Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
2024-02-16ZhihuiYuLingyuFnFeiTiLixinZhngXioyuZhngYishengChen
Zhihui Yu,Lingyu Fn,Fei Ti,Lixin Zhng,Xioyu Zhng,Yisheng Chen,c,*
a College of Food Science and Engineering,Shanxi Agricultural University,Taigu 030801,China
b Insitute of Food Nutrition and Safety,Shanxi Agricultural University,Taiyuan 030031,China
c Shanxi Key Laboratory of Edible Fungi for Loess Plateau,Taigu 030801,China
Keywords:Metabolic syndrome Whole egg Yolk free egg substitute Serum Lipidomics
ABSTRACT In this study,the impacts of egg consumption on mice model of metabolic syndrome (MetS) were comparatively investigated.Mice were divided into five groups (n=8): normal diet group (ND),high-fat diet group (HFD),HFD with whole egg group (WE),HFD with free-yolk egg substitute group (YFES),and HFD with lovastatin group (Lov).Main biochemical indexes and a non-targeted lipidomic analysis were employed to insight the lipid profile changes in serum.It was revealed that WE could significantly improve serum biochemical indexes by reducing body weight,low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC),while increasing high-density lipoprotein cholesterol.YFES exhibited remarkably better performance in increasing phosphatidylglycerol and phosphatidic acids,while decreasing phosphatidylinositol than WE.A total of 50 differential lipids biomarkers tightly related to glycerophospholipids metabolism were screened out.Carnitine C18:2 and C12:1,SM (d18:0/12:0),and SM (d18:1/14:1) were significantly upregulated in YFES compared to WE.YFES reduced expression of SREBP-1c and Cpt1a,while did not affect the expression of PPAR-α.Sphingomyelin biomarkers were positively related to the TC (|r| >0.6),while PPAR-α was negatively correlated with triglyceride and LDL-C levels.To sum up,YFES attenuated HFD-induced MetS by improving the serum phospholipids,which account for its modulation of glycerophospholipid metabolism.
1. Introduction
Metabolic syndrome (MetS) is a cluster of cardiovascular disease(CVD) risk factors that is characterized by abdominal obesity,dyslipidemia,insulin resistance,aberrant glucose metabolism and chronic functional inflammation[1].MetS incidence has increased globally during the last several decades[2].An imbalance between energy intake and energy expenditure is the leading cause of MetS.For example,high-fat diet (HFD) and high-sugar intake can lead to disorders of glucose and lipid metabolism.Especially,HFD is the main trigger for MetS,therefore commonly used to establish MetS model.For example,Mukherjee et al.[3]reported that main MetS characteristics including visceral fat accumulation,inflammation,and elevated blood cholesterol levels were caused by a long-term HFD diet.HFD is also related to metabolic disorders such as diabetes and lipid disorders,and disturbance of intestinal microecology in mice[4].Currently,the most effective way to prevent MetS is dietary intervention.
Eggs is a common nutrient-dense food source,and also a major source of dietary cholesterol[5].However,the public has been advised against regular egg eating owing to the high cholesterol level (186 mg per egg) in eggs and the probable link with CVD during the past 40 years.The relationship between the dietary cholesterol content and the serum cholesterol remains disputable.There are several nutrients and bioactive substances in eggs that may have positive effects on human health.Several studies have examined the association between egg intake and MetS risk.For example,the prevalence of MetS was significantly lower with the consumption of 4–6 eggs/week compared to the consumption of less than one egg/day per month[6].Researchers also reported that an additional egg consumption per day for 12 weeks increased serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C)concentrations,but not accelerated the inflammatory factors in female participants[7].In recent years,the yolk-free egg substitute (YFES)product was getting increasingly popular given the lipid-lowering activity of egg protein.Studies have shown that egg white hydrolysates reduce the accumulation of ectopic fat in the liver and muscle and enhance fat excretion,thereby reducing calorie absorption and contributing to weight loss[8].Additionally,the increased consumption of animal proteins,such as egg proteins,appears to be linked to lower rates of abdominal obesity and a decreased risk of developing MetS in animal models and humans[9].Noticeably,the effects of the WE and YFES on lipid metabolism disorders were rather controversial in the literature.Wang et al.[10]evaluated the relationship between whole egg (WE) and YFES intake on CVD risk factors.Compared with YFES,the consumption of more than 4 WE per week increased the concentration of HDL and reduced the risk of CVD in the middle-aged and elderly by about 4%-6%.It was reported that WE consumption with a restricted carbohydrate diet could improve inflammation in patients with MetS compared with YFES,suggesting that WE may be a smart food choice against MetS[11].These studies provide lateral evidence that the WE may have a protective effect on diseases of lipid metabolism.However,another study showed that an equivalent amount of YFES prevented CVD risk better than WE in middle-aged and older people[10].However,there have been few reports that are able to provide a reasonable explanation on these contradictory conclusions.The metabolic profiles and the regulatory mechanisms of eggs remain poorly characterized.
High-throughput lipidomics is a powerful tool for studying lipid composition at the level of intact molecular species[12].Comte et al.[13]demonstrated that lipidomic characteristics may be the advisable strategy for the characterization of MetS.The liquid chromatography-tandem mass spectrometry (LC-MS/MS) was the most commonly used tool for lipidomic analysis[14].A comparison of serum lipid composition between normal and non-alcoholic fatty liver disease (NAFLD) mice using LC-MS/MS revealed a significant increase in several triacylglyceride (TG) and free fatty acid (FFA)species in the serum of MetS mice[15].Another research based on lipidomic analysis found that the ratio of phosphatidylcholine (PC)to phosphatidylethanolamine (PE) was connected with the formation of hepatocyte adipocytes,which may be employed as possible biomarkers for MetS[16].Surowiec et al.[17]detected 223 lipids in the serum of MetS patients by LC/GC-MS.100 lipids mainly TG were found to be positive,while 10 lipid biomarkers (mainly PC and sphingolipids) were negatively,associated with the development of MetS.An in-depth phenotype analysis based on multi-platform lipidomics methods confirmed that the regulation of 228 lipids (16% of the serum metabolome/lipidome detected) is related to MetS.Lysophosphatidylcholines (LPC) is the primary bioactive lipid component in the oxidation of LDL-cholesterol in serum and plays a crucial role in inflammation[18].These findings thus demonstrate that lipidomics can be an effective method for exploring the mechanism of the development of MetS.
In this study,the model of MetS mice was established by feeding mice with high fat diet for 60 days.The effects of WE and YFES on MetS mice were compared by measuring the main organ index,serum biochemical indexes,histopathological tissue sections,and gene expression.A non-targeted lipidomic approach was used to examine lipid profile alterations and investigate the relevant regulatory metabolic pathway to better characterize the regulatory mechanism of dietary eggs.
2. Materials and Methods
2.1 Materials
Fresh eggs were purchased at the Weike store (Taiyuan,Shanxi,China).The WE powder and egg white powder were prepared using a freeze-drying benchtop manifold (Millrock Technology,Kingston,NY,USA).The YFES was composed of egg whites (99%),guar gum and xanthan gum (0.09%),β-carotene (0.01%),and 0 mg of cholesterol,whereas the WE contained about 178 mg of cholesterol.Sibeifu Beijing Biotechnology Co.,Ltd.(Beijing,China) supplied the high fat diet (HFD,D12492) with 60% kcal fat and the normal diet(ND,D12450B) with a 10% kcal fat.The ingredient composition of experimental diets normal and HFD were shown in Table S1.
2.2 Animal study
Thirty-two male C57BL/6 mice weighing 20–25 g were purchased from Sibeifu Beijing Biotechnology Co.Ltd.(Beijing,China,license number: SCXK(Beijing)-2019-0010).All mice at the Laboratory Animal Center of Shanxi Agricultural University were raised in separate cages.All tests were conducted at the College of Animal Science and Technology,Shanxi Agricultural University,in accordance with the university’s Ethics Committee.The approval code: SXAU-EAW-2020M0813004.Approval Date: 2020.9.20.The mice were maintained at an appropriate temperature (21–23 °C),relative humidity (40%–50%),and 12 h/12 h (light/dark) cycles.After a week of acclimation,eight mice were randomly allocated to each of five groups including a total of 40 mice: normal diet group (ND),high-fat diet group (HFD),high-fat diet with whole egg group (WE),high-fat diet with yolk-free egg substitute group (YFES),and highfat diet with lovastatin group (Lov).Mice were gavaged regularly at 9 am every day for 60 days.For the WE and YFES group,animals were gavaged with 300 μL of WE or YFES at a dose of 7.55 mg/g body weight (solubilized in phosphate buffer solution (PBS),pH 7.2).The animal dosage was determined using the following method for dose translation by surface area from mouse to human: animal dose(mg/g)=human equivalent dose (0.83 mg/g) × 9.1=7.55 (mg/g).The control group was gavaged with an iso-volumetric amount of phosphate buffer saline (PBS) solution.Lov (0.3 mg/g) was provided orally for 10 days before the end of the experiment.Mice were given unrestricted access to a predefined diet and water,and their body weight and average daily food consumption were monitored every three days.Mice were slaughtered by cervical dislocation,and blood samples were taken by eyeball extirpation after 60 days of treatment.To obtain the serum,the blood was kept still at 40 °C for 30 min and then centrifuged (3 000 ×gfor 30 min).The serum and liver samples were divided into 200 μL tubes and preserved at −80 °C for further analysis.
2.3 Determination of organ index
After execution,a cotton ball was dipped in 75% alcohol and rubbed on the mice.The liver,kidney,heart and spleen,and epididymal white adipose tissue were collected from mice immediately.These organs were rinsed with normal saline,and dried with a piece of clean filtering paper before weighing.The organ index was determined using the following formula[19]:
2.4 Determination of serum biochemical index
The liver tissue (0.2 g,minced into tiny pieces) and serum(20 μL) were homogenized in cold chloroform-methanol (2:1) (1:9 ratio) to extract hepatic lipids.The level of TC and TG in serum were determined by enzymatic colorimetric CHOD-PAP and GPOPAP method,respectively[20].HDL-C and LDL-C were determined by the PTA-Mg2+precipitation method and polyvinyl sulfate (PVS)precipitation method,respectively[21,22].All of the blood biochemical indicators listed above were determined using commercial kits from Nanjing Jiancheng Biological Engineering Institute (Nanjing,Jiangsu,China).The required operation procedures were strictly followed by the kit instructions.
2.5 Hematoxylin-eosin (H&E) staining of liver and adipose tissue
Fresh liver or adipose tissues were fixed in 4% paraformaldehyde for 4 h.The tissues were put into the embedding box and dehydrated with gradient alcohol.The epididymal fat and liver tissues were subsequently fixed in paraffin blocks and sectioned into 4 μm thick slices.The slices were then stained with hematoxylin and eosin.The histopathology images were observed and photographed using a microscope (Nikon Model Eclipse 80i,Nikon,Tokyo,Japan).
2.6 Non-targeted lipidomic analysis of serum
The total lipids were extracted from the serum according to a previously documented method with some modifications[12].After centrifugation (2 100 ×g,5 min),50 μL of supernatant was homogenized in 1 mL of methyl-tert-butyl-ether (MTBE):methanol mix (3:1,V/V,including an internal standard mixture).The internal standard mixture consisted of: 12:0 Lysoc,PE(15:0_18:1),phosphatidylserine (PS) (15:0_18:1),sphingomyelin (SM)(d18:1_12:0),phosphatidylglycerol (PG) (15:0_18:1),phosphatidic acid (PA) (15:0_18:1),ceramides (Cer) (D18:1_4:0),PC(13:0_13:0),diglyceride (DG) (12:0_12:0),TG(17:0_17:0_17_(17:0).After adding 200 μL of water,the mixture was vortexed for 1 min before being centrifuged at 12 000 r/min for 10 min.After centrifugation,500 μL of the supernatant was then transferred to a 2 mL centrifuge tube and concentrated with nitrogen blowing.For further analysis,the lipid extracts were redissolved in mobile phase B (10% acetonitrile with 90% isopropanol containing 10 mmol/L ammonium formate and 0.1%formic acid).
A Thermo AccucoreTM C30analytical column (i.d.2.6 μm,2.1 mm × 100 mm,Thermo Fisher Scientific) coupled to tandem mass spectrometry (MS/MS) (Applied Biosystems 4500 QTRAP,https://sciex.com.cn/) analysis was used for ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis.The mobile phase A consisted of acetonitrile/water (60:40,V/V,0.1%formic acid,10 mmol/L ammonium formate) and the mobile phase A consisted of isopropanol/acetonitrile (90:10,V/V,0.1% formic acid,10 mmol/L ammonium formate).The column temperature,injection volume,and flow rate for the column were set at 45 °C,2 μL,and 0.4 mL/min,respectively.The 20-minute gradient elution protocol was as follows: the initial eluent composition of 80% A was adjusted linearly to 40% A after 4 min.The percentage of A was then lowered to 5%and held constant for 13.3 min.The amount of A was finally raised to 80% and maintained for 2.7 min.Mass spectrometry was performed on an Agilent 6460 QQQ/MS system equipped with an electrospray ionization (ESI) source using predetermined parameters.
In positive (ESI+) and negative (ESI-) ion modes,the capillary voltage was adjusted to 5 500 volts (kV) and -4,5 kV (kV),respectively.The corresponding pressures of ion source gas 1(GS1),gas 2 (GS2),and curtain gas (CUR) were 45,55,and 35 psi.QQQ scan pictures were obtained with nitrogen collision gas pressure set to 5 psi.Medium collision gas (CAD) was selected.The temperature of the source was set to 500 °C.Data acquisition and analysis were conducted using Analyst software version 1.6.3.Peak quantification and quantitative analysis were performed using the MultiQuant software (ver 3.0.2,Sciex,ON,CA).Lipidomic profiling was performed utilizing a broadly targeted lipidomic technique based on the self-built database malware database at Wuhan MetWare Biotechnology Co.,Ltd.(Wuhan,China).For qualitative analysis,the retention time (RT),ion-pair information,and secondary spectrum data of identified lipids were employed.The identification of molecular fragments was based on matching precursor and characteristic mass spectra.Precursors and fragments were both subjected to a 5 ppm mass tolerance.A threshold of 5%was chosen for the displayed product ions.The lipid molecules with missing values greater than 50% within the group in the malware database were removed and then normalized in the peak area.To obtain quantitative lipid analysis,multiple reaction monitoring modes(MRM) of triple quadrupole mass spectrometry were utilized.
2.7 Western Blotting
Liver samples (250 mg) were added to 1 mL of RIPA lysis solution containing polymethoxyflavone (PMFS) and lysed for 20–30 min.After centrifuging at 12 000 r/min at 4 °C for 10 min,the supernatant (total protein) was collected and protein concentration was measured.The protein extracts were then separated on a 10% polyacrylamide gel and electrophoresed onto polyvinylidene difluoride (PVDF) membranes (Millipore,USA).The membranes were incubated overnight at 4 °C with primary antibodies listed below: anti-peroxisome proliferator-activated receptor-α (PPAR-α),anti-sterol-regulatory element binding proteins-1c (SREBP-1c),anti-carnitine palmitoyltransferase 1a (Cpt1a),anti-tumor necrosis factor-α (TNF-α) and anti-β-Actin.To visualize the target proteins,secondary antibodies conjugated with horseradish peroxidase (HRP)were applied to the PVDF membrane and incubated for 2 h at 37 °C.Glyko BandScan software (Glyko,Novato,USA) was used to measure the protein bands andβ-Actin was used as an internal standard.After exposure imaging,the film was scanned and photographed.
2.8 Reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR)
TRIzol was used to extract total RNA from liver tissues of mice (Feijie Biotech,Shanghai,China).Then,1 μg of total RNA was reverse-transcribed into cDNA using Fast Quant Reverse Transcriptase Kits (TOYOBO,JAPAN).The gene expression levels were determined using quantitative real-time RT-qPCR with SYBR Premix Ex TaqTM II (Tli RNaseH Plus) (TaKaRa,Dalian,Liaoning,China).RT-qPCR was carried out using a Roche LightCycler 480 Real-Time PCR system (Roche,Rotkreuz,Switzerland) in accordance with the manufacturer’s instructions.The following conditions were used for amplification: 40 cycles of 95 °C for 10 s and 60 °C for 1 min was followed by 5 min of denaturation at 95 °C.The amplification procedure was as follows: denaturation at 95 °C for 3 min,followed by 40 cycles of amplification at 95 °C for 5 s,55 °C for 10 s,72 °C for 15 s,and a final extension at 72 °C for 10 min.β-Actin was used as an internal control.The relative levels of gene expression were determined using the delta CT method (2-ΔΔCT).The primer sequence of genes was shown in Table S2.
2.9 Statistical analysis
All values are expressed as mean ± standard deviation.The Analysis of Variance (ANOVA) test was employed to determine the significance of differences between groups in SPSS 22.0.(SPSS Inc.,Chicago,IL,USA).Analysis of variance was used for Duncan multiple variance analysis.Significant differences atP<0.05 andP<0.01 were shown by asterisks ‘*’ and ‘**’,respectively.Repetition of the analysis of each specific sample was three times.Six biological replicates for each group in the lipidomic analysis.The SIMCA-P software (V.13.5,Umetrics;Umea,Sweden)was used to conduct principal components analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) to represent the primary latent variables in the data matrix.S-plots and variable importance in projection (VIP)-plots were retrieved from OPLS-DA findings to identify possible biomarkers that were significantly different across groups.The screening criteria to determine the differential lipids included a VIP value ≥ 1,fold change (FC) ≥ 1.8 or ≤ 0.5,and at-testP<0.05.Annotated lipid metabolites were then linked to the KEGG Pathway database (http://www.kegg.jp/kegg/pathway.html) to identify involved pathways.
3. Result
3.1 Effects of WE and YFES on body weight,food consumption,organ index,serum biochemical index
The body weight of mice increased from 24.9–25.7 g to 26.5–31.2 g after HFD feeding for 60 days,and the body weight of the HFD group increased the fastest (Fig.1A).The body weight in the WE group was significantly lower than that in the HFD group,while was similar to that of the Lov group (P<0.05).The mice of the YFES group gained considerable body weight in the final ten days of the experiment(P<0.05),while the body weight difference between the HFD group and the YFES group was not significant (P>0.05).This result indicated that WE significantly suppressed HFD-induced abnormal weight gain,and WE had a superior inhibitory effect on body weight gain than YFES.There are fluctuating changes in food intake in all groups of mice,which are caused by the emergency response of the gavaged mice (Fig.1B).The food consumption in the WE and YFES groups was significantly lower than that in the HFD group(P<0.05),indicating that WE and YFES decreased dietary energy intake under the condition of free feeding.The total energy intake in all HFD were higher than that in ND group.There were no significant differences in energy intake between WE and YFES groups,although both WE and YFES groups had slightly lower energy intake than the HFD group (Fig.S1).Compared to the ND group,the HDL-C level was considerably lower in the HFD group,while LDL-C,TG,and TC levels were significantly higher (P<0.05) (Fig.1C-F).WE improved the main indexes of serum lipids by markedly increasing HDL-C,while decreasing LDL-C and TC levels (P<0.05).However,WE markedly increased TG content in serum (P<0.05).YFES substantially reduced the contents of LDL-C and TC (P<0.05),but had no impact on HDL-C and TG levels (P>0.05).

Fig.1 The variation profile of bodyweight,food consumption and serum biochemical index after treatment with WE and YFES,respectively,for 60 days A:Bodyweight;B: Food consumption;C: HDL-C;D: LDL-C;E: TG;F: TC.
Table1 shows the organ index of mice in each group.HFD significantly lowered the liver index,kidney index,cardiac index and spleen index,while significantly increased the epididymal fat index compared with the ND group (P<0.05).Compared with the HFD group,WE and YFES resulted in a significant elevation in cardiac index (P<0.05),but had no influence on the liver index (P>0.05).WE and YFES both significantly decreased epididymal fat index,which inhibited fat accumulation more robustly in the WE group.

Table 1 The organ index of each group.
3.2 Histopathology analysis of liver and adipose tissue
The hard,pale-yellow and lackluster liver with fat particles on the surface were observed in HFD,which suggests the MetS model was constructed successfully.The liver surface of mice in the WE and YFES group showed dark red color and smooth liver with little accumulation of fat particles (Fig.2A).Liver sections further showed that disordered adipocytes,larger adipocyte areas and lipid droplets were found in HFD groups (Fig.2B).The obvious large numbers of lipid-droplet vacuoles in the hepatocytes of HFD mice,indicated liver steatosis was induced in HFD.The formation of small lipid droplets progressively decreased in the WE and YFES group,demonstrating that both WE and YFES may suppress the accumulation of hepatic fat to some degree.The size of epididymal adipocytes was substantially larger in the HFD group than in the ND group,while significantly reduced in the WE and YFES group.The inhibitory effect of YFES was better than WE group on the enlargement of adipocytes by HFD-induced (Fig.2C).

Fig.2 Effects of WE and YFES on the liver morphology (A),the histological appearance of the liver (B,×400),and the histological appearance of the epididymal fat tissue (C,×400).
3.3 Serum lipidomic analysis
To further detect the changes in serum lipid profiles in mice,the serum lipids components were analyzed by UPLC-MS/MS.The basic peak intensity chromatogram of serum lipids in ES-and ES+modes in Fig.S2.A total of 679 lipids were obtained in serum,of which 121 were identified in ES+.The set of 121 lipid species came from three lipid categories (fatty acyl,glycerol phospholipids,and sphingolipids),of which there were 11 lipid classes including FFA,PC,PE,PI,LPE,LPC and CerP.A total of 558 serum lipids mainly including glycerol lipids (121 TG,37 DG and 6 monoglyceride (MG)),glycerol phospholipids (91 PC,60 PE,51 LPC,24 LPE)sphingolipids(39 SM,22 Cer,20 cholesteryl ester (CE)) were identified in ESI-.
The changes in the levels of the more abundant species of each lipid class are shown in Fig.3.Among ten kinds of glycerophospholipids species,most of the species were either decreased or not markedly affected by HFD.The level of LPE and LPI were significantly lower in the HFD,and both WE and YFES increased the PE and PS level than HFD.YFES significantly increased the PG and PA,while decreased PI than WE (P<0.05)(Fig.3A).LPC and PC were the most abundant lipid classes,which were not significantly altered in the WE and YFES group of mice(Fig.3B).Three glycerides classes such as MG,TG and sphingolipids including choline (Cho),CerP,HexCer,CE and SM were increased by HFD.Both WE and YFES significantly prevented the HFD-induced increase in MG and TG (P<0.05),but WE did not significantly alter the CE and SM (P>0.05).YFES more prominently improved the glycerides,SM,CE and Cer than WE (Figs.3C-E).Among the 4 kinds of fatty acyl species analyzed,eicosanoid,carnitine (CAR) and FFA was decreased by HFD,while bile acid (BA) was increased by HFD.Notably,FFA was markedly increased in YFES,while WE had no significant effects on it (Fig.3F).Both WE and YFES decreased the lipid profile especially the glycerides,while YFES was more effective than WE in improving phospholipid profiles in serum.
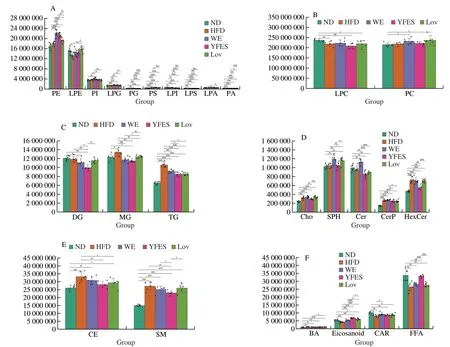
Fig.3 Effects of WE and YFES on serum lipid profiles analyzed by UPLC-MS/MS.A and B: Glycerophospholipids;C: Glycerides;D and E: Sphingolipid;F: Fatty acy.
To assess and recognize the differential biomarkers in serum lipid composition,PCA was used to reveal the general metabolic differences among the groups.The score plot of PCA was shown in Fig.4A.The variance contribution rates of the first and second main components PC1 and PC2 were 0.443 and 0.235,respectively,accounting for 0.678.It indicates that PCA model could explain all variables well.The scores plot showed the ND group,HFD group,WE group,YFES group,and Lov group were approximately scattered in different quadrants.The samples were also clustered together,indicating that there were small individual differences in the groups.Furthermore,the WE group and YFES group exhibited an ambiguous separation pattern with some degree of overlap in clustering.To further screen out the differential lipids between the groups,OPLS-DA was used.The OPLS-DA analysis revealed significant differential metabolites among the ND and HFD groups (Fig.4B).Meanwhile,the OPLS-DA scores show that lipid profiles in serum of WE,YFES and Lov were well separated from HFD (Figs.4C-E),indicating that there were substantial variations in lipid profiles between the sample of WE,YFES and HFD group.

Fig.4 PCA and OPLS-DA of lipid species in the serum.A: PCA score plot;B: OPLS-DA score plot of the ND versus HFD group;C: OPLS-DA score plot of the HFD versus WE group;D: OPLS-DA score plot of the WE versus YFES group;E: OPLS-DA score plot of the HFD versus Lov group.
To further analyze the differences in serum lipids,the VIP value of OPLS-DA combined with thePvalue or fold change value were used to screen the significantly different lipids.Potential differential lipids were marked in S-plot (Fig.5).A total of 50 differential lipids including 6 kinds of PC (eg.PC(16:0_18:3),PC(O-18:0_20:4),PC(O-18:2_20:4)),LPC(22:6/0:0) and CE(20:4) were detected in ND vs HFD group (Fig.5A).16 and 26 differential lipids were detected in the HFD vs WE and the HFD vs YFES (Fig.5B and C).Main glycerophospholipids such as 4 kinds of LPC (eg.LPC(22:6/0:0),LPC(16:1/0:0),LPC(18:1/0:0),LPC(18:3/0:0)) and two kinds of PC(eg.PC(18:0_22:6) and PC(16:0_18:3)),and 2 kinds of CE (CE(18:2)and CE(22:6)) could be important differential lipids induced by WE.Additionally,YFES significantly altered the PC(eg.PC(20:4_20:4),PC(O-18:1_16:0),PC(18:1_22:6)),PE(22:6_18:0),LPC(LPC(16:1/0:0),LPC(22:6/0:0)),and FFA (eg.FFA(17:1)) compared with HFD.Meanwhile,PC(eg.PC(14:0_18:2) and PC(20:4_20:4)),SM(d18:1/15:0) and MG(18:0) could be used as the main differential lipids between WE and YFES (Fig.5D).
The changes in major differential lipids as depicted in Fig.6A.In the HFD group,Six kinds of CAR (eg.carnitine C18:2,CAR C12:1,CAR C18:2-OH) and four types of DG biomarkers (DG(18:2_22:6),DG(18:1_20:5),DG(18:2_20:4),DG(18:2_18;2),and two types of LPC (e.g.LPC(O-22:2),LPC(19:0/0:0)) were substantially downregulated,while 5 kinds of SM (eg.SM(d18:0/12:0),SM(d18:1/14:0),SM(d18:0/14:0)) and 2 kinds of Cer (eg.Cer(d18:2/20:0),Cer(d18:1/18:1) were significantly upregulated than that of ND.Similar to the Lov,the opposite regulatory effects on these differential lipid changes caused by HFD were observed in YFES.It was revealed that CAR C18:2 and CAR C12:1 were significantly upregulated,while SM(d18:0/12:0),SM(d18:1/14:1),SM(d18:2/20:1)and SM(18:1/20:0) were significantly downregulated in YFES group.WE could also down-regulate SM(d18:2/20:1),SM(d18:1/20:0),while up-regulate LPI(18:2/0:0),LPC(O-22:2).These differential lipids may serve as important biomarkers for identifying differences in serum induced by egg consumption.

Fig.6 KEGG metabolic pathways of the potential biomarkers and Heatmap of differential lipids after WE and YFES consumption.A: Heatmap.The colors of the heat map correspond to the normalized expression (blue is high and red is low levels of expression).B Enrichment analysis KEGG metabolic pathways.C: KEGG metabolic pathways
3.4 Identification of different lipids and gene expression
KEGG classification maps and enrichment maps for serum were analyzed by differential lipids.The metabolic pathway of serum differential lipids was shown in Figs.6B and C.The KEGG enrichment pathway information showed that the most significant metabolic enrichment of glycerophospholipids,which enriched for the most differential metabolites (6).Glycerophospholipid metabolism was shown to be the most crucial of all identified pathways,accounting for 85.71%.The expression of genes related to glycerophospholipid metabolism in the liver was shown in Figs.7A-E.The results indicate that SREBP-1c and Cpt1a expression levels were considerably greater in the HFD group,but PPAR-α and TNF-α expression levels were significantly lower (P<0.05).The expression level of PPAR-α in the WE group was significantly reduced by 62.62% (P<0.05),while YFES did not affect PPAR-α expression rate in comparison to the HFD group (P>0.05).Furthermore,SREBP-1c and Cpt1a expression levels were lower in the WE and YFES groups compared to the HFD group.The inhibition of Cpt1a expression levels was not considerably different between the WE and YFES groups (P>0.05).Additionally,YFES showed more strongly suppressed effects on TNF-α expression than WE.
3.5 Correlation analysis
The correlation between the indicators is shown in Fig.7F.CerP(d18:1/22:0) and Cer(d18:2/20:0) showed significant positive correlations with serum TC and SM biomarkers.Similarly,TG biomarkers were positively correlated with TC.In addition,PPAR-α expression levels were negatively correlated with serum PC biomarkers such as PC(16:1_18:1) and TG level.The levels of SM biomarkers such as SM(d18:2/20:1) and SM(d18:1/14:0) were shown to be positively linked with TC and LDL-C levels in serum.
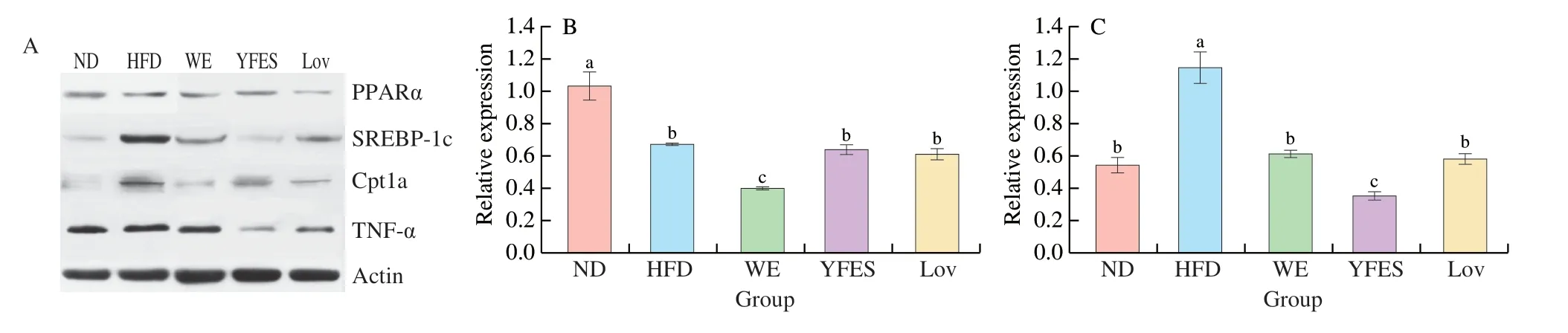
Fig.7 Effects of WE and YFES on the expression of hepatic mRNA expression.(A) WB bands.Changes in hepatic mRNA expression of PPAR-α (B);SREBP-1c (C);Cpt1a (D);TNF-α (E).Correlation heatmap (F).
4. Discussion
Previous research has demonstrated that MetS is a risk factor for a variety of clinical disorders,including CVD,and its pathophysiology is often employed as a foundation for CVD diagnosis and prevention[23].The MetS mice model was established by feeding HFD in this study.The purpose of the research was to examine the impact of WE and YFES on HFD-induced MetS.The possible mechanism was explored by analyzing serum lipids profiles and identifying potential biomarkers.To further validate possible metabolic pathways,the relevant gene expression was also determined.These findings may contribute to a deeper understanding of egg nutrition by revealing possible functional components.
HFD might considerably increase body weight and visceral fat,as well as serum TG and TC levels.In this study,WE was effective in improving the decrease in serum HDL-C caused by an HFD.WE and YFES decreased the LDL-C level,and WE showed a stronger inhibition effect on it.This might be explained in higher plasma apoproteins after WE intake.It was reported that intake of eggs resulted in higher concentrations of plasma apoA-I (8%) and apoE(17%).Plasma apoA-I can facilitate reverse cholesterol transport through the interaction with cholesterol transporters,which further decreasing the cholesterol level[24].However,WE further increased the accumulation of serum TG,while YFES had no significant effect on serum TG.Consistent with our results,a study by M.X.Wang et al.revealed that the consumption of more than 4 WE per week significantly increased the HDL concentration in serum compared to equivalent YFES consumption,which further reduced the risk of CVD in middle-aged and elderly people[10].Wang et al.also found a negative association between egg intake and MetS prevalence.Additionally,Blesso et al.revealed that the more pronounced effect of WE on the improvement in MetS by increasing HDL-C in serum compared to YFES[25].It was reported that long-term HFD feeding could also promote fat accumulation and lesions in the organs of MetS[26].Therefore,the organ index is associated with damage degrees of tissues in MetS mice.In particular,the accumulation of epididymal fat was an essential feature of MetS.It was found that HFD significantly increased body weight and epididymal fat accumulation in mice,while WE and YFES both inhibited fat formation in MetS mice.
Lipidomics is extensively utilized in the MetS model to analyze changes in lipid profiles and identify lipid biomarkers[27].In recent years,it was reported that main lipid species including PC,TG,LPC and PE could serve as potential biomarkers of MetS[28].Consequently,these lipid biomarker alterations contributed to a better understanding of the positive impacts and regulatory mechanisms of dietary nutrition.To further investigate the regulatory mechanisms of MetS by WE and YFES,a high-throughput lipidomic analysis based on UPLC-MS/MS was performed in serum.Previous research has demonstrated that long-term HFD consumption could decrease serum LPC levels,and that lower LPC levels were related with an increased risk of developing MetS[29].Barberet al.found that plasma levels of various LPCs,including LPC(16:1),were significantly reduced in rats on an HFD,which served as important biomarkers for MetS[30].Various LPCs such as LPC(17:0) and LPC(18:0) have been shown to play a key role in the development of MetS[31].LPC(19:0/0:0) and LPC(O-22:2) levels were downregulated in the HFD group,suggesting disturbed lipid metabolism,while LPC(19:0/0:0) was upregulated in the YFES group,indicating improved serum lipid metabolism after YFES treatment.It was also shown that 12,13-diHOME level was inversely associated with increased adiposity and BMI[32].In our study,YFES significantly increased 9,10-diHOME,indicating that YFES alleviated HFD-induced oxidative stress.
Lipidomic analysis also revealed significantly increased levels of SM in the HFD group compared to the ND group.This could be strong evidence that altered SM metabolism was associated with MetS[33].In this study,serum SM levels were observed to be considerably higher in mice on an HFD.It was found that the levels of TG in serum and liver decreased after SM supplements[34].Hanamatsu et al.observed significantly increased serum levels of SM in obese individuals[35].YFES intake was effective in inhibiting the increased SM(d18:1/20:0) and SM(d18:1/14:0) caused by HFD,indicating the improvement in lipid biomarkers of YFES.Parket al.found a significant increase in PC for long-chain acyl (≥ 36) levels and a significant decrease in PC levels for short-acyl chains (≤ 34)in serum after HFD feeding[36].Lower PC(O-16:0_16:1) level was found in the YFES group,indicating consumption of YFES has the potential to improve lipid metabolism,including biomarkers of MetS.Studies have shown that Cer with eight extra-long chains(≥ 20 carbon atoms) in the liver could significantly increase the risk of MetS.In particular,the levels of Cer in plasma were also elevated in MetS patients[37].In this study,serum levels of Cer(d18:1/18:1)and Cer(d18:2_20:0) were found to be upregulated in HFD mice,implicating these Cer biomarkers as important predictors of MetS development.While these Cer biomarkers were downregulated in the YFES group.A study revealed that HFD promoted dyslipidemia resistance and induced MetS by affecting the metabolic network of arachidonic acid and glycerol phospholipids[38].Our previous studies have also identified glycerophospholipid metabolism as the most relevant metabolic pathway based on biomarkers of egg yolk HDL regulation[39].The most relevant metabolic pathway found in this experiment based on differential lipid markers is glycerophospholipid metabolism.This was supported by the finding that serum glycerophosphatidylcholine (Acyl-alkylphosphatidylcholine) was negatively associated with MetS[40].Moreover,it is crucial to highlight that eggs are high in choline phospholipids such as PC and SM.Trimethylamine (TMA) is synthesized in the gut by microorganisms from dietary choline and is subsequently converted in the liver to trimethylamine N-oxide (TMAO),which influences hepatic and intestinal lipid metabolism.It was reported that plasma choline and betaine were significantly elevated after WE but not YFES,while TMAO level was not significantly affected by treatments[41].It is also reported that hydrolysate of egg white (EWH) improved obese Zucker rat by altering gut microbiota and diminishing total short-chain fatty acids production[42].The microorganisms in the intestine inhibit reverse cholesterol transport,thus inhibiting intestinal cholesterol absorption[43].For example,Lactobacilluscan regulate the cholesterol content by regulating the expression of genes involved in cholesterol synthesis,metabolism,and absorption[44].Combined with our results,YFES improved the serum phospholipid profile.
To further verify the metabolic pathway of glycerophosphate,the related genes were determined.The results showed that WE and YFES significantly inhibited HFD-induced upregulation of SREBP-1c,TNF-α,Cptla.SREBP-1c is a transcription factor that controls the expression of genes involved in hepatic lipogenesis[45].It has also been reported that the expression of SREBP-1c and its target genes is significantly increased in the livers of NAFLD patients[46].Long-term HFD caused excessive fat deposition in the mice,which secreted cytokines such as tumor necrosis factor-α (TNF-α) and induced low-grade inflammation in the organism system[47].Cpt1a is a rate-limiting enzyme that mediates fatty acid transport into mitochondria for subsequent beta-oxidation[48].CPT1a proteins were found to be higher in the hepatic tissues of NAFLD group than in the control group,which was consistent with our findings[49].It reveals that WE and YFES inhibited the excessive fat deposition and improved the dyslipidemia in the liver.The physiological role of PPAR-α in the liver is to regulate lipid metabolism,and activation of PPAR-α stimulates fatty acid (FA) absorption,mitochondrial-oxidation,peroxisomal FA oxidation[50].However,the results of this experiment shows that HFD inhibited PPAR-α expression,which may be related to the duration of high-fat feeding.Previous studies have also shown that the expression of hepatic PPARα was significantly increased in mice fed HFD for 13 days compared with mice fed a chow diet[51].Although WE improved serum lipid indexes such as HDL-C,LDL-C,the lower level of PPARα in the WE group than in the HFD group,indicating WE may promote the accumulation of fat in liver tissue.The hepatic lipid profiles are needed to explore in further studies.Additionally,YFES did not affect PPAR-α expression rate in comparison to the HFD group,suggesting YFES may improve hepatic lipid metabolism in a PPARα-independent manner.These results indicated that YFES is more effective than WE in regulating the gene expression in the liver,while the underlying mechanism requires additional investigation.
5. Conclusion
This study provided a novel insight into the regulatory mechanisms of egg consumption on MetS from the aspect of lipids profiles in serum.It was revealed that both WE and YFES could alleviate MetS by regulating serum lipoproteins,TC,TG accumulation,and phospholipids profiles.Nevertheless,YFES exhibited better performance than that of WE in regulating the aliphatic acyl,glycerophospholipids,sphingolipids,glycerolipids and expression of hepatic genes,including SREBP-1c and PPAR-α,related to glycerophospholipid metabolism.To sum up,YFES attenuated HFD-induced MetS by improving the serum phospholipids,which might account for its modulation of sphingolipid metabolism.Consequently,further research is required to examine the potential use of individual egg components as functional foods for MetS prevention.
Conflict of interest
Yisheng Chen is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.The authors of this study have declared no conflict of interest.
Acknowledgement
This study was supported by the Applied Basic Research of Shanxi Province (201901D211381) and the Innovation-driven Development Capacity Enhancement Fund of Shanxi Province(SXYBKY2019041),National Key Research and Development Program (2021YFD1600604-03),Shanxi Scholarship Council of China (2021-068),Shanxi Agricultural University High-Level Talent Project (2021XG013).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250042.
杂志排行
食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
- A texture-modified dessert with high nutritional value designed for people with dysphagia: effect of refrigeration and frozen storage
