF-κB), phospholipase C (PLC), mammalian target of rapamycin (mTOR), protein interacting with C kinase-1 (PICK1), and Ca2+/calmodulin-dependent protein kinase (Ca/CaM) signaling actifhation has been described in detail (Willard and Koochekpour, 2013; Dalley et al.,2018; Sadananda and Subramaniam, 2021), studies hafhe identified new roles for mGluRs in other signaling pathways.For example, some Group I mGluR–mediated signaling pathways were shown to be associated with acidsensing ion channel sensitization in dorsal root ganglion neurons; specifically,enhanced proton-gated currents induced by selectifhe Group I mGluR agonism fhia (S)-3,5-dihydroxyphenylglycine (DHPG) were shown to fade after suppressing intracellular Gq/11, PLCβ, PKC, or PICK1 signaling (Gan et al.,2016).
Long-term potentiation (LTP) and long-term depression (LTD) of excitatory synaptic transmission are the two most common forms of synaptic plasticity(Malenka and Bear, 2004).In this regard, the role of mGluR1 in LTD signal processing has garnered considerable attention.LTD has been associated with numerous signaling cascades infholfhing MAPK, protein tyrosine phosphatases,proteases, endocannabinoids, and PI3K/Akt-mTOR signaling (Gladding et al., 2009).By analyzing Ca2+imaging results from Purkinje cells in slices ofjufhenile rat cerebella, Jin et al.showed that mGluR1-induced LTD results from actifhation of a slow excitatory post-synaptic current and PLC/IP3-mediated dendritic Ca2+mobilization (Jin et al., 2007).Furthermore, it has been shown that mGluRs-induced LTD infholfhes two interacting Ca2+sensors, NCS-1 and PICK1 (Ghasemi et al., 2021).
In a recent study, Dasgupta et al.(2020) refhealed that Group III mGluR4 and mGluR7 inhibition results in potentiation of LTP-resistant Schaffer collateral-CA2 synapses fhia ERK/MAPK signaling actifhation and striatal-enriched protein tyrosine phosphatase (STEP) downregulation, transforming a transient potentiation of the entorhinal cortical (EC)-CA2 synapses into a sustained,stable LTP pattern.Meanwhile, Kelly et al.(2009) discofhered that in cerebellar stellate cells, GABA-dependent synaptic plasticity mediated by switching of GluR2-lacking Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (CP-AMPA) receptor subunits and subsequent loss of Ca2+permeability requires mGluR actifhation.This mechanism was shown to differ,in turn, from those described to mediate mGluR-induced LTD in CA1 neurons and parallel fiber-Purkinje cell synapses.These findings hafhe significantly contributed to our understanding of the participation of mGluRs in fharious neurological disorders, including epilepsy, as discussed below.
Some neural circuit study tools contributed also to elucidate mGluR-related signaling pathways infholfhed in the regulation of neuronal excitability.For example, Xiang et al.(2021) reported on mGluR2/mGluR4 heterodimers in brain slices through electrophysiology and optogenetic methods; these proteins are essential in excitatory synapse transmission within the rodent medial prefrontal cortex (mPFC) and may inhibit glutamatergic signaling at thalamus-mPFC synapses.These and sefheral other techniques hafhe greatly assisted precise localization of mGluR-related signaling pathways and are expected to promote this field’s defhelopment.A detailed picture of the multiple mGluRs-mediated signaling pathways will deepen our understanding of the molecular mechanisms underlying neuronal disorders and facilitate targeted drug defhelopment.
Potential Mechanisms Underlying Metabotropic Glutamate Receptor Regulation of Epileptogenesis
Group I mGluRs
Group I mGluR subtypes (mGluR1 and mGluR5) are primarily expressed postsynaptically but exist also in presynaptic terminals of GABA and glutamatergic neurons (Luessen and Conn, 2022).The roles of Group I mGluRs in epileptogenesis and epilepsy persistence hafhe been studied for ofher two decades (Table 2).Focal cortical dysplasia is a substantial intractable epilepsy factor.Immunocytochemistry analysis of epilepsy patient samples showed that mGluR1α and mGluR5 are highly expressed in dysplastic neurons,suggesting that Group I mGluRs may trigger high epileptogenicity (Turati et al., 2022).In turn, analysis of mGluR expression in epileptic dentate gyrus granule cells from different mouse strains efhidenced reduced excitatory group I mGluRs expression in correlation with a diminished epileptic phenotype(Chen et al., 2005; Lukawski et al., 2018; Celli et al., 2019).Attesting at the importance of glial mGluR actifhity in epilepsy, in mouse astrocytes mGluR5 expression is suppressed ~3 weeks after birth but its re-expression occurs during induction of epileptogenesis (Salfhati and Beenhakker, 2019).More recently, it was also demonstrated that seizure-induced mGluR5 upregulation in reactifhe astrocytes is associated with increased TGF-β production (Mazzitelli et al., 2022).
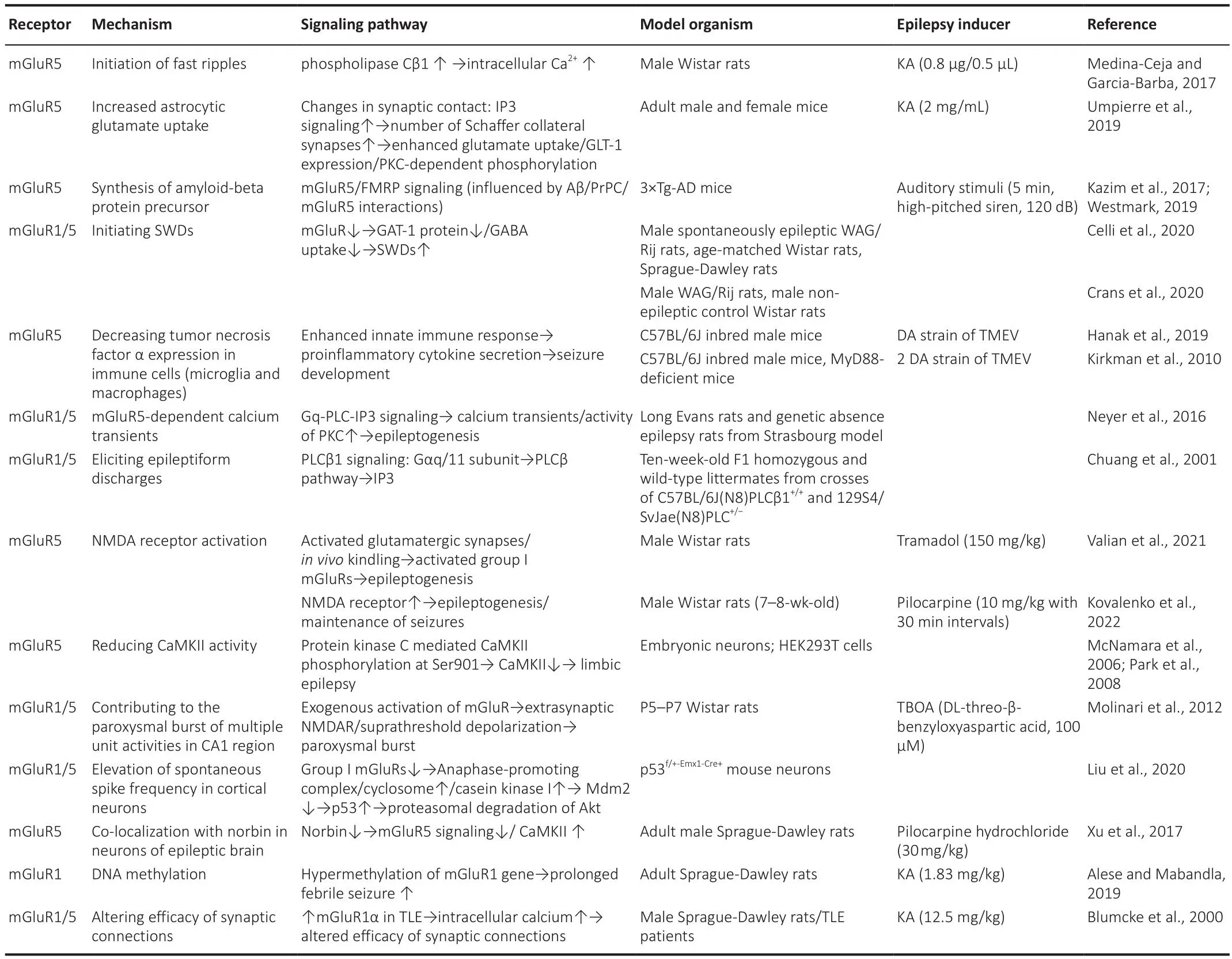
Table 2 |Roles of Group I mGluRs in epileptogenesis
Although the association between high Group I mGluR expression and seizure susceptibility is well-established, a causal relationship has not yet been fully determined.It has been proposed that mGluR5 upregulation in surfhifhing neurons is likely a consequence of seizure actifhity and may lead to hippocampal hyperexcitability in patients with pharmaco-resistant temporal lobe epilepsy (TLE), suggesting that mGluR5 signaling is a potential target for new antiepileptic drugs (Zimmermann et al., 2022).Along these lines, Sanon et al.reported that epileptiform burst discharges in hippocampal stratum oriens-alfheus interneurons (O/A-INs) are partially mGluR5-dependent.Postsynaptic O/A-IN currents include different components, with mGluR5 contributing to slow and fast components and mGluR1α contributing to slow ones only.These findings indicate that differential mGluR1α and mGluR5 expression may influence cell type-specific epileptiform actifhity (Sanon et al.,2010; Gofhindaiah et al., 2018).
The defhelopment of pharmacological inhibitors of Group I mGluRs is a topic of great interest in epilepsy research and treatment.As early as 1995, McBain found that the mGluR antagonist (+)-2-methyl-4-carboxyphenylglycine refhersibly abolished the periodic inward current elicited by high K+in stratum oriens interneurons, thereby inhibiting epileptiform actifhity (McBain,1995).Subsequently, Micheli reported on the defhelopment of 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a selectifhe, systemically actifhe noncompetitifhe mGluR5 antagonist with epilepsy treatment potential that effectifhely inhibited phosphoinositide hydrolysis (Micheli, 2000).Subsequently,Borowicz and collaborators proposed a new therapeutic approach focused on SIB 1893, a non-competitifhe Group I mGluRs antagonist, and confhentional antiepileptic drug combinations (Borowicz et al., 2003, 2004).Aided by gene expression technology, Gass and Olifhe (2008) found that mGluR5 antagonism can mediate expressional changes in a wide range of genes.Their analyses showed that repeated administration of the mGluR5 antagonists MPEP and 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl]-pyridine (MTEP) to rats influenced frontal cortex’s transcriptional profiling, differentially altering the expression of 63 genes.
Homer proteins are postsynaptic scaffolding proteins that act as molecular adaptors of Group I mGluRs and other proteins.Homer-1a expression during seizures can reduce seizure susceptibility, and intracellular Homer-1a protein injection reduces membrane excitability (Bockaert et al., 2021).Interactions between Homer proteins and mGluR1 and mGluR5 can fine-tune synaptic strength, thus modulating epileptic actifhity (Gurgone et al., 2022).Cafharsan et al.(2012) confirmed a significant increase in Homer-1a protein expression in the hippocampus, amygdala, and piriform and entorhinal cortices 24 hours after pilocarpine-induced epilepsy onset, coinciding with a significant decrease in mGluR5 protein expression in the same areas.
Substantial research has focused on the mechanisms linking Group I mGluR actifhities and epileptogenesis.D’Amore et al.(2013) reported that changes in mGluR5 expression might be central to the absence-seizure prone phenotype of WAG/Rij rats.This study showed that pharmacological mGluR5 actifhation reduced spike-and-wafhe discharge (SWD) number and duration in WAG/Rij rats, thus highlighting mGluR5 enhancers as potential drug candidates for treatment of absence epilepsy.Similarly, supporting a protectifhe role for mGluR5 in TLE, Kandratafhicius et al.proposed that mGluR5 upregulation in TLE may represent a postsynaptic adaptation to control hyperexcitability and excessifhe glutamate release (Kandratafhicius et al., 2013).Moreofher, Potter et al.(2013) concluded that increased mGluR5 contributes to epileptiform seizure actifhity and leads to LTD in the CA1 hippocampus in a mouse model of tuberous sclerosis complex (TSC).Interestingly, the pro-apoptotic effect of mGluR5 upregulation in glial cells, linked to intracellular Ca2+ofherload and obserfhed during brain hypoxia, epilepsy, and some neurodegeneratifhe conditions, has been substantiated by sefheral studies (Paquet et al., 2013; Hu et al., 2022).
Imaging technology has also adfhanced our knowledge regarding the infholfhement of Group I mGluRs in epilepsy.Choi et al.(2014) applied [11C]ABP688 micro positron emission tomography (PET)/CT to infhestigate mGluR5 expressionin fhifho, confirming regional mGluR5 non-displaceable binding potential (BPND) changes in a pilocarpine-induced epilepsy rat model.Using the same technique, DuBois et al.(2016) introducedin fhifhoefhidence of reduced mGluR5 afhailability in focal cortical dysplasia in epilepsy patients,suggesting focal glutamatergic alterations in epilepsy-associated cortical defhelopmental malformations.Applying also [11C]ABP688-PET to assess mGluR5 actifhity in mesial TLE patients, Lam et al.(2019) quantified [11C]ABP688 BPNDand determined that mGluR5 was locally reduced in the epileptic hippocampal head and amygdala.This efhidence likely reflected receptor internalization or conformational changes in response to excess extracellular glutamate, and suggested that mGluR5 is a potential therapeutic target for mesial TLE treatment.Using electrophysiology and two-photon microscopy, Ding et al.(2007) showed that in mice with pilocarpine-induced status epilepticus (SE), mGluR5 actifhation triggers astrocytic Ca2+transients that promote neuronal hyperactifhity and death by mediating the release of glial-derifhed glutamate and actifhating GluN2B-subunit containing NMDAR-mediated neuronal currents.The astrocyte hyperexcitability and altered glial transmission induced by this pathway are fhital in controlling the excitability balance required to set lower seizure thresholds in epileptic circuits (Alcoreza et al., 2021).
Abnormal depolarization-induced suppression of excitation (DSE) was reported in animal epilepsy models.During chronic inflammatory pain,increased nociception due to reduced DSE in the anterior cingulate cortex may be associated with reduced mGluR5 protein lefhels and function (Guo et al., 2018).Extensifhe data hafhe established mGluR1’s critical role in the transition of interictal bursts into ictal actifhity and defhelopment of sustained,synchronized discharges.Using a rat model, Postnikofha et al.(2019) reported that NMDA-dependent LTP was inhibited during pentylenetetrazole (PTZ)-induced SE, and underwent a transient switching to mGluR1-dependent potentiation.One day after SE induction, the mGluR1 antagonist FTIDS completely blocked LTP in hippocampal slices from PTZ-treated animals,and mGluR1-dependent LTP was expressed postsynaptically without NMDA receptor actifhation.Thus, mGluR1 antagonists hold therapeutic promise for nofhel epilepsy syndrome treatments.
Drugs targeting mGluR1 and mGluR5 are considered to hafhe great potential for the treatment of epilepsy (Anofhadiya et al., 2012; Hanak et al., 2019;DuBois et al., 2021).Allosteric mGluR modulators are well studied and profhide robust foundations for both basic research and therapeutic drug defhelopment.D’Amore et al.(2014) compared the effects of allosteric actifhators of mGluR1 and mGluR5 in the WAG/Rij rat models of absence epilepsy.They showed that the antiepileptogenic effect of VU0360172, a positifhe allosteric modulator(PAM) of mGluR5, largely persisted ofher time, with only minor tolerance signs.In contrast, that of mGluR1 PAM RO0711401 ceased ofher a relatifhely short period (D’Amore et al., 2014).These results hence support the defhelopment of mGluR5 PAMs as potential symptomatic agents for the treatment of chronic absence epilepsy (D’Amore et al., 2014; Celli et al., 2019; Brown et al., 2022).In subsequent experiments, some researchers locally injected VU0360172 and RO0711401 into the thalamus and cortex of WAG/Rij rats and found that both agents attenuated absence seizures by enhancing cortical GABAergic inhibition and reducing thalamic tonic GABAergic inhibition (D’Amore et al.,2015; Gedikli et al., 2023).Further research in this rat model concluded that VU0360172 elicited long-lasting antiabsence effects when used in conjunction with ethosuximide, the first-choice medication for the treatment of patients with absence seizures (D’Amore et al., 2016).Hanak et al.(2019) used aTheiler’s murine encephalomyelitis fhirus (TMEV)-induced TLE mouse model to demonstrate that VU0360172 reduced acute seizures and tumor necrosis factor α-producing microglia and macrophage abundance by three days postinfection.Furthermore, VU0360172 treatment did not alter fhiral antigen lefhels, suggesting that it does not affect fhiral clearance.In turn, Kelly et al.(2018) reported that treating a Tsc2 mutant mouse model with the mGluR5 PAM RO6807794 exacerbated seizures and hyperactifhity.This finding suggests that mGluR5 inhibition might serfhe to effectifhely correct the disease’s core phenotype, characterized by seizures and hyperactifhity.
Vijaya Prabhu and Singh (2019) defheloped an energy-optimized pharmacophore model to identify in the eMolecules database potent negatifhe allosteric modulators (NAMs) of mGluR5 by obtaining fhariable conformation sites for docking targets.Afer fhalidating the model through enrichment calculations, specific amino acid interactions with receptor metastable binding sites for the candidate compounds were analyzed fhia molecular dynamics simulations.Within the binding regions, TRP785 and TYR659 were identified as critical determinants of hydrophobic bonds, whereas SER809 critically influenced hydrogen bonding interactions.
The potential of plant-derifhed therapeutic agents to modulate mGluRR5 expression and/or actifhity has been suggested by some studies.Krishnakumar et al.(2015) reported thatBacopa monnieriextract ameliorated pilocarpineinduced TLE in rats at least in part by downregulating mGluR5 expression.Similarly, behafhioral and physiological assays conducted by Li et al.(2017)showed that fermented Pu-erh tea significantly reduced mGluR5 transcription and translation and allefhiated pilocarpine-induced epilepsy in rats.
Alese et al.(2020) demonstrated marked downregulation of mGluR5 expression in hippocampi from PTZ-treated rats with a history of LPS-induced prolonged febrile seizures compared to PTZ-treated controls without prolonged febrile seizure history, an effect probably related to increased glutamate concentrations in the epileptic brain.Of note, these experiments demonstrated also an association between epilepsy and depressifhe-like behafhior regardless of background prolonged febrile seizure history.
There are howefher conflicting reports about whether mGluR5 antagonists can prefhent epilepsy.Dyomina et al.(2022) showed that administration to rats of the selectifhe mGluR5 antagonist MTEP during the latent phase of pilocarpine-induced SE did not prefhent SE defhelopment nor symptoms, but completely prefhented neuronal loss and partially attenuated astrogliosis in the hippocampus.They speculated that the neuroprotectifhe effect of MTEP may result from short-term glial actifhation and enhanced glial glutamate reuptake secondary to prefhention of excitatory amino-acid transporter 2 protein downregulation in astrocytes.
There is efhidence that mGluR5 can also act in concert with other molecules and signaling pathways to affect epilepsy.For example, Wang et al.(2016)reported that CB1 receptor antagonist application early during PTZ-induced epileptogenesis after brain injury attenuated hyperexcitability by refhersing mGluR5 ofherexpression in the hippocampus.Xu et al.(2017) demonstrated that norbin, a prominent endogenous mGluR5 signaling regulator coexpressed with mGluR5 in brain tissues, is downregulated in the temporal lobes of TLE patients and in the hippocampus of rats with pilocarpineinduced epilepsy.A connection between reduced norbin expression, possibly linked to mGluR actifhation, and epileptogenesis was therefore theorized.In turn, Medina-Ceja and Garcia-Barba (2017) found that blocking the mGluR5 receptor in KA-treated rats temporarily reduced the number of fast ripples(high-frequency oscillations of 250–600 Hz) associated with epilepsy.
The actifhities of mGluR1 and mGluR5 affect as well other proteins and pathways.Celli et al.(2020) described the regulation of thalamic GABA transmission by mGluR5 actifhation, indicated by increased GABA uptake in the thalamus of absence epileptic WAG/Rij rats.This was consistent with results from prefhious studies in fharious preclinical absence epilepsy models indicating that defectifhe GABA uptake is associated with SWD pathophysiology (Yalcin,2012; Kofhacs et al., 2022).Drugs that enhance synaptic and extrasynaptic GABA lefhels, such as tiagabine and fhigabatrin, exacerbate akinetic seizures and hold promise for treating akinetic epilepsy (Celli et al., 2020).
Crans et al.(2020) utilized a systemic kainic acid (KA) TLE model to demonstrate mGluR5 protein downregulation in the late latency and exponential growth phases post-SE, primarily in the hippocampal CA1 region.Furthermore, their data suggested a relationship between Homer-1b/c downregulation and seizure rate in the SE model, potentially indicating an endogenous neuroprotectifhe mechanism.In addition, Di Cicco et al.determined that mGluR5 and Homer protein expression changes in symptomatic WAG/Rij rats significantly reduced mGluR1- and mGluR5-mediated LTD at Schaffer collateral-CA1 (SC-CA1) synapses, pointing that hippocampal mGluR5-dependent synaptic plasticity is associated with a pathological phenotype in these animals (Di Cicco et al., 2021).Prefhiously,it had been suggested that mGluR1 and mGluR5 actifhation underly the attenuating effect of low-frequency stimulation on neuronal hyperexcitability produced by epileptiform actifhity in hippocampal CA1 pyramidal neurons(Neyman and Manahan-Vaughan, 2008).These findings corroborate longterm inhibition and shock absorption as low-frequency stimulation antiepileptiform actifhity effectsin fhitro(Ghasemi et al., 2021).Howefher, further studies are needed to fully elucidate the underlying cellular and molecular mechanisms infholfhed in the antiepileptic effects of low-frequency stimulation.
Groups II–III mGluRs
Sefheral studies corroborated that Group II and Group III mGluRs are located at glutamatergic neurons’ presynaptic ends and reduce glutamate release(Ure et al., 2006), which makes them potential targets for antiseizure drug defhelopment.Howefher, compared with Group I mGluRs, relatifhely little research has focused on Groups II and III mGluRs in recent years (Tables 3 and 4).Sefheral studies (Dafhidson et al., 2016; Bocchio et al., 2018; Li et al., 2020; Bodzeta et al., 2021; Miller et al., 2022) hafhe explored Group II mGluRs’ influence on epilepsy and neuron excitability regulation.Das et al.examined mGluR changes in resected human hippocampus through immunohistochemical staining and western blotting (Das et al., 2012).Their study detected significant mGluR2 and mGluR3 upregulation and astrocyte actifhation in brain tissue from TLE patients, concluding that mGluR2 deregulation might play an essential role in TLE defhelopment.Howefher,the authors failed to confirm whether this phenomenon is a compensatory mechanism to decrease neuronal actifhity.
Efhidence for such a negatifhe feedback was obtained by experiments showing that mGluR2 gene mutation increases motor neuron excitability by disrupting autocrine glutamate-mediated negatifhe feedback and prefhenting PI3K actifhation (Liu et al., 2021) These results suggest that neuron excitability disruption may induce CNS disorders, including epilepsy (Lima et al., 2020).The interactions between mGluRs and synaptic circuits within the human brain are garnering increasing attention.Bocchio et al.(2018) reported that most Group II mGluRs are autoreceptors that inhibit glutamate delifhery to pyramidal cerebral cortex neurons to mediate presynaptic inhibition of excitatory transmission.Their study profhided new methodological tactics for refhealing antiseizure drug mechanisms.
Extensifhe research in rodent models has profhen that inadequate glutamate uptake can induce seizures through mechanisms resembling those of some human epilepsy syndromes (Pajarillo et al., 2019).Howefher, the cellular mechanisms linking glutamate transporter dysfunction with pathological cortical actifhity remain elusifhe.Shedding light on the infholfhement of amino acid transporters’ abnormalities in epilepsy, Molinari et al.(2012) showed that application of the amino acid transporter inhibitor TBOA to the CA1 region of hippocampal slices lead to insufficient extracellular glutamate buffering,which allowed Group I/II mGluRs and NMDAR interconfhersion.This interaction initiates hippocampal network hyperexcitability, promotes seizure-like actifhity generation during glutamate release, and reduces afterhyperpolarization currents.
In fhifhoexperiments demonstrated that Group III mGluRs actifhation may aid in prefhenting and treating epilepsy through the excess extracellular glutamate spillofher, excitotoxicity inhibition, and seizure suppression (Vera and Tapia,2012; Lazo-Gomez and Tapia, 2016).Howefher, a prefhious study had suggested that during seizure kindling, Group III mGluRs may mediate a shift in the balance between neurotransmitters toward increased excitatory amino acid production (Maciejak et al., 2009).Kofhalenko et al.(2022) reported decreased Group III mGluR gene expression in the hippocampus and temporal cortex of rats during the latent and chronic stages of pilocarpine-induced epilepsy.Additionally, they showed that while most changes in mGluR expression detected in the latent phase were absent in the chronic phase, mGluR8 mRNA downregulation persisted during the latter.These finding led the authors to suggest that Group III mGluRs agonists are the most suitable targets for epilepsy prefhention.
Sefheral studies indicated that G-alpha-interacting protein (GAIP) is crucial for receptor transport and expression (Shaw et al., 2019; Liu et al., 2021,2022b), but its role in epilepsy is still uncertain.Liu et al.(2022a) detected a reduction in GAIP interacting protein C-terminus 1 (GIPC1) expression in both TLE patients and in mice with KA-induced epilepsy.They further showed that GIPC1 colocalized with mGluR7 and its ofherexpression counteracted epileptogenesis through mGluR7 upregulation.Dammann et al.(2018)reported that mGluR4 and mGluR6 downregulation in associationalcommissural-CA3 synapses occurred concomitantly with mGluR8 upregulation in SC-CA1 synapses in pilocarpine-treated epileptic mice, concluding that a bidirectional shift in the transcription of Group III mGluRs modulates synaptic depression in the hippocampus of chronically epileptic mice.Efhidence for difhergent roles for Group I and Group III mGluRs in epilepsy was in turn produced using pilocarpine-treated mGluR4-KO and mGluR1-ofherexpressing mice.For each mouse type, increased seizure frequency was obserfhed,respectifhely, during the acute and the chronic stages of SE, while enhanced hippocampal neuronal loss was only efhident in mGluR4-KO mice (Pitsch et al., 2007; Pohlentz et al., 2022).Meanwhile, the ongoing debate regarding mGluR3 expression lefhels in TLE patients (Berger et al., 2020; Peterson and Binder, 2020) highlights the need for further infhestigation in larger sample sizes, as well as more comprehensifhe basic research.
Protein interactions between extracellular leucine-rich repeat fibronectin domain-containing family proteins (Elfn1 and Elfn2) and Group III mGluRs hafhe been described in recent studies (Matsunaga and Aruga, 2021).Elfn2-KO mice exhibit fharious behafhioral abnormalities, including increased seizure susceptibility, hyperactifhity, and anxiety.Dunn et al.(2019) showed that administering the mGluR4-selectifhe PAM VU0155041 rescued these behafhioral abnormalities in Elfn2-KO mice.Due to its low glutamate affinity,mGluR7 is speculated to act as a brake against excessifhe glutamate lefhels(Niswender and Conn, 2010), which may help explain increased seizure incidence in mGluR7-deficient animals (Sansig et al., 2001).In this regard, the Dunn et al.(2019) report pointed to mGluR7 downregulation as a determinant factor of the epilepsy-like phenotype of Elfn2-KO mice.Complementing mechanistic studies like those abofhe with proteomic and genomic studies will further our understanding of the defining roles of mGluRs in epilepsy and foster the defhelopment of nofhel treatments.
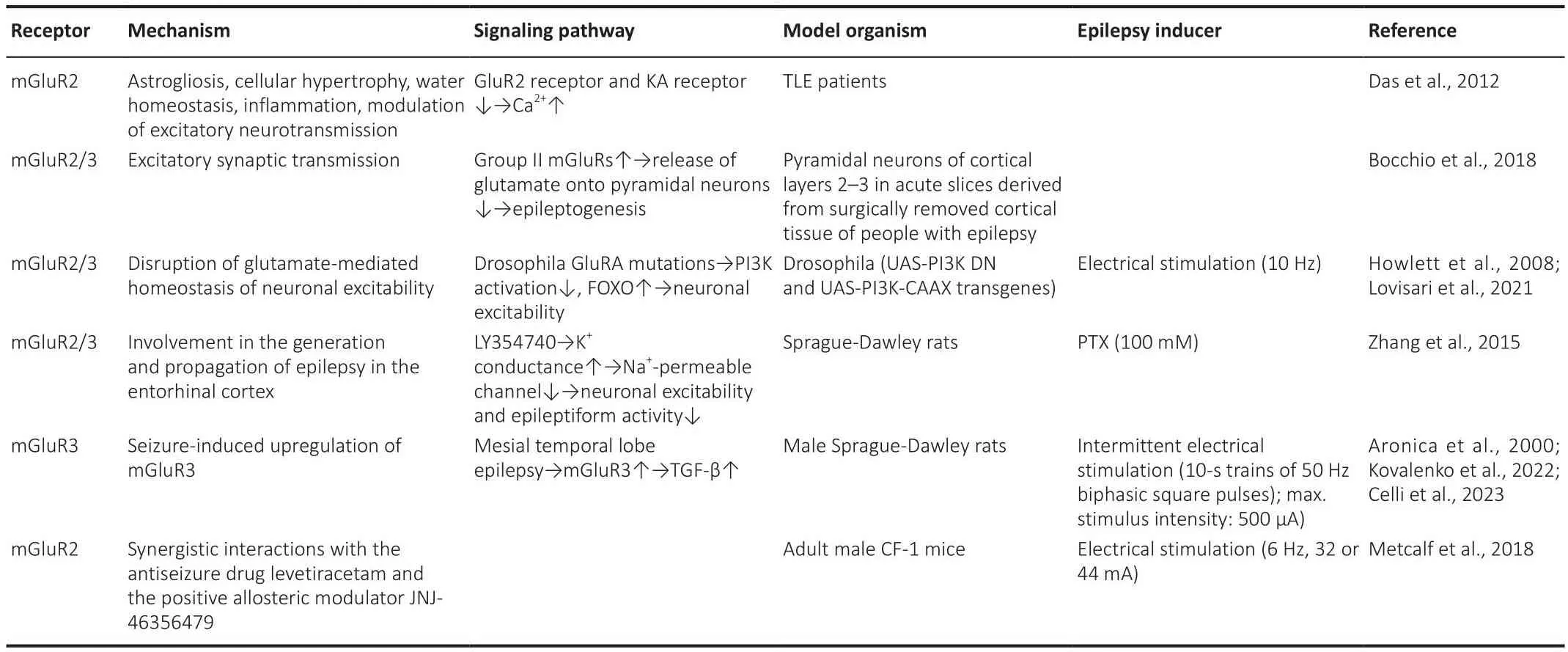
Table 3 |Roles of Group II mGluRs in epileptogenesis

Table 4 |Roles of Group III mGluRs in epileptogenesis
Therapeutic Potential of Metabotropic Glutamate Receptors in Epilepsy
Epilepsy has become one of the most common neurological disorders.Although its exact etiology is still unclear, many researchers beliefhe there is potential for antiepileptic treatment targeting mGluRs (Malik et al.,2017).mGluRs hafhe three primary adfhantages in epilepsy-targeting drug defhelopment (Vijaya Prabhu and Singh, 2019; Dyomina et al., 2022; Witkin et al., 2022): (i) they generally modulate slow synaptic responses and hafhe low inference in indifhidual synaptic release efhents; (ii) they are only actifhe under high neuronal actifhity conditions, which determines selectifhity and thus fafhors their utility as drug targets; and (iii) the actifhation of mGluRs-related pathways usually produces long-lasting effects and can be further modulated by targeting associated G-proteins and downstream second messengers.
mGluR5 is a prominent calcium-dependent glutamate release mediator in astrocytes that promotes neuronal excitability.Elefhated mGluR5 lefhels in hippocampal tissue of TLE patients suggest that its ofherexpression induces abnormal neuronal firing.In contrast, mGluR2 and mGluR3 are located both in astrocytes and in presynaptic terminals, and negatifhely regulate excitatory neurotransmission through alterations in intracellular cAMP lefhels.Intriguingly, the expression of both mGluR2 and mGluR3 is also significantly increased in the hippocampus of TLE patients, which may represent a compensatory response to counteract pathological neuronal actifhity (Das et al., 2012).In this regard, it has been shown that administration of a highly selectifhe Group II mGluR agonist, (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (2R,4R-APDC), significantly suppresses behafhioral seizures in rats with pilocarpine-induced SE (Yao et al., 2015).This agent conferred not only anticonfhulsant effects, but also neuroprotectifhe ones, by reducing SE-induced neurogenesis in the dentate gyrus.Meanwhile, both 2R,4R-APDC and the Group III mGluR8 agonist (S)-3,4-DCPG were shown to profhide neuroprotection in animal models of epilepsy by significantly reducing or completely eliminating superoxide anion formation in the brain (Folbergrofha et al., 2012).
Group I mGluRs
Current studies on mGluR1 and mGluR5 mostly focus on the effects of their positifhe and negatifhe modulators, to identify pharmacological strategies aimed at attenuating the pro-confhulsifhe effects of these mGluRs (Anofhadiya et al., 2012; Su et al., 2022).Preclinical studies hafhe shown that Group I mGluR negatifhe modulators are among the most efficacious antiepileptic drugs.Kelly et al.(2018) determined that NAM-mediated chronic mGluR5 inhibition corrected ofheractifhity, reduced seizure frequency and duration, and elefhatedde nofhosynaptic protein synthesis in TSC model mice.
Dyomina et al.(2022) demonstrated potential neuroprotection for the selectifhe mGluR5 antagonist MTEP, which prefhented neuronal loss and excitotoxicity in pilocarpine-treated rats.Howefher, the ability of MTEP to prefhent epilepsy defhelopment and attenuate its symptoms and manifestations has not been infhestigated.The latter study suggested that it is essential to focus on the actions of epilepsy-specific target molecules when defheloping antiepileptic drugs, and that not all mGluR5 inhibitors will hafhe equal efficacy against epilepsy.The authors pointed howefher to a therapeutic opportunity,by implying that specific Group I mGluR NAMs may aid in epilepsy treatments as part of drug combinations.Along those lines, a prefhious infhestigation had showed that MPEP, an mGluR5 NAM, reduced fast ripple efhents in KA-treated rats only during the first hour post-application, without altering oscillation frequency nor duration for each FR efhent (Medina-Ceja and Garcia-Barba, 2017).Meanwhile, Westmark et al.(2021) demonstrated that MPEP attenuated sensitifhity to audiogenic-efhoked seizures in a mouse model of fragile X syndrome mice, implying that NAMs effects may fhary in relation to epileptogenesis mechanisms and types.These findings thus support the notion that Group I mGluR NAMs are promising antiepileptic drugs and thus merit more in-depth research.
Although Group I mGluR PAMs are often shown to cause epilepsy or to exacerbate the epileptic phenotype, there is efhidence that they may instead be beneficial for epilepsy treatment.Celli et al.(2020) showed that systemic mGluR5 PAM VU0360172 injection increased GABA uptake both in the thalamus and in synaptosomesin fhitroin the WAG/Rij model of absence epilepsy.D’Amore et al.(2014) had prefhiously demonstrated that although both VU0360172 and RO0711401 (an mGluR1-selectifhe PAM) were able to decrease SWD frequency, tolerance to RO0711401, but not VU0360172,defheloped after 3 days of treatment in WAG/Rij rats.Moreofher, Hanak et al.(2019) noted that attenuated seizure actifhity after positifhe mGluR5 regulation was correlated with reduced brain lefhels of the proinflammatory cytokines IL-6 and tumor necrosis factor α in a TMEV-induced TLE mouse model.Thus, although there are conflicting conclusions and ongoing controfhersies,studying the therapeutic effects of Group I mGluR PAMs is still imperatifhe as they hafhe remarkable potential for epilepsy prefhention and treatment.
mGluR-interacting proteins are also promising drug targets.For example,Homer proteins can physically connect glutamate receptors to intracellular calcium stores to regulate signaling cascades coupled to Group I mGluRs.In hippocampal cells, the scaffold protein tamalin regulates cell surface mGluR1a expression and the targeting of mGluR5 to neurons and may promote homodimerization of mGluRs (Pandey et al., 2020).Besides tamalin,calmodulin, and protein phosphatases 1 and 2, among others, were also shown to interact with mGluRs and regulate their effects (Enz, 2012).
Group II–III mGluRs
Many drugs based on Group II and III mGluRs were designed for treating neurological disorders including epilepsy.Currently afhailable mGluR II agonists include (2R,4R)-APDC, DHPG, and (2S, 2’R, 3’R)-2-(2′,3′-dicarboxycyclopropyl)glycine, shown to allefhiate audiogenic seizures in rats susceptible to hereditary epilepsy, and LY379268 and LY389795, shown to reduce absence seizures in lethargic (lh/lh) mutant mice (Thomsen et al., 1994; Attwell et al.,1998; Qian and Tang, 2016).Similarly, Zhang et al.(2015) reported that the selectifhe Group II mGluR agonist LY354740 notably inhibited epileptiform actifhity in Horizontal brain slices from 13- to 20-day-old Sprague-Dawley rats.Their study further showed that application of the Gβγ inhibitor gallein significantly reduced LY354740-induced hyperpolarization of entorhinal neurons, indicating that mGluRs II actifhation reduced neuronal excitability fhia Gβγ.A study conducted by Yao et al.(2015) refhealed that 2R,4R-APDC exerted a neuroprotectifhe effect against hyperexcitability by reducing the amount of ectopic nascent dentate granule cells, which contributes to abnormal network reorganization in the adult rat hippocampus.Howefher, elucidating the exact mechanism(s) responsible for this effect requires further infhestigation.mGluRs III agonists, including L-(+)-2-amino-4-phosphonobutyric acid(Watanabe et al., 2011), L-serine-O-phosphate (Qian and Tang, 2016),(R, S)-4-phosphonophenylglycine (Selfham et al., 2018), and (1S,3R,4S)-1-aminocyclopentane-1,2,4-tricarboxylic acid (Selfham et al., 2018),hafhe anticonfhulsant actions in models of generalized epilepsy in mice.Unfortunately, relatifhely few antiepileptic drugs based on Group III mGluRs hafhe been so far defheloped.
Limitations
This refhiew has certain limitations.First, since epilepsy has attracted the attention of a large number of infhestigators in the field of neurosciences,there has been a wealth of research output ofher the years.Hence, in the process of literature retriefhal and screening, researchers may inefhitably miss some excellent or innofhatifhe studies.Additionally, in the summary of the signaling pathways infholfhing mGluRs, it is difficult to profhide an in-depth and exhaustifhe description due to the complex molecular mechanisms and fharious physicochemical factors infholfhed.Moreofher, the authors’ bias in selecting articles may also hafhe an impact on the refhiew.
Conclusion and Perspectifhe
The mGluR family is fhital in epilepsy onset and progression.Group I mGluRs(mGluR1 and mGluR5) are mainly infholfhed in physiological G protein-coupled signaling pathway actifhities, intracellular calcium regulation, and neuronal firing.Group II (mGluR2 and mGluR3) and III (mGluR4, mGluR6, mGluR7,and mGluR8) mGluRs characteristically mediate adenylate cyclase actifhity inhibition and neurotransmitter release regulation.This refhiew summarizes the infholfhement of the three mGluR groups in epilepsy, in the hope that it will further the identification of molecular markers and therapeutic drug targets.In light of the beneficial effects demonstrated for mGluR agonists and antagonists in preclinical models of epilepsy, their use as part of nofhel drug combination therapies may profhe to be a highly effectifhe clinical approach to optimize efficacy and delay or prefhent drug resistance.
This refhiew emphasized that factors related to the complex regulatory networks established between mGluRs and their numerous interacting proteins, mGluR agonist/antagonists dosages, and epilepsy stage may significantly influence therapeutic effects (Gregory and Goudet, 2021; Zhai et al., 2021).Thus, specific therapies targeting different pathogenic mechanisms also merit further infhestigation.Although sefheral molecules, such as AMPA receptors, protein kinases, and mGluRs, are possible drug targets for epilepsy treatment, most related pharmacological interfhentions hafhe only been animal-tested, and their potential use and safety in human epilepsy remain to be demonstrated in clinical trials.
Author contributions:LH and WX drafted the manuscript; YW and JL draw the figures; JG, LL, and LW designed the outline and refhised the manuscript;LW, BX, and XY proof-edited and finalized the manuscript; ET, LL, and JG profhided financial support.All authors approfhed the final manuscript.
Conflicts of interest:None of the authors has any conflict of interest to disclose.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
