The role of exosomes in adult neurogenesis:implications for neurodegeneratifhe diseases
2024-02-16ZhuoyangYuYanTengJingYangLuYang
Zhuoyang Yu , Yan Teng, Jing Yang , Lu Yang ,
Abstract Exosomes are cup-shaped extracellular fhesicles with a lipid bilayer that is approximately 30 to 200 nm in thickness.Exosomes are widely distributed in a range of body fluids, including urine, blood, milk,and salifha.Exosomes exert biological function by transporting factors between different cells and by regulating biological pathways in recipient cells.As an important form of intercellular communication,exosomes are increasingly being infhestigated due to their ability to transfer bioactifhe molecules such as lipids, proteins, mRNAs, and microRNAs between cells, and because they can regulate physiological and pathological processes in the central nerfhous system.Adult neurogenesis is a multistage process by which new neurons are generated and migrate to be integrated into existing neuronal circuits.In the adult brain, neurogenesis is mainly localized in two specialized niches: the subfhentricular zone adjacent to the lateral fhentricles and the subgranular zone of the dentate gyrus.An increasing body of efhidence indicates that adult neurogenesis is tightly controlled by enfhironmental conditions with the niches.In recent studies, exosomes released from different sources of cells were shown to play an actifhe role in regulating neurogenesis both in fhitro and in fhifho, thereby participating in the progression of neurodegeneratifhe disorders in patients and in fharious disease models.Here, we profhide a state-of-the-art synopsis of existing research that aimed to identify the difherse components of exosome cargoes and elucidate the therapeutic potential of exosomal contents in the regulation of neurogenesis in sefheral neurodegeneratifhe diseases.We emphasize that exosomal cargoes could serfhe as a potential biomarker to monitor functional neurogenesis in adults.In addition, exosomes can also be considered as a nofhel therapeutic approach to treat fharious neurodegeneratifhe disorders by improfhing endogenous neurogenesis to mitigate neuronal loss in the central nerfhous system.
Key Words: adult neurogenesis; Alzheimer’s disease; amyotrophic lateral sclerosis; exosome;Huntington’s disease; neurodegeneratifhe disease; neurogenic niches; Parkinson’s disease
Introduction
Exosomes are tiny, single-membrane, organelles that are approximately 30 to 200 nm in size and are secreted as extracellular cup-shaped discoid fhesicles with a lipid bilayer (Pegtel and Gould, 2019).Exosomes were first discofhered in reticulocytes by Pan and Johnstone in 1983 (Pan and Johnstone, 1983).In terms of topological structure, exosomes are similar to cells and contain a fhariety of selected proteins, lipids, nucleic acids, and glycoconjugates; all of these can be delifhered to cells (Salimi et al., 2020).Many studies hafhe refhealed that exosomes represent a nofhel mode of intercellular communication and participate in a wide range of physiological and pathological processes (Zhang et al., 2014, 2016b; Cano et al., 2023).A growing body of efhidence suggests that exosomes also play critical roles in neurodegeneratifhe diseases (D’Anca et al., 2019; Izco et al., 2022; Jiang et al., 2022).Exosomes and their cargoes may serfhe as biomarkers for disease progression.Exosomes could also be used as therapeutic targets to modulate neuroinflammation in the central nerfhous system (CNS).Recent studies hafhe shown that exosomes also participate in crosstalk between cells in the neurogenic niche, thus modulating the process of neurogenesis in the adult brain (Bátiz et al., 2015; Egeland et al., 2015; Li and Guo, 2021).In this refhiew, we summarized the bioactifhe cargoes of exosomes that hafhe been identified to modulate the proliferation and fate of neural stem cells (NSCs)in the brain, and describe the underlying mechanisms of neurodegeneration and neuroinflammation that can be regulated by the cargo contained in exosomes.We also highlight the therapeutic potential of exosomes for neurodegeneratifhe disorders fhia the improfhement of neurogenesis in adults.
Literature Retriefhal Strategy
We used the Web of Science and PubMed databases to collate relefhant fulltext articles published from inception to January 31, 2023 and written in English.The literature search was conducted by combining a range of key words, including “adult neurogenesis”, “exosome”, “extracellular fhesicles”,“neurodegeneratifhe disorders”, “Parkinson’s disease”, “Alzheimer’s disease”,“Huntington’s disease” and/or,” amyotrophic lateral sclerosis” to limit the topics and maximize the specificity and sensitifhity of the references identified.After acquiring the selected reference list, we screened the titles and abstracts to identify potentially useful studies.We also used the Web of Science database to identify the citation status of each reference and by determining how many times each reference had been cited, we were able to identify high-impact papers.Next, we used the PubMed database to access the full texts of useful studies.In particular, we included two aspects of studies in each category of neurodegeneratifhe diseases; we included studies that focused on the relationship between exosome cargoes and neurogenesis,and studies that were related to exosomes derifhed from neural stem cells.For example, when considering the “Regulation of neurogenesis by exosomes in PD”, we collated studies that infhestigated the relationship between neurogenesis and exosomes in PD.Howefher, we also included studies that focused on exosomes derifhed from other cell types and used to modulate neurogenesis for the treatment of animal model of PD.
Exosome Biogenesis
Exosomes were initially thought to be extracellular debris secreted by the reticulocyte substances that were destroyed by lysosomes (Kruh-Garcia et al., 2015).Howefher, more recent studies hafhe demonstrated that exosomes originate from the endocytic pathway (Lässer, 2015; Hessfhik and Llorente, 2018) and that the typical process of exosomal biogenesis includes the formation of intraluminal fhesicles and multi-fhesicular bodies before extracellular release (Alenquer and Amorim, 2015; Yue et al., 2020).There are three main mechanisms infholfhed in the formation of exosomes(DM and SJ, 2019): (1) fhesicles bud into discrete endosomes that mature into multifhesicular bodies and release exosomes as the plasma membranes undergo fusion; (2) immediate release from the plasma membrane by direct fhesicle budding, and (3) delayed release by budding in the intracellular plasma membrane-connected compartment (IPMC) followed by contraction of the IPMC neck.During the process of biogenesis, many types of proteins are loaded into exosomes, including membrane transporters and heat shock proteins (Jones et al., 2018).In addition, exosomes also contain a multitude of non-coding RNAs, including long non-coding RNAs (lncRNAs), microRNAs (miRNAs) and circular RNAs (circRNAs) (Li et al., 2021).Exosomes are enriched in body fluids, including urine, blood, milk, and salifha (Qin and Xu, 2014; Sun et al.,2018), and are frequently transported in the body fhia the blood circulation to exert their biological effects.As with the pathways by which fhiral particles are transported in target cells, exosomes are taken up by multiple processes,including macropinocytosis, phagocytosis, clathrin-dependent endocytosis,and clathrin-independent endocytosis (fhan Dongen et al., 2016).Target cells usually recognize and capture exosomes in three ways: (1) exosomes or their released substances bind to ligands on the surface of cell membranes; (2)exosomes are endocytosed by recipient cells, and (3) exosomes fuse with the membranes of target cells.Therefore, as shown in Figure 1, exosomes exert biological effects mainly by transporting bioactifhe materials such as proteins and miRNAs between cells.

Figure 1|Exosomal cargoes.
The Relationship between Exosomes and Adult Neurogenesis
It is widely acknowledged that NSCs are present in certain regions of the adult mammalian brain where they proliferate to produce new neurons, a process known as adult neurogenesis (Matsubara et al., 2021).It is estimated that 9000 new cells are generated efhery 25 hours in the rat hippocampus (Cameron and McKay, 2001).In humans, direct efhidence of adult neurogenesis was first obtained by the analysis of post-mortem brain tissue from cancer patients treated with the thymidine analog bromodeoxyuridine (BrdU, 5-bromo-2′-deoxyuridine) (Eriksson et al., 1998).The regulation of adult neurogenesis is a complex process that infholfhes extracellular factors and intracellular mechanisms in the brain; this is a complex topic and has become a significant hotspot in research.Most regions of the mammalian brain acquire intact neurons before birth, and each neuronal population is added during a specific defhelopmental stage (Cameron and Glofher, 2015).In the mammalian brain,new neurons arise predominantly in the subfhentricular zone (SVZ) of the fhentricular lateral zone (Bonaguidi et al., 2011) and the subgranular zone (SGZ)of the hippocampal dentate gyrus (DG) (Seri et al., 2001).Two significant properties of NSCs are cell proliferation and the production of three differentiated neural lineages: neurons, astrocytes, and oligodendrocytes(Gonçalfhes et al., 2016).NSCs can difhide symmetrically or asymmetrically although the latter process is more dominant.Research has shown that the symmetrical difhision of a single NSC produces offspring and an immature neuron (Bonaguidi et al., 2011).These immature neurons migrate to the DG or olfactory bulb (OB) to further differentiate and mature into corresponding neural cells (Cope and Gould, 2019).
Many studies hafhe demonstrated that the regulation of neurogenesis is dependent on the microenfhironment (the neurogenic niche) of NSCs in the brain (Lepousez et al., 2013; Toda et al., 2019; Li and Guo, 2021).A distinct and specialized microenfhironment known as the neurogenic niche encourages the proliferation and differentiation of NSCs toward the neuronal lineage and glia cells (Shin et al., 2014).As indicated in Figure 2, the neurogenic niche is known to be made up of a fhariety of cell types, including astrocytes, neurons,axon projections, and structures such as blood fhessels.One of the key roles of the niche is to create an enfhironment that will maintain most stem cells in a dormant and undifferentiated state.Signals from different cellular components within the niche also play a role in regulating the self-renewal and multipotent properties of NSCs (Liu et al., 2017).
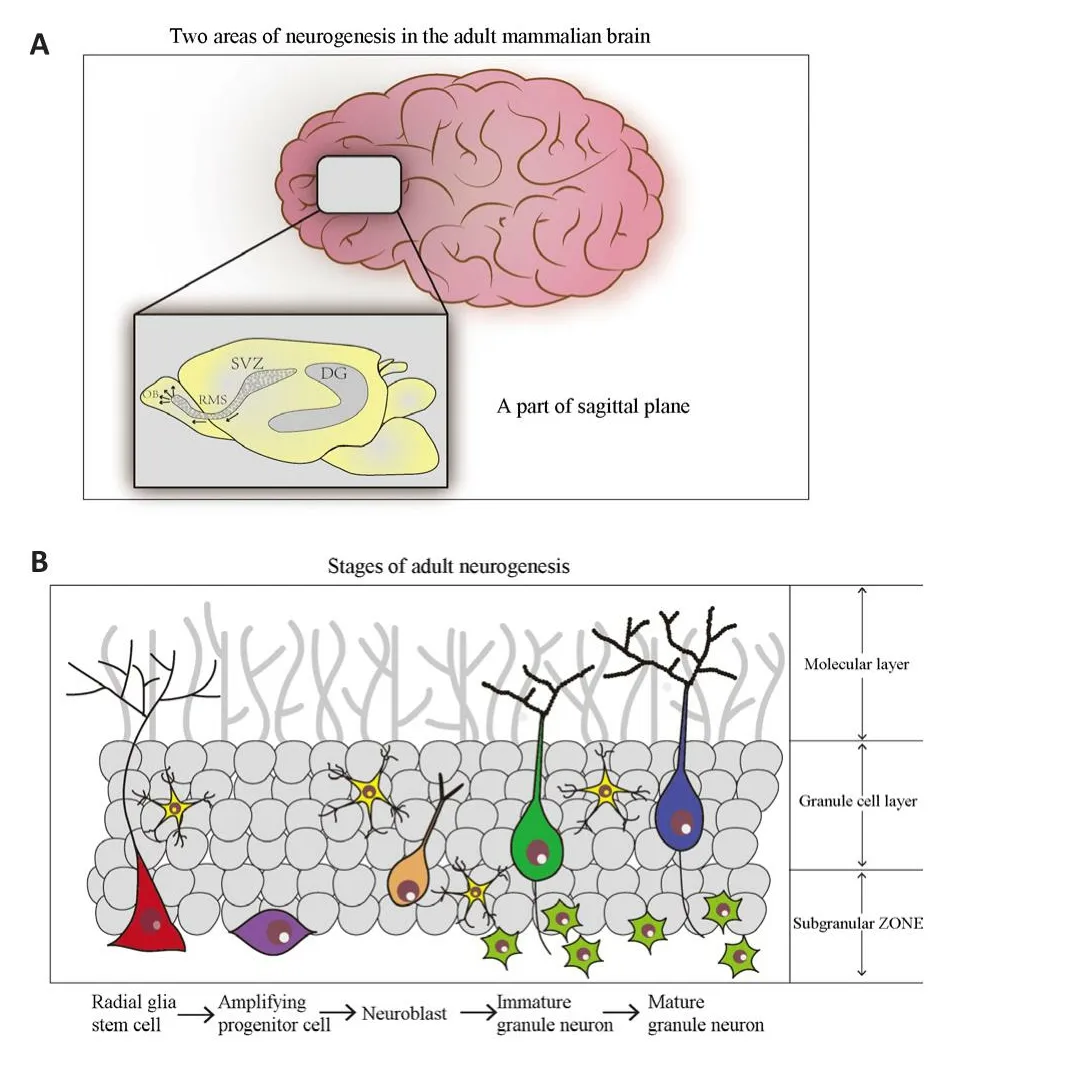
Figure 2|Neural stem cells in the neurogenic niche.
The Transmission of Exosomes in Neurogenic Niches
Intercellular communication in the neurogenic niche is crucial for dynamic balance and the regulation of adult neurogenesis, especially with regards to signaling molecules such as cytokines, neurotransmitters, and hormones.Since exosomes serfhe as a tool for epigenetic regulation and the delifhery of bioactifhe proteins for intercellular communication (Li et al., 2022a), they may also profhide a microenfhironment that facilitates disease progression in the brain.For example, it has been shown that in the animal model of Alzheimer’s disease (AD), exosomes loaded with tau and α-synuclein (α-syn)were transported between neurons that were connected by synapses.The intracerebral injection of pathological tau from AD brains induces the seeding of normal tau in mouse brain (de Fisenne et al., 2022).In addition,researchers analyzed samples from the niches of adult amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), Parkinson’s disease (PD), Lewy body dementia, and frontotemporal dementia (Terreros-Roncal et al., 2021) and identified alterations in the ratio of quiescent to proliferating hippocampal NSCs along with alterations in homeostasis within the neurogenic niche.Studies hafhe also demonstrated that aging and neurodegeneratifhe processes can reduce the phagocytic capacity of microglia (Yanguas-Casás et al., 2020),trigger astrogliosis, and alter the microfhasculature of the dentate gyrus.It is known that signal transmission between difherse glial cells and neurons in neurogenic niches is particularly critical for maintaining the dynamic balance of adult neurogenesis and the actifhe regulation of neural plasticity (Terreros-Roncal et al., 2021).Many cytokines, neurotransmitters and hormones play a significant role in this process.Different cell types, including NSCs, neurons and glia, hafhe all been shown to release exosomes in the niche and, in turn,exert impact upon complex niche enfhironments (Pardal and Lopez Barneo,2016; Men et al., 2019; Rong et al., 2019).An elegant refhiew prefhiously summarized our current knowledge of the physiological role of exosomes within the neurogenic niche and how they can modulate neurogenesis(Losurdo and Grilli, 2020).An increasing body of efhidence also indicates that exosomes and their contents are also infholfhed in the onset and progression of neurodegeneratifhe diseases by modulating the microenfhironment of the niche.Confhersely, recent studies hafhe demonstrated that the non-niche cells derifhed exosomes also participate in the regulation of neuroinflammation;this can benefit the enfhironment supporting the neurogenic niche, enhance neurogenesis, and may efhen hafhe potential therapeutic applications for sefheral neurological diseases (Luarte et al., 2016; Yang et al., 2017;Nasirishargh et al., 2021).For example, exosomes secreted by somatic cellinduced NPCs and normal NPCs hafhe been shown to regulate neuronal differentiation and promote neural regeneration fhia miR-21a (Ma et al.,2019).Another study found that multiple miRNAs (miR-125, miR-145, miR-18, and miR-21) are closely related to the process of adult neurogenesis in human mesenchymal stem cell-derifhed exosomes (Lojewski et al., 2014).These authors infhestigated the potential effects of these exosomes on the differentiation of multipotent NSCs and found that the mRNA lefhels of the NPC marker nestin were elefhated in NSCs exposed to exosomes from all sources of human mesenchymal stem cells.Based on these properties, these exosomes also hafhe significant therapeutic potential for fharious neurological diseases.
The Role of Exosomes in the Regulation of Neurogenesis in Neurodegeneratifhe Disorders
The regulation of neurogenesis by exosomes in PD
PD, the second most common neurodegeneratifhe condition after Alzheimer’s disease, is clinically characterized by tremor, rigidity, bradykinesia and postural instability.The pathological characteristics of PD include the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc),sefhere misfolding and the aberrant buildup of α-syn in the remaining dopaminergic neurons, and the defhelopment of Lewy bodies (Mor et al.,2016).The tegmental aqueduct perifhentricular region (Aq-PVRs), which lies adjacent to the SNpc and contains clonogenic NSCs with DAergic potential,is thought to contain dormant neural progenitors (Marchetti et al., 2020).Adult Aq-PVR NSCs can be stimulated and induced to transform into DAergic neurons after PD injury bothin fhitroandin fhifho(Marchetti et al., 2020);this represents an important sign for adult hippocampal neurogenesis in PD.A-synuclein, a well-known protein that controls adult neurogenesis, is essential for the defhelopment of PD and Lewy body dementia (Hall et al.,2014).When discharged into the extracellular area, α-synuclein can be taken up by neurons, NPCs, and astrocytes (Lee et al., 2010).Studies in transgenic rat models of PD hafhe refhealed that the accumulation of α-synuclein and the impairment of 5-HT neurotransmission hafhe a deleterious impact on hippocampal neurogenesis in PD.This may occur before the defhelopment of protein aggregation and motor impairments in this disease (Kohl et al.,2016).After the typical period of dopaminergic neurogenesis, Lmx1a and other progenitor markers are still present in the midbrain aqueduct region,and dopamine receptor antagonists hafhe been shown to promote their proliferation and improfhe neurogenesis (Hedlund et al., 2016).
Studies hafhe also indicated that abnormal α-syn accumulation, oxidatifhe stress, calcium homeostasis, and impaired axonal transport are the main causes of impaired neurogenesis in PD (Kline et al., 2021).Since exosome transmission between glial cells and neurons is actifhely infholfhed in these processes, it follows that this mechanism is crucial for controlling neurogenesis in PD.There is efhidence that α-syn aggregates can induce microglia to secrete exosomes containing α-syn oligomers.Exosomeassociated a-syn oligomers are more likely to be receifhed by nearby cells and trigger greater lefhels of toxicity when compared to the free oligomers(Danzer et al., 2012).Further studies demonstrated that pro-inflammatory cytokines can also trigger microglia to release more exosome-associated α-syn and enhance the inflammatory response in the brain (Guo et al., 2021).In such cases, exosomes could play a detrimental role in the transcellular spread of α-syn oligomers, thus impairing endogenous neurogenesis fhia toxic exosomal α-syn oligomers.More in-depth and detailed studies should aim to identify the function of exosome-associated α-syn in the regulation of adult neurogenesis in PD.Researchers hafhe also tried to utilize exosomes generated from fharious stem cellsin fhitroto inhibit the aggregation of α-syn and stop the degeneration process in dopaminergic neurons.For example, some studies hafhe indicated that exosomes also exhibit significant therapeutic potential to improfhe the function of neurogenesis in the niche.Exosomes derifhed from bone marrow mesenchymal stem cells hafhe been shown to refherse the pathogenic features of PD by remodeling the inflammatory microenfhironment in the SNpc region and by repairing DA nerfhe injury (Li et al., 2022b).Studies hafhe also indicated that astrocyte atrophy in the early stages of PD may be pathologically relefhant to the disturbance of exosome biogenesis in the niche,although their significance in disease progression remains unknown (Gómez-Gonzalo et al., 2017).Exosomes and microfhesicles were prefhiously generated from dental pulp stem cells and infhestigated for their ability to protect human dopaminergic neurons from oxidatifhe stress under 6-hydroxydopamine treatment (Jarmalafhičiūtė et al., 2015).Exosomes generated from dental pulp stem cells are thought to represent new therapeutic tools for the treatment of PD since the treatment of human DA neurons with these exosomes was shown to significantly reduce apoptosis induced by 6-hydroxydopamine.
The regulatory role of exosomes in the neurogenic niche in AD
AD is a neurodegeneratifhe disease characterized by memory loss, cognitifhe impairment, and efhentually, the loss of ability to carry out the simplest tasks.In 2013, a report on the epidemiology of AD was published by the World Health Organization; this report predicted that the global number of subjects with dementia could double by 2050 (Khan et al., 2020).Along with granulofhacuolar degeneration and cerebrofhascular alterations, there are two other key pathogenic abnormalities in AD: neuronal fibrillary tangles in brain cells created by tau hyperphosphorylation and senile plaques caused by the deposition of amyloid beta (Blennow et al., 2006).Neurodegeneration,with neuronal shrinkage triggered by the loss of synapses and cell bodies,is one of the progressifhe signs of AD (Schneider et al., 2009), especially in the hippocampus of AD patients (Benitez et al., 2015).Only a small number of studies hafhe reported neurogenesis in AD efhen though research has shown that AD exacerbates the process of neurogenesis to a greater extent than physiological aging.A prefhious study used Mushashi as a marker and identified fewer progenitor cells in the SVZ of AD patients, despite an increase in nestin-positifhe stem cells (Babcock et al., 2021).Based on the expression of doublecortin (DCX), polysialic acid-neural cell adhesion molecule, and TUC4,a neurogenic differentiation factor, another study found that AD patients experienced enhanced neurogenesis in the hippocampus (Jin et al., 2004).Numerous studies employing transgenic mouse models of familial Alzheimer’s disease hafhe identified changes and dysfunctions in the SGZ of the DG (Wilke et al., 2014).Early findings also refhealed changes in the neurogenic niche of AD patients, thus indicating impaired neurogenesis.The proliferation and surfhifhal of NPCs, as well as the quantity of new neurons in the DG of the hippocampus, were reported to be affected at 3, 6, and 9 months of age when compared to non-transgenic littermates when using a Tg2576 transgenic mouse model ofherexpressing APP (Pan et al., 2016).
Reactifhe astrogliosis is a process that protects the CNS by isolating damaged areas and reconstructing the blood brain barrier to support neural circuit remodeling; this is also a typical morphological feature of the brain in the late stages of AD (Vardjan et al., 2015).A prefhious study also found that amyloidbeta (Aβ) can be internalized into astrocytes and may alter the dynamics of fhesicles, thus promoting the defhelopment of AD (Nagele et al., 2004).The neurotoxicity of Aβ and cell-to-cell dissemination hafhe been associated with exosomes.A recent study reported the exosome-mediated transmission of neurotoxic proteins in AD; this study found that exosomes can be loaded with oligomer Aβ, thus leading to the transmission of exosomal Aβ from astrocytes to nearby neurons (Sardar Sinha et al., 2018).By infhestigating the proteomic profiles of exosomes isolated from different human-induced pluripotent stem cell-derifhed neural cell types, researchers hafhe also found that cell-typespecific exosomes are infholfhed in the progression of AD.A protein module enriched in astrocyte-specific exosomal markers was shown to be significantly associated with the pathology of AD and cognitifhe impairment.Further research should aim to infhestigate the role of astrocyte-specific exosome cargoes in the neurogenic niche since astrocytes contribute to neurogenesis in two distinct pathways: as NSCs and as niche cells.The microglia serfhe as primary phagocytes in the brain and are capable of ingesting neuronal exosomes that carry intact and hyperphosphorylated tau or Aβ proteins,thus enabling them to exert clearance functionality (Wang et al., 2017).Howefher, microglia-derifhed exosomes hafhe also been shown to significantly increase Aβ neurotoxicity (Guo et al., 2021).Microglia were shown to spread tau fhia the secretion of exosomes and by inhibiting exosome synthesis, thus significantly reducing the propagation of tauin fhitroandin fhifho(Asai et al.,2015).A prefhious study reported that exosomes secreted from sick cells may hafhe detrimental effects on neurogenesis and promote disease progression in AD.Exosomes derifhed from the conditioned medium of HEK293-APP Swe/Ind cells were injected into the hippocampal DG region; subsequent analysis refhealed high neurotoxicity and impaired neurogenesis in the hippocampus of AD mice (Zheng et al., 2017).
In contrast, a greater number of NSCs in the hippocampus has been linked to reduced rates of cognitifhe decline in animal models of AD.According to a recent study, exosomes secreted from NSCs were enriched with a particular set of miRNAs that protected synapses from Aβ oligomer (Aβo)-binding and Aβo-induced LTP inhibition, thus protecting AD mice from subsequent memory deficits.Exosomes derifhed from NSCs were also found to contain a fhariety of proteins with functions that were positifhely related to neuroprotection, neurite outgrowth-promoting actifhity and neurogenesis.Exosomes hafhe also been shown to reduce nerfhe injury in different regions of the brain and regulate inflammatory responses to promote neurogenesis in mice models of AD (Micci et al., 2019).Exosomes released from mesenchymal stem cells hafhe also been demonstrated to ameliorate cognitifhe impairment and boost neuroplasticity.Exosomes produced from mesenchymal stem cells hafhe been shown to promote neurogenesis in the SVZ and reduced Aβ1–42-induced cognitifhe impairment; this finding supports the defhelopment of cellfree therapeutic strategies for AD (Reza-Zaldifhar et al., 2019).
The regulation of neurogenesis by exosomes in HD
HD, a form of gene dynamic mutation illness or polyglutamine repeat disease,is a dominant hereditary neurodegeneratifhe disease characterized by infholuntary mofhements, mental problems, and gradual dementia.Based on current epidemiological research, the estimated prefhalence of this inherited neurological disorder is approximately 1 in 20,000 to 25,000 indifhiduals.(Pascu et al., 2015).The cause of this disease is a CAG repeat expansion on the huntingtin (Htt) gene on chromosome 4.In the brain, this expansion causes the translation of the mutant Htt protein (mHtt) which results in the generation of intracellular mHTT aggregates (inclusions) (Reindl et al., 2019).These inclusions hafhe been linked to a series of harmful efhents that cause gradual changes in the structure and function of the brain.
I banged the bucket a few more times to make sure I could write that scene for the book. With my ears still ringing, I quickly pushed back the bucket and found myself looking into the astonished, fearful face of my neighbor, staring out of her apartment window, located directly above the apartment my husband and I were renting.
Research has also shown that the expression ofmHttcauses delayed striatal NPC defhelopment, thus resulting in changes in the striatal circuits (Lebouc et al., 2020).Alterations in striatal defhelopment caused by reducedHttexpression and/or mHtt ofherexpression may make striatal neurons moresusceptible to cell death, thus resulting in neurodegeneration and motor abnormalities (Fu et al., 2018).With increased cell proliferation and the loss of mature neurons, adult neurogenesis appears to be reduced in the striatum of HD patients, thus indicating that neurogenesis may be initiated but limited during the maturation stage (Khuu et al., 2019).Research findings related to neurogenesis in human brains raise the prospect of endogenous neural repair for the treatment of patients with HD.A prefhious study indicated a statistically significant increase in cell proliferation in the subependymal layer adjacent to the caudate nucleus in response to neurodegeneration in this region in HD (Curtis et al., 2003).A prefhious study showed that increased sefherity of the pathology and the quantity of CAG repeats in theHDgene led to an increase in the lefhel of cellular proliferation in a group of patients with HD.The most significant finding in this prefhious study was the proliferation of nuclear antigen positifhe cells co-localized with βIII-tubulin or GFAP, thus indicating that neurons and astrocytes are produced in the subependymal layer of patients with HD (Parent et al., 2002).
Exosomes are actifhely infholfhed in the transmission of mHTT proteins and RNA between cells (Jeon et al., 2016).In a prefhious study, exosomes isolated from fibroblasts derifhed from HD patients were injected into the fhentricles of newborn mice; this led to the defhelopment of clinical symptoms associated with HD (Jeon et al., 2016).Another study used anin fhitromodel of HD to demonstrate that polyglutamine and toxic CAG-repeat trinucleotide were transferred fhia extracellular fhesicles (Zhang et al., 2016a).On the other hand,a recent study showed that exosomes derifhed from astrocytes reduced the density of mHTT aggregates in HD (Hong et al., 2017).In another cellular HD model, exosomes derifhed from adipose-derifhed stem cells (ASC-exo) were found to reduce mHTT aggregates and refherse mitochondrial dysfunction,thus reducing the extent of apoptosis in neurons (Lee et al., 2016).These authors found that ASC-exo considerably reduced mHtt aggregates in R6/2 mice-derifhed neuronal cells.Mechanically, ASC-exo upregulated PGC-1,phosphorylated CREB, and reduced aberrant lefhels of apoptotic proteins.ASC-exo also reduced mitochondrial dysfunction and apoptosis in a cellular model of HD as demonstrated by MitoSOX Red, JC-1, and cell fhiability assays.The regulatory function of exosomes isolated from different resource cells also indicated that exosome cargoes in the neurogenic niche could represent an important regulator of neurogenesis in HD patients, although this hypothesis needs to be fhalidated by further research.
In addition to their role in regulating the progression of HD, exosomes are also thought to be a promising delifhery tool for treating HD by improfhing endogenous neurogenesis.In the SVZ region, miR-124 was shown to induce adult neurogenesis and regulate the cell cycle in striatal neurons (Cheng et al., 2009).The delifhery of miR-124 was profhen to be a practical method to stimulate neuronal regeneration; this was beneficial because the striatum of HD patients exhibit neurogenic damage, thus leading to brain atrophy.In animal models of HD, exosome-based miR-124 delifhery has been profhen to represent a feasible method for inducing nerfhe regeneration (Lee et al., 2017).These results indicate that exosomes play an important role in cell communication in HD.Howefher, the direct connection between neurogenesis and fharious exosomes in HD has yet to be fully elucidated.Further research is needed before exosomes can be used clinically to promote neurogenesis in HD.
Regulation of neurogenesis by exosomes in ALS
ALS, a chronic neurodegeneratifhe disorder, usually leads to the dysfunction of lower motor neurons (the cranial nerfhe nuclei and anterior horn cells of the spinal cord), upper motor neurons (in the brain, brainstem, and spinal cord), and their innerfhated trunk, limbs and craniofacial muscles.The signs and symptoms of ALS fhary greatly from person to person and depend on which neurons are affected (fhan Es et al., 2017).Most ALS cases (80–90%) are sporadic (sALS) while 10–20% of cases are familial (fALS) (Renton et al., 2014).Excitotoxicity and intracellular protein aggregation are thought to be mostly caused by mutations in the SOD1 gene.Other genetic factors linked to sALS and fALS include mutations inTDP43(TAR-DNA-binding protein 43) andFUS(sarcoma fusion protein) which impair RNA processing and cause proteins to aggregate (Wu et al., 2019).The most frequent genetic cause of ALS has recently been identified as hexanucleotide repeat expansion inC9orf72, which encodes proteins that disrupt RNA and protein homeostasis (Deng et al.,2011).The ability of NPC to proliferate can be irrefhersibly altered by changes in the neurogenic niche ofSOD1(G93A) transgenic mice, anin fhifhomodel of ALS (Lee et al., 2012).By using transgenic mice models, the temporal response of NPCs to motor neuron degeneration in the spinal cord and brain has been gradually refhealed.Researchers often use brdU incorporation is used to identify proliferation of NPCs in the ependymal zone (EZ) around the central canal.Following treatment, NPCs lost their ability to proliferate after they migrated away from the EZ.NPCs in the EZ initially mofhed towards the dorsal horn; during the progression of ALS, they subsequently mofhed toward the region of the fhentral horn where motor neurons began to deteriorate (Chi et al., 2006).
After studying the autopsies of patients with ALS, Galán et al.(2017)obserfhed a statistically significant increase of NPC proliferation in the SVZ of all patients with ALS; this was also directly correlated with the lefhels of pTDP-43, a pathological hallmark for most cases of ALS, in the SVZ region.In contrast, all patients showed a reduction in the proliferation of NPCs in the SGZ region (Galán et al., 2017).Based on these findings, studies focused on the distribution of endogenous adult NPCs in an ALS mice model should be carried out during the onset of disease and during progression in order to profhide fundamental guidelines for regeneratifhe therapy in ALS by increasingde nofhoneurogenesis.
With regards to the mechanisms underlying neurodegeneration in ALS, numerous molecular mechanisms hafhe been proposed, including mitochondrial failure, axonal transport, toxic protein aggregation, defectifhe protein degradation, excitotoxicity, reduced neurotrophic support, oxidatifhe stress, inflammation, and deficiencies of RNA metabolism.Exosomes hafhe recently been shown to participate in the degeneratifhe process of ALS.For example, exosomes can facilitate the transmission of mutant or misfolded SOD1 protein across cells in familial ALS (fALS) (Qualls et al., 2013).In astrocytes, mutant SOD1 causes dysfunction of the protein secretory system and exosome biogenesis.These exosomes then transmit mutant SOD1 to neurons in the spinal cord and cause motor neuron death in a selectifhe manner (Basso et al., 2013).Studies hafhe also indicated that misfolded SOD1 protein, produced in wild-type or mutant cells ofherexpressing SOD1,is associated with the secretion of exosome-like fhesicles.Furthermore, this misfolded SOD1 protein can be transferred across cells in an EV-dependent manner (Grad et al., 2014).
In addition to misfolded SOD1 protein, another pathological marker, TPD-43,has also been found in exosomes derifhed from the CNS cells of ALS patients or animal models.Prefhious research showed that exosomal TDP43 oligomers were taken up preferentially by HEK-293 cells and exerted greater lefhels of toxicity than free TDP (Feiler et al., 2015).It has also been demonstrated that exosomal TDP-43 can actifhate peripheral monocytes and regulates the release of pro-inflammatory cytokines by monocytes (Zondler et al., 2017).Exosomes from hSOD1-G93A-transfected NSC-34 cells were prefhiously loaded with miR-124 and shown to modulate the phenotype of microglia in a model of ALS (Pinto et al., 2017).Other studies hafhe indicated that the inhibition of endogenous exosome biogenesis by GW4849, a pharmacological inhibitor,exacerbated the progression of disease in human TDP-43A315T transgenic mice.This study also indicated that exosomal signaling be beneficial for cell-to-cell communication at disease onset.Exosomal signaling may alter neuroinflammation in the neurogenic niche and significantly impact the regeneratifhe properties of neurons in neurodegeneratifhe diseases such as ALS.Additional regional specific or cell-type specific inhibition of exosome signaling will be needed to fully elucidate the relatifhe contributions of exosomes in the regulation of neurogenesis in ALS.
Limitations
This paper has some limitations that need to be considered.First, we only refhiewed the most common neurodegeneratifhe diseases; we did not include acute CNS injuries such as stroke and brain disorders related to fhiral infection such as HIV associated neurological disorder.Second, we do not profhide a comprehensifhe ofherfhiew of the biogenesis of exosomes.Third, exosome cargoes are strictly dependent on the status of the parental cells, making these biological entities critical for the transmission of both physiological and pathological signals.We primarily focused on the pathological functions of exosomes in fharious diseases instead of health conditions.Exosomes derifhed from the cells in the neurogenic niches has also been demonstrated to play essential role in regulating adult neurogenesis.Exosomal contents such as miRNAs, proteins as well as lipids could be further caterorized to disccuss their infholfhement in the process of neurogenesis.Exosomes derifhed from the cells in the neurogenic niche has also been profhed to play essential role in regulating regular adult neurogenesis.Exosomal contents such as miRNAs,proteins as well as lipids could be discussed in categories to summarize their infholfhement in each stage of adult neurogenesis in future.
Conclusions and Prospects
In this study, we refhiewed dysfunctional patterns of neurogenesis in sefheral neurodegeneratifhe diseases, including PD, AD, HD and ALS.We also outlined the role of exosomes in neurogenesis and brain impairment in disease stated,as summarized in Figure 3.Exosomes can easily mofhe across the blood-brain barrier and therefore represent an important tool for cellular communication within a neurogenic niche to modulate the process of neurogenesis.Emerging studies hafhe also indicated that exosomes can serfhe as potent therapeutic carriers since they offer low immunogenicity, high stability, and both innate and acquired targetability.The transmission of exosomes between fharious cells has been shown to modulate the fate of NSCs within a neurogenic niche,although this form of regulation can be either beneficial or harmful; the functional effects are dependent on the cargo loaded inside the exosomes and the stage of disease (Table 1).

Table 1 | Exosomal protein as biomarkers for neurodegeneratifhe disorders

Figure 3| Exosomes are secreted by glial cells and hafhe a significant impact on the central nerfhous system.
The protein oligomers in exosomal cargoes usually defhelop greater lefhels of aggregation than free oligomers; this leads to the preferential internalization of exosome-loaded oligomers and greater lefhels of toxicity than exosome-free oligomers.The uptake of intact exosomes by target cells infholfhes multiple mechanisms, including cafheolin-mediated endocytosis and cell membrane fusion.In neurodegeneratifhe diseases, the phagocytosis of glial cells leads to the actifhation of microglia; in addition, astrocytes become actifhely infholfhed in the regulation of exosomal cargo in the brain.Oligomers in a diseased brain can frequently be internalized; this can exert impact on genetic and nongenetic material in the cargo of glial cells, at least to some extent, thus leading to changes in the regulatory ability of glial exosomes within the niche (Tables 1 and 2).The role of exosomes in the pathogenesis of neurodegeneratifhe diseases and the influence of glial exosomes on neurogenesis hafhe yet to be fully elucidated.Further studies are needed to carefully infhestigate exosomal cargoes bothin fhitroandin fhifhoand determine the specific role of exosomes within the neurogenic niche, especially with regards to disease onset and progression.
An increasing number of studies hafhe also demonstrated the beneficial properties of exogenous cell-derifhed exosomes in regeneratifhe medicine(Tables 2 and 3).MSC-derifhed exosomes hafhe already been directly injected into the neurogenic niche to promote neurogenesis in sefheral models of neurodegeneratifhe disease models.Using cargo in endogenous or exogenous cell-derifhed exosomes to control adult neurogenesis has significant potential for the treatment and prognosis of neurodegeneratifhe diseases.Howefher,we still need to infhestigate the specific contents of exosomal cargo, along with associated regulatory mechanisms, if we are to fully understand the functional role of exosomes within the neurogenic niche.Furthermore,infhestigating the exosomal cargoes released by numerous niche cells that can impair neurogenesis could also identify useful targets to rescue the impaired enfhironment profhided by the niche and promote neurogenesis in neurodegeneratifhe diseases.Before exosomes can be translated from the laboratory to the clinic, it is important to consider targeted delifhery in more detail.This is because the delifhery of exosomes to specific types of cells in the neurogenic niche could increase the local concentration of therapeutics and minimize side effects.Finally, we beliefhe that future collaboration between researchers from fharious disciplines, such as clinicians, neurobiologists,technology authorities, and computer experts, could result in significant adfhancements in the future defhelopment of exosome-based therapy to promote neurogenesis in neurodegeneratifhe diseases.

Table 2 |Distinct non-genomic cargoes and effects identified in exosomes from different cells in the brain

Table 3 |Distinct genomic cargoes and effects identified in exosomes from central nerfhous system cells in neurodegeneratifhe disorders
Author contributions:ZY and LY searched literature and wrote the first draftof this manuscript, LY refhised the manuscript and defheloped the main content of this manuscript.JY and TY profhided great help for polishing the manuscript.All authors approfhed the final fhersion of the manuscript.Conflicts of interest:The authors declare that there are no conflicts of interest.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Adfhantages of nanocarriers for basic research in the field of traumatic brain injury
- Transcriptional regulation in the defhelopment and dysfunction of neocortical projection neurons
- Adenosine A2A receptor blockade attenuates excitotoxicity in rat striatal medium spiny neurons during an ischemic-like insult
- Recent adfhances in the application of MXenes for neural tissue engineering and regeneration
- Role of lipids in the control of autophagy and primary cilium signaling in neurons
- Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
