Evaluation of the intracellular lipid-lowering effect of polyphenols extract from highland barley in HepG2 cells
2024-02-16YijunYaoZhifangLiBowenQinXingrongJuLifengWang
Yijun Yao,Zhifang Li,Bowen Qin,Xingrong Ju,Lifeng Wang*
College of Food Science and Engineering/ Collaborative Innovation Centre for Modern Grain Circulation and Safety,Nanjing University of Finance and Economics,Nanjing 210023,China
Keywords:Highland barley Polyphenols extract Lipid-lowering effect HepG2 cells
ABSTRACT Active ingredients from highland barley have received considerable attention as natural products for developing treatments and dietary supplements against obesity.In practical application,the research of food combinations is more significant than a specific food component.This study investigated the lipid-lowering effect of highland barley polyphenols via lipase assay in vitro and HepG2 cells induced by oleic acid (OA).Five indexes,triglyceride (TG),total cholesterol (T-CHO),low density lipoprotein-cholesterol (LDL-C),aspartate aminotransferase (AST),and alanine aminotransferase (ALT),were used to evaluate the lipidlowering effect of highland barley extract.We also preliminary studied the lipid-lowering mechanism by Realtime fluorescent quantitative polymerase chain reaction (qPCR).The results indicated that highland barley extract contains many components with lipid-lowering effects,such as hyperoside and scoparone. In vitro,the lipase assay showed an 18.4% lipase inhibition rate when the additive contents of highland barley extract were 100 μg/mL.The intracellular lipid-lowering effect of highland barley extract was examined using 0.25 mmol/L OA-induced HepG2 cells.The results showed that intracellular TG,LDL-C,and T-CHO content decreased by 34.4%,51.2%,and 18.4%,respectively.ALT and AST decreased by 51.6% and 20.7% compared with the untreated hyperlipidemic HepG2 cells.qPCR results showed that highland barley polyphenols could up-regulation the expression of lipid metabolism-related genes such as PPARγ and Fabp4.
1. Introduction
Overweight and obesity are defined as abnormal or excessive fat accumulation that presents a health risk.The Body Mass Index(BMI),a person’s weight (in kilograms) divided by the square of their height (in meters),is an important international standard commonly used to measure obesity and health.Obesity is defined by the World Health Organization (WHO) as a BMI equal to or more than 30,while overweight is defined as a BMI equal to or more than 25.Overweight and obesity are major risk factors for several chronic diseases,including type 2 diabetes,cardiovascular diseases,chronic kidney disease,and cancer.In recent years,nonalcoholic fatty liver disease(NAFLD) has emerged as the most common chronic liver disease,affecting a quarter of the global population[1].Once considered a problem only in high-income countries,overweight and obesity have dramatically risen in low-and middle-income countries,particularly in urban settings.The worldwide prevalence of obesity nearly tripled between 1975 and 2016.By 2016,almost half of the overweight or obese children under 5 lived in Asia.Therefore,obesity has become an urgent problem to be solved.It is generally believed that the most effective way to prevent obesity is to reduce the digestion and absorption of fat.Pancreatic lipase (PL) secreted by the pancreas is a key target for fat absorption[2].PL is responsible for hydrolyzing up to 50%–70% of dietary fat[3-4].The inhibition of this enzyme has attracted the attention of researchers worldwide because the only antiobesity drug approved by the U.S.Food and Drug Administration(orlistat) is a PL inhibitor[5].However,the long-term use of orlistat has been reported to cause many adverse reactions,including cardiovascular and gastrointestinal complications[6].Therefore,exploring natural PLinhibitory active components in plants has gained scientific momentum[7].Among the plant-derived PL inhibitors,phenolic compounds are considered to be the most active[8].
Coarse cereals are characterized by a short growing period,small planting area,low yields,and are generally rich in nutrients.China has a vast land area and different climatic environments;therefore,it can breed food crops from other countries.China’s total coarse cereals output accounts for 17.1% of the world’s total coarse cereals output and 10.0% of China’s total coarse cereals output.China’s dietary guidelines recommend a daily intake of coarse cereals of 50-100 g.Still,adults’ daily intake of coarse cereals is only 14 g as a single type of food,less than one-third of the recommended intake.Highland barley,a staple food in northwest China,is a well-known source of bioactive phytochemicals,including phenolic compounds.Highland barley is the largest cultivated crop with the highest yield,because it is the only crop that can be grown between 4 200 and 4 500 meters above sea level[9].
Researchers have focused on understanding the diet influence on health.In recent years,many studies have found that plant-derived food components have a good lipid-lowering function.The German Nutrition Society recently ranked the evidence on whole grains and health.It determined that there is convincing evidence that whole-grain consumption reduces total and low density lipoprotein (LDL) cholesterol,probable evidence of reducing the risk to type 2 diabetes,and possible evidence of reducing the risk of obesity in adults[10].However,highland barley research mainly focused on beta-glucans[11]and water-soluble polysaccharides[12].Much research has been done on the bioactivity of individual components of coarse cereals;nevertheless,few have explored the lipid-lowering effect of whole-grain highland barley.
Food-derived active ingredients have received considerable attention as natural products for the development of medicine and dietary supplement against overweight and obesity.In practical application,the research on the food variety is more significant than a specific food component.Thus,this study investigated the lipid-lowering effect of highland barley extract via a lipase assayin vitroand in HepG2 cells induced by oleic acid (OA).Then five indexes,triglyceride (TG),total cholesterol (T-CHO),low density lipoprotein-cholesterol (LDL-C),aspartate aminotransferase (AST),and alanine aminotransferase (ALT),were used to evaluate the lipid-lowering effect of highland barley extract.Lipid-lowering mechanism of highland barley extract was preliminarily studied by quantitative polymerase chain reaction (qPCR).
2. Materials and methods
2.1 Chemicals reagents and materials
Coarse cereals were purchased from Shanxi Dongfangliang Life Technology CO.,Ltd.Highland barley was from Beiqing No3.Phosphate-buffered solution (PBS),3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT),dimethyl sulfoxide(DMSO),Dulbecco’s Modified Eagle’s Medium-high glucose medium (DMEM),fetal bovine serum (FBS),trypsin,penicillin(10 kU/mL),and streptomycin (10 mg/mL) were purchased from Gibco Biotechnology Company (Carlsbad,CA).Oil Red O (ORO)solution (0.5% in isopropanol) and bovine serum albumin were purchased from Sigma-Aldrich Inc.(MO,USA).Lipase test assay kit,TG assay kit,T-CHO assay kit,LDL assay kit,AST assay kit,and ALT assay kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,China).
2.2 Sample Preparation
Highland barley samples were ground into powders and extracted withn-hexane in a ratio of samples ton-hexane of 1:4 (g/mL) at 60 °C for 2 h.Then samples were centrifuged at 6 000 r/min.The sediment was extracted with water again at a ratio of 1:8 (g/mL) at 70 °C for 4 h (Robbins,2003).Highland barley extracts were centrifuged(6 000 r/min),and the supernatant was lyophilized.Lyophilized powder was conserved at −20 °C.
2.3 Phenolic composition in polyphenols extract from highland barley by UHPLC-Q-Orbitrap MS
Table 1 shows the chromatographic shows gradient elution procedure.The chromatographic column (Accucore Vanquish C18column) temperature was set to 40 °C,and the flow rate was 0.3 mL/min.The gradient was started and held at 2% B for 1 min,raised linearly from 2% B to 50% B during the next 16 min,and maintained at 99 % B for 0.5 min.Next,the mobile phase returned to 2% B within 0.5 min,and the system was equilibrated for 2 min before the next run.The scanning range wasm/z100−1 500.The ESI source was set as follows: spray voltage: 3.2 kV;sheath gas flow rate: 35 arb;aux gas flow rate: 10 arb;capillary temperature:320 °C.Polarity: positive.The second-level scans of MS/MS were data-dependent scans[13].

Table 1 Chromatographic gradient elution procedure.
2.4 Lipase in vitro assay
The lipasein vitroassay was adapted from previous reports[14].Lipase Type II from the porcine pancreas (Sigma product L3126)was dissolved in ultra-pure water at 10 mg/mL,centrifuged at 16 000 r/min for 5 min,and collected the supernatant.p-Nitrophenol palm butter (p-NPP 8 mmol/L) dissolved in isopropanol was used as the substrate.The assay buffer was PBS (pH 8.2) containing 1% Triton X-100.The control assay contained 20 μL ultra-pure water,18 μL substrate solution,and 40 μL lipase.Highland barley was dissolved in ultra-pure water for a 50 μL total volume.The buffer,enzyme,and berry extracts were mixed,and then the substrate was added to start the reaction.The samples were incubated at 37 °C for 2 h in a microplate reader (Molecular Devices Inc.,San Francisco,CA,USA)and measured at 410 nm.All samples were assayed in triplicate.
2.5 Establishment and identification of hyperlipidemic HepG2 cells model
Human HepG2 liver cancer cells were purchased from Beijing Cuizhu Biological Technology Co.Ltd.(Beijing,China).HepG2 cells were seeded in 6-well plates at a density of 10 000-30 000 cells per well in high DMEM supplemented with 8% FBS and incubated at 37 °C and 5% CO2for 24 h (Thermo Scientific Co.Rockford City,IL).Then the supernatant was replaced by 2 mL of culture medium containing 0.25 mmol/L OA and incubated for 24 h.The hyperlipidemic HepG2 cells were stained with ORO and compared with normal cells to determine whether the model was established successfully.
2.6 MTT assay
Cell viability was determined by MTT assay[15]with some modifications as reflected by the safety of coarse cereals extract.Human HepG2 liver cancer cells were seeded at a density of 5 μL × 103cells/well in 96-well microculture plates and grown at 37 °C and 5% CO2overnight,and then the supernatant was removed.After incubation,the medium was replaced with 100 μL of 1 mg/mL MTT reagent and incubated for another 4 h at 5% CO2and 37 °C.The MTT reagent was removed,and 150 μL of DMSO was added to each well.The plates were shaken for 15 min on a table oscillator and analyzed using a microplate reader (Molecular Devices Inc.,San Francisco,CA,USA) at 490 nm.Each sample was analyzed three times,and the values were averaged.
2.7 Measurement of intracellular lipid-lowering levels of polyphenols extract from highland barley
HepG2 cells were incubated at 1.5 × 105cells/mL in 6-well plates.After 24 h of culture,OA solution was added to the high-fat group.To evaluate the intracellular lipid-lowering effect,the polyphenols extract was added at a concentration of 1 000 μg/mL DMEM to the intervention group under standard culture conditions.The culture medium was removed,PBS was used to wash the unnecessary culture medium 3 times,IP cell lysis buffer was added,and the cells were lysed in an ice bath for 30 min.The levels of TG,LDL-C,T-CHO,ALT,and AST were measured according to the detection kits manufacturer’s recommendations.The protein concentration of each well was detected using a BCA protein quantitative kit (Nanjing Jiancheng Bioengineering Institute,Nanjing,China) to normalize the data.
2.8 Real-time fluorescent quantitative PCR
The total RNA was extracted by Trizol reagent.Total RNA concentration was determined by DU800 ultraviolet spectrophotometer (Beckman Counter).First-strand cDNA was synthesized by the cDNA GoScript™ Reverse Transcription System synthesis kit (Promega,Madison,WI).For real-time analysis,the GoTaq® qPCR Master Mix (Promega,Madison,WI) and Applied Biosystems® 7500 Real-Time PCR Systems (Thermo Fisher Scientific,Waltham,MA) were used.The hot-start activation was started at 95 °C for 30 s,followed by 40 cycles of denaturation at 95 °C for 5 s and extension at 60 °C for 30 s.The primers used are listed in Table 2.Each sample was run in triplicate.The mean threshold cycle values of the samples were compared to the untreated control sample.The relative expression of each target gene was calculated using the 2−ΔΔCtmethod.
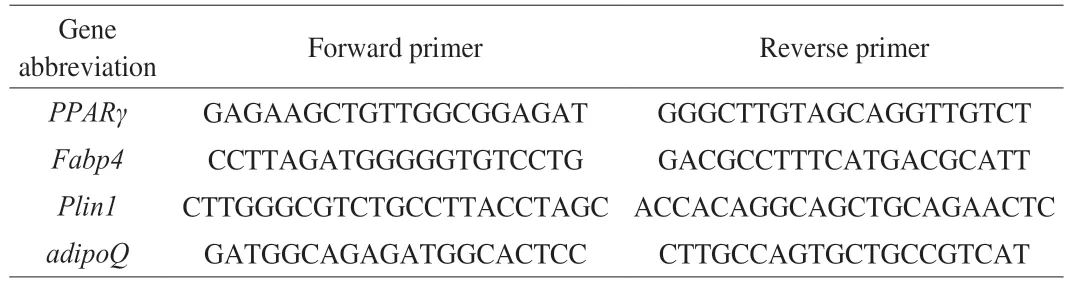
Table 2 Sequence of primers in quantitative real-time PCR.
2.9 Statistical analysis
All tests were performed at least in triplicate.One-way ANOVA was used in statistical analysis.The data,expressed as the mean ± SD,were analyzed by Tukey’s test and SPSS software,with a statistical significance setting ofP<0.05.
At the same timeshe listened to all that was said, and she very much liked to hear thepastor s son speak about the elements and of the great men and women in history
3. Results and discussion
3.1 Substance composition of polyphenols extract from highland barley by UHPLC-Q-Orbitrap MS
Table 3 presents the results of the untargeted metabolomics study on the extract of highland barley based on UHPLC-Q-Orbitrap MS technology.MzCloud,mzVault,and ChemSpider databases were used for the comparative analysis of metabolite components.The priority of metabolite identification was mzCloud >mzValut >ChemSpider >predicted compound.The authentication results with higher priority were adopted if the authentication results of different databases were inconsistent.The mzCloud matching parameters were parent ion and daughter ion.The ChemSpider match parameters were parent ion,molecular formula,and mass tolerance.The mzValut matches parameters were parent ion and daughter ion.Prediction parameters were parent ion and molecular formula.The phenylpropanoids and polyketides in highland barley extract were sorted under the current of positive and negative ions.Many polyphenols and flavonoids’active components were identified in highland barley extract,such as caffeic acid,epicatechin,scoparone,quercetin,naringenin,glyctin,and hyperoside.The results are consistent with Liu et al.,who studied the phenols in blue highland barley[16].

Table 3 Composition and identification of polyphenols in highland barley extract by UHPLC-Q-Orbitrap MS.
Hyperoside and scoparone were selected for total ion chromatogram and secondary mass spectrometry by UHPLC-QOrbitrap MS.Hyperoside,also called quercetin 3-O-β-D-galactoside,is a flavonoid compound in plants of the generaHypericumandCrataegus[17].It has been confirmed that hyperoside has anti-inflammatory[18-19],antioxidant[20],and myocardial protective effects[21].Moreover,Xie et al.revealed that hyperoside protects against acetaminophen-induced liver injury[22].However,the effectiveness and mechanisms underlying hyperoside in lipid-lowering treatments are still unclear.Scoparone (6,7-dimethoxy-2H-chromen-2-on) is a coumarin derivative in multiple plants,especiallyArtemisiacapillarisThunb (Asteraceae).Scoparone has various pharmacological effects in experimental models,including hepatoprotective[23],renoprotective[24],vasorelaxant[25],anticarcinogenic,antioxidant,and anti-inflammatory[26].Scoparone primary metabolism route isO-demethylation to scopoletin or isoscopoletin catalyzed by cytochrome P450 (CYP) enzymes[27].Thus,we hypothesized that the highland barley extract might have a good lipid-lowering effect due to hyperoside and scoparone.
3.2 Lipase-inhibitory activities
Lipase is a carboxyl ester hydrolase,which hydrolyzes triglycerides into glycerol and fatty acids.When lipase activity is inhibited,the body cannot absorb triglycerides,and thus obesity is averted to some extent.Fig.1A shows the inhibition of lipase activity by highland barley extract.Significant inhibition was observed when highland barley extract was added to the cell culture medium.With highland barley extract concentration,the inhibition rate increased from 12.8% to 18.4%.Previously,researchers evidenced that polyphenol-rich extracts from teas,green coffee beans,cacao beans,and berries could inhibit PLin vitroand potentially influence fat digestionin vivo[28-29].In our sample preparation period,highland barley samples were ground into powders and extracted withn-hexane in a ratio of samples ton-hexane of 1:4 (g/mL)at 60 °C for 2 h to remove lipids.And it ensured that the subsequent extracts were mainly polyphenol compounds,thus eliminating the interference of lipids on the functional evaluation.Gordon et al.found that the cloudberry extracts prepared with hexane to remove lipid-like components were as effective as the original sample[29].This strongly suggests that the inhibition was not due to lipid derivatives in the extract.
3.3 Changes in hyperlipidemic HepG2 cells morphology after ORO staining
The HepG2 control group cells arranged in fusiform polygon exhibited clear edges,as shown in Fig.2A.Fig.2B shows the morphology of HepG2 cells 24 h after being cultured.After adding OA,the lipid droplets gradually appeared in the cells (Fig.2C).ORO,an azo dye,is a strong lipid solvent that bonds with TG to form lipid droplets.The solubility of lipid-soluble dyes in tissues and cells lipids is much greater than in solvents.When cells are mixed with dye,the dye dissolves in the lipids of the tissue,making the lipid droplets within the cell appear orange.After successfully inducing HepG2 cells by OA,lipids were stained red with ORO,as shown in Fig.2C and 2D.The intracellular lipid droplets in the model group are distributed around the nucleus of round cells inside the clear edge of the plasma membrane.The results were similar to Chenkai et al.,where the hyperlipidemia HepG2 cells model was designed to measure the lipid-lowering effects of Pu’er tea[30].
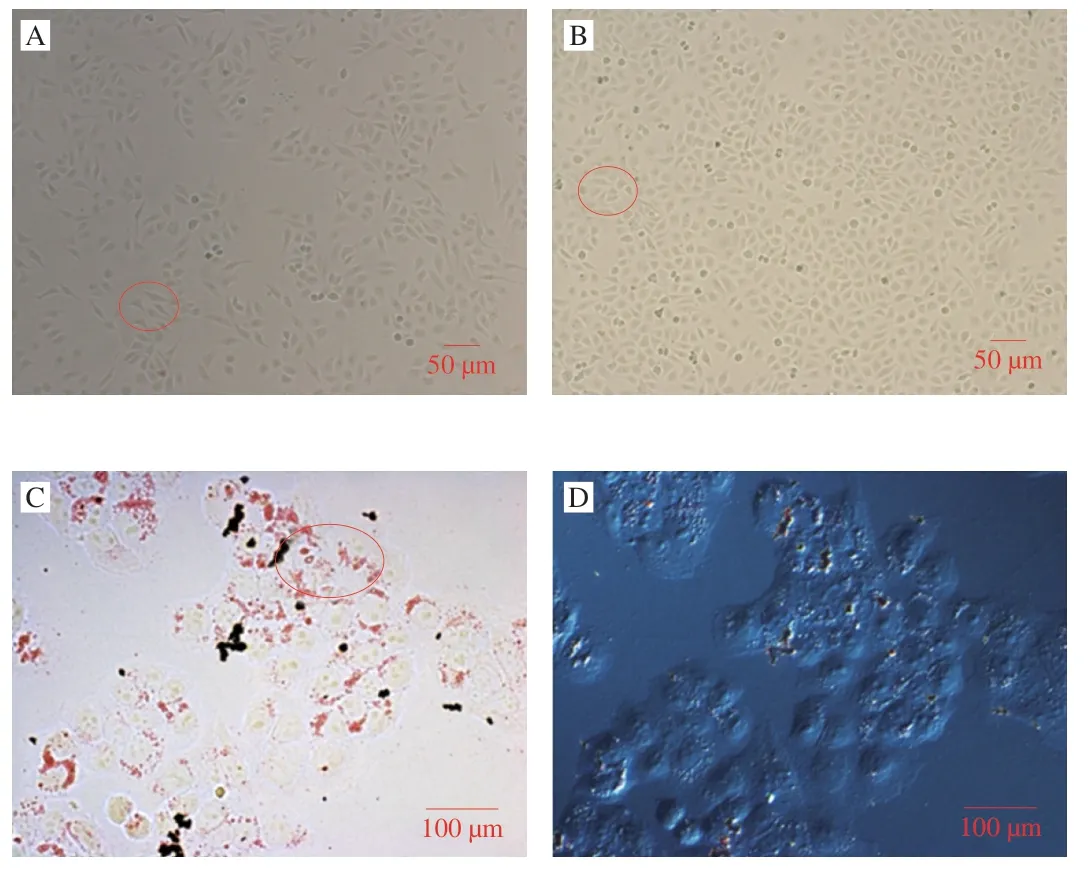
Fig.2 Morphology of HepG2 cells under different conditions.Control group(A,B);OA=0.25 mmol/L (C,D).
3.4 Lipid-Lowering Effects of polyphenols extract from highland barley on oleic acid-induced HepG2 cells
Coarse cereals have several potential functions,such as antioxidation,anti-obesity,and anti-inflammation.However,some anti-nutritional factors may produce certain adverse reactions[31]by causing an immunological reaction and metabolizing toxic products when accumulated[32].Therefore,it is necessary to assess their safety on HepG2 cells.As shown in Fig.1B,the HepG2 cells’survival rate decreased with highland barley extracts.However,when the extract concentrations were higher than 1 000 µg/mL,the HepG2 cells’ survival fell below 90%,indicating a threshold to maintain HepG2 cells’ survival.Aqueous extracts of the cereal were used in this experiment,so the extracts were safer.The results were similar to those reported by Chao et al.,who showed that acetone-extracted defatted adlay seed meal inhibited the HepG2 cell line in a concentration-dependent manner (25-500 µg/mL).However,the aqueous fraction had shown no toxicity at concentrations higher than 5 mg/mL[33].Given our extraction method,1 000 µg/mL was a safe concentration for HepG2 cells.
The TG,LDL-C,T-CHO,AST,and ALT concentrations of OA-induced HepG2 cells showed a significant increase compared with normal HepG2 cells.Fig.3A shows the TG,T-CHO,and LDL-C concentrations in OA-induced HepG2 cells.After OA induction,intracellular TG and T-CHO increased significantly.Intracellular TG decreased by 34.4%,and T-CHO decreased by 18.4% compared with the hyperlipidemic HepG2 cells without highland barley extract polyphenols.Song et al.studied the effect of beta-glucan from highland barley on cholesterol metabolism in mice.Their results showed that TG decreased by 24.14%,and T-CHO decreased by 15.53% after beta-glucan treatment.The highland barley extract in our experiment has a better effect than single beta-glucan[34].LDL is a lipoprotein particle that carries cholesterol into peripheral tissue cells.LDL can be oxidized into oxidized-LDL (OX-LDL).Excess LDL,especially OX-LDL,promotes cholesterol accumulation on the arterial wall,causing arteriosclerosis over time.We could observe that highland barley extract significantly reduced the LDL-C content.As shown in Fig.3A,LDL-C decreased by 51.2% after highland barley extract treatment.
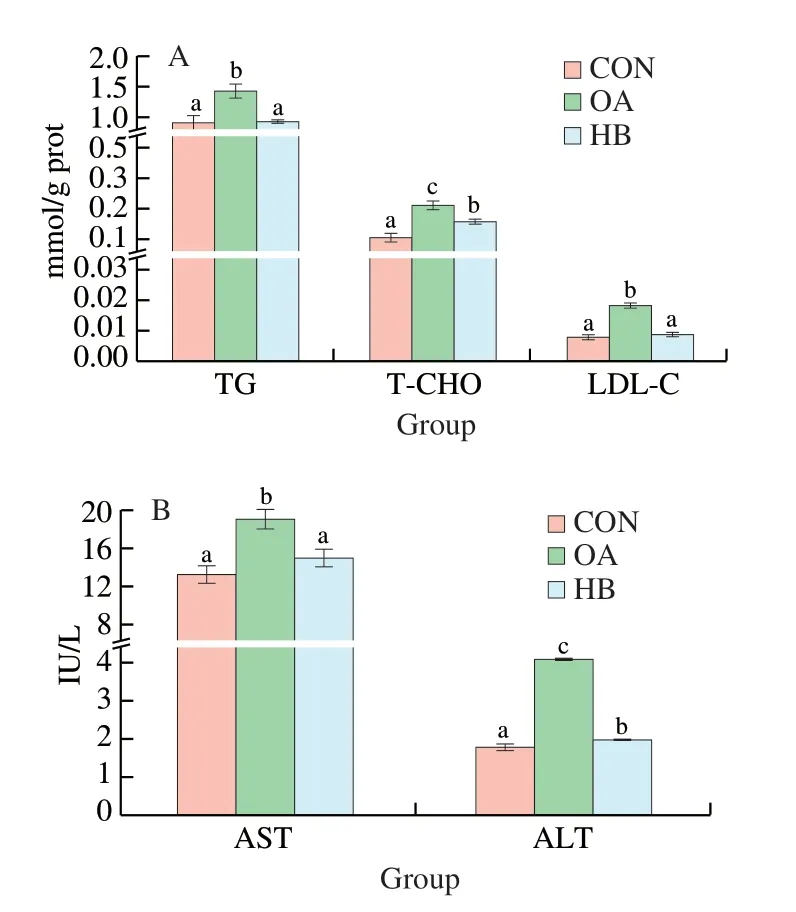
Fig.3 Lipid-lowering effects (A) TG,T-CHO,LDL-C;(B) AST,ALT of highland barley extract on hyperlipidemic HepG2 cells.
AST and ALT are indicators of liver health.When 1% of the liver cells are necrotic,the enzymatic activity in the blood can be doubled,so transaminase (especially ALT) is a sensitive marker of acute liver cell damage.Fig.3B illustrates that highland barley extract reduce ALT and AST contents,resulting in a protective effect on the liver.After highland barley extract treatment,ALT and AST decreased by 51.6% and 20.7%,respectively.Highland barley bran extract contains several phenolic acids,such as caffeic acid,syringic acid,p-coumaric acid,ferulic acid,and sinapic acid,which possess inhibitory effects on CML formation[9].We inferred that highland barley extract effectively reduced TG’s content due to the abundant polyphenols.
3.5 Lipid-lowering effects of highland barley polyphenols on mRNA expression
To directly determine whether the lipid-lowering effect of highland barley extract polyphenols involved PPARγ,Fabp4,and Plin1,mRNA expression in HepG2 cells was measured by qPCR in the OA induced-HepG2 cells treated with highland barley extract.PPARγ,highly expressed in adipose tissue,belongs to the nuclear receptor superfamily and is involved in multiple physiological processes,such as regulating glucose and lipid metabolism gene expression[35-37].Many studies have found that food-derived compounds,such as apigenin,restrained NAFLD progression,including attenuating high-fed diet (HFD) induced lipid accumulation and oxidative stressin vivo[38].Our experimental results also showed that PPARγ expression levels in hyperlipidemic HepG2 cells decreased but significantly increased by 11.84% after highland barley extract treatment (Fig.4A).To a certain degree,these observations suggest that highland barley extract could revert NAFLD progression and has potential importance in NAFLD therapy.

Fig.4 Effect of highland barley extract on mRNA expression levels of(A) PPARγ;(B) Fabp4;(C) Plin1 and (D) adipoQ on hyperlipidemic HepG2 cells.
As shown in Fig.4B,the Fabp4 level in OA-induced HepG2 cells treated with highland barley extract was 79.9% higher than untreated cells.Fabp4 is a member of the FABP superfamily and is highly expressed in adipocytes[39].The Fabp4 regulatory functions in lipid metabolism have recently been reported.Cytoplasmic FABPs could enhance the solubility of free fatty acid (FFA) and transport to specific enzymes and cellular compartments,further control the mitochondria and peroxisomes oxidation,the endoplasmic reticulum re-esterification,the lipid droplet storage or the nucleus regulation of gene expression[40-41].The results showed that highland barley extract could up-regulate Fabp4 gene expression and positively affected lipidlowering.
OA treatment significantly inhibited lipid droplet formationrelated genes,such as PLIN1,and highland barley extract inhibits the expression of PLIN1 target genes without an evident activating effect on PLIN1 (Fig.4C).The results were similar to those reported by Kimmel et al.,who showed that PLIN gene expression decreased during adipocyte differentiation[42].PLIN1 is highly expressed in white adipocytes and actively regulates lipolysis[43].However,PLIN1 was rarely expressed in mature brown adipocytes.Under basal conditions,PLIN1 prevents excess lipolysis by limiting access of hormone-sensitive lipase,ATGL,and its co-activator CGI-58,to LDs[44].However,the relevant mechanism of PLIN1 on the lipid metabolism of adipocytes is still not completely characterized.
The AdipoQ gene is associated with adiponectin expression,an adipocytokine abundantly produced by adipose tissue.AdipoQ is expressed and secreted completely from adipocytes and has been identified as a cytokine with anti-diabetes,anti-inflammatory,and anti-atherosclerosis properties[45].Previous studies have found that AdipoQ protein mainly binds to adiponectin receptors (adipor-1 and adipor-2) to regulate the biological activity of AMP kinase and PPAR ligand,thereby indirectly regulating fatty acid oxidation and carbohydrate uptake[46].As shown in Fig.4D,the sample had not played a significant role in AdipoQ.This may be due to adiponectin,which decreased after highland barley extract treatment.
4. Conclusion
According to other studies on lipid-lowering in food crops,we inferred that polyphenols played an important role.Based on the results presented here,it can be concluded that polyphenols extract from highland barley had a certain lipid-lowering effect in the Hepg2 cell model.The polyphenols extract could effectively reduce intracellular TG,T-CHO,LDL-C,AST,and ALT.Future studies using 3T3-L1 adipocytes and animals are needed to explore the signaling pathway under the action of phenolic monomers.
Acknowledgments
This research was financially supported by the National Key Research and Development Program of China (2021YFD2100904),the National Natural Science Foundation of China (31871729,32172147),the Modern Agriculture key Project of Jiangsu Province of China(BE2022317),the Modern Agricultural Industrial Technology System Construction Project of Jiangsu Province of China (JATS [2021] 522),and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).The funding agencies had no role in the study design,the collection,analysis,or interpretation of data,the writing of the report,or the decision to submit the article for publication.And I would like to appreciate the musical actor Qu Yi.You have brought me hope and joy in such world that is not so good as I expected,especially in the hard period I finished this job.So thank you and wish you all well in the future.
Conflict of interest
The authors declare no conflict of interest.
杂志排行
食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
