Structure and immunomodulatory activity of Lentinus edodes polysaccharides modified by probiotic fermentation
2024-02-16JingjingLingMeinZhngXiohnLiYunYueXioweiWngMengzhenHnTinliYueZhouliWngZhenpengGo
Jingjing Ling,Mein Zhng,Xiohn Li,Yun Yue,Xiowei Wng,Mengzhen Hn,Tinli Yue,Zhouli Wng,Zhenpeng Go,*
a College of Food Science and Engineering,Northwest A&F University,Yangling 712100,China
b Xi’an GaoXin No.1 High School,Xi'an 710065,China
Keywords:Lentinus edodes polysaccharide Lactobacillus fermentum fermentation Structural analysis Immunoregulatory activity Nuclear magnetic resonance
ABSTRACT Plant-based fermentations provide an untapped source for novel biotechnological applications.In this study,a probiotic named Lactobacillus fermentum 21828 was introduced to ferment Lentinus edodes.Polysaccharides were extracted from fermented and non-fermented L.edodes and purified via DEAE-52 and Sephadex G-100.The components designated F-LEP-2a and NF-LEP-2a were analyzed by FT-IR,HPGPC,HPAEC,SEM,GC-MS and NMR.The results revealed that probiotic fermentation increased the molecular weight from 1.16 × 104 Da to 1.87 × 104 Da and altered the proportions of glucose,galactose and mannose,in which glucose increased from 45.94% to 48.16%.Methylation analysis and NMR spectra indicated that F-LEP-2a and NF-LEP-2a had similar linkage patterns.Furthermore,their immunomodulatory activities were evaluated with immunosuppressive mice.NF-LEP and F-LEP improved immune organ indices,immunoglobulin (IgG and IgM) and cytokines concentrations;restored the antioxidation capacity of liver;and maintained the balance of gut microbiota.F-LEP displayed better moderating effects on the spleen index,immunoglobulin,cytokines and the diversity of gut microbiota than NF-LEP (200,400 mg/kg).Our study provides an efficient and environment-friendly way for the structural modification of polysaccharides,which helps to enhance their biological activity and promote their wide application in food,medicine and other fields.
1. Introduction
Lentinusedodes,an edible mushroom mainly cultivated in East Asia for about 2000 years,has been extensively served as food and medicinal ingredients[1].L.edodesis reported to contain various bioactive compounds,such as proteins,fibers,lipids,vitamins,carbohydrates,niacin and minerals[2,3].L.edodespolysaccharide is the main component ofL.edodesfruiting body and mycelium cell wall.Over the past decade,increasing research evidence reported thatL.edodespolysaccharide is an important macromolecular substance with multiple biological activities such as anti-oxidation,anti-tumor and immune regulation[4-6].The bioactivities of polysaccharides are affected by their physiochemical characteristics and structure,including monosaccharide composition,molecular weight (mw),type of glycosyl linkage and degree of branching[3].For example,the elimination of the triple-helix conformation ofL.edodespolysaccharide significantly reduced its anti-tumor activity[7].Lentinan with a molecular weight of about 1 × 106Da could inhibit S-180 tumor in mice,while the activity of small molecular weight lentinan fragments degraded by formic acid decreased[8].A study showed that the inhibition rate of lentinan on lipid peroxidation was related to the monosaccharide composition of polysaccharides[9].
Plant fermentations,in which lactic acid bacteria (LAB) often dominate,provide an under-exploited source for new biotechnology applications.LAB fermentation technology has been used for food preservation and improvement of sensory and functional properties,including antioxidant,hypoglycemic and lipid-lowering[10-12].Studies published to date have indicated that fermentation affects the structure and function of plant polysaccharides.For example,Lactobacillus plantarumNCU116 was used to fermentMomordicacharantia,leading to a change in polysaccharide structure that was associated with stronger anti-diabetic activity[13].Similarly,L.plantarumNCU 116 fermentation led to a decrease in average molecular weight and enhanced anti-diabetic functionality of carrot polysaccharides[14].Bacillussp.DU-106 fermentation altered themwand monosaccharide composition ofDendrobium officinalepolysaccharide,which madeD.officinalepolysaccharide a potential immunoregulator to alleviate diseases[15].However,little information is available on the impact of LAB fermentation on the structure and biological activity of edible mushroom polysaccharides.
The immune system plays a significant role in monitoring,defending and regulating body functions[16].Recently,studies on food or bioactive components for improving human immunity have attracted intense global debate.The immune regulation ability of lentinan is proven in many aspects.Lentinan can regulate and stimulate T cells to proliferate and differentiate,leading to a series of immune responses;promote the synthesis of immunoglobulins G (IgG) and immunoglobulins M (IgM) by B lymphocytes;improve serum antibody titer;activate natural killer (NK) cells[17,18].Recently,polysaccharides with immunoregulatory activity are proven to maintain host health by modulating gut microbiota[19].On the one hand,undigested polysaccharides regulate the gut microbial composition,meanwhile,the effects of polysaccharides on immune response and intestinal barrier function are related to major metabolites[20].Lentinan is a polar macromolecular compound,and its special structure is closely related to immune activity.Therefore,obtaining modified polysaccharides with immunomodulatory functions by LAB fermentation is of great significance for designing disease intervention strategies.In this study,L.fermentum21828 was selected to fermentL.edodesby determining the acid and bile salt tolerance,intestinal adhesion and fermentability of 9 probiotic strains inL.edodesliquid (data not shown).L.fermentum21828 was applied to fermentL.edodes,and the fermentation alteration on structure and immunoregulatory activities ofL.edodespolysaccharide was measured.Our study aimed to modify the structure ofL.edodespolysaccharide by microbial fermentation to improve its biological activity,and further elucidate the structure-activity relationship between the immunomodulatory activity and structure ofL.edodespolysaccharide.
2. Materials and methods
2.1 Materials and reagents
L.edodeswas purchased from a local shopping mall in Yangling(Shaanxi province,China).DEAE-52 cellulose and Sephadex G-100 were purchased from Shanghai Yuanye Bio-Technology Co.,Ltd.Monosaccharide and dextran standards were obtained from Sigma Chemical Company (St.Louis,MO,USA).Trifluoroacetic acid(TFA),deuterium oxide (D2O),cyclophosphamide (CTX) and levamisol hydrochloride were purchased from Macklin Biochemical Co.,Ltd.(Shanghai,China).The superoxidase dismutase (SOD) kit,catalase (CAT) kit and malon dialdehyde (MDA) kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,China).ELISA kits of IgG and IgM were obtained from MultiSciences(Hangzhou,China).
2.2 Preparation of fermented of L.edodes
L.edodeswas washed,dried and powdered with a grinder.The obtainedL.edodespowder was homogenized with distilled water at a ratio of 1:25 (m/V) and subjected to ultrasound pretreatment (500 W,15 min).Then the mixture was autoclaved and cooled before adding the microorganism.For fermentation,CICCLactobacillusfermentum21828 (1%V/Vinoculum) was inoculated into the non-fermentedL.edodesliquid (NFL),and seed liquid was obtained after fermentation at 37 °C for 10 h.Subsequently,the seed liquid(107CFU/mL) was inoculated into NFL with 0.8% (V/V) inoculum and fermented at 37 °C for 10 h to acquire the fermented liquid (FL).Both the fermented group and the unfermented groups were heated at 80 °C to stop the fermentation reaction.They were further used for the polysaccharide extraction.
2.3 Extraction and purification of L.edodes polysaccharide
The NFL and FL were extracted and enzymatically treated at 45 °C (4.5% (m/m)) for 90 min at a pectinase (EC 3.2.1.15) to cellulase (EC 3.2.1.4) ratio of 2 : 1.The supernatant was collected by centrifugation (2 286 ×g,15 min) and concentrated under vacuum at 55 °C.Then the concentrate was mixed with four volumes of ethanol overnight at 4 °C to precipitate polysaccharides.The precipitate was obtained by centrifugation (4 770 ×g,10 min) and then redissolved in ultrapure water.The polysaccharide solutions were deproteinized with Sevag reagent[21]and decolorized using the H2O2method[22].Finally,the polysaccharide solutions were dialyzed for 24 h in dialysis bags (MWCO 3.5 kDa) at 4 °C and lyophilized to acquire the fermented and non-fermentedL.edodespolysaccharides,named as F-LEP and NF-LEP,respectively.
The crude polysaccharide was purified with a DEAE-52 cellulose column (2.6 cm × 30 cm) equilibrated with 0,0.1,0.3,0.5 mol/L NaCl solutions at a flow rate of 1 mL/min.The eluted fractions(6 mL/tube) were collected and the phenol-sulfuric acid method was utilized to measure the total carbohydrate contents.The fraction eluted with 0.1 M NaCl solution was collected due to the high yield,named as F-LEP-2 and NF-LEP-2,respectively.The F-LEP-2 and NF-LEP-2 were further purified by a Sephadex G-100 column(1.6 cm × 60 cm) using ultrapure water as the eluent at a flow rate of 0.3 mL/min.The eluents were collected in 3 mL/tube,and a total of 50 tubes were collected and determined for carbohydrate content.A dialysis bag (MWCO 3.5 kDa) was applied to remove the salt with distilled water for 24 h.The retentates were lyophilized and designated as F-LEP-2a and NF-LEP-2a,respectively.
2.4 Basic chemical composition analysis
The total polysaccharides and protein contents were detected using the phenol-sulfuric acid method[23]and Bardford’s method[24],respectively.
2.5 Structure characterization
2.5.1 Molecular weight
Themwof polysaccharides was determined by HPGPC (Shimadzu LC-10A HPLC system) equipped with a refractive index detector.Sample separation was operated by three columns connected in series (Shodex Ohpak SB-805/804/803 HQ,8 mm × 300 mm,Showa Denko K.K.).Mobile phase was 0.05 mol/L NaCl solution with a flow rate of 0.6 mL/min.We adopted T-series dextran standards to establish the calibration curve (mw=1 152,5 000,11 600,23 800,48 600,80 900,148 000,273 000,409 800 and 667 800 Da).According to the fitted curve of standard dextrans,the molecular weights of NF-LEP-2a and F-LEP-2a were calculated.
2.5.2 Monosaccharide composition
Polysaccharide samples (5 mg) were mixed with 1 mL of trifluoroacetic acid (TFA,2.5 mol/L) and then kept at 121 °C for 2 h to accomplish acid hydrolysis.The reactant was dried under a stream of N2,and the hydrolysate was repeatedly dissolved in methanol(1 mL) and dried with nitrogen three times.Finally,the reactants were dissolved in sterile water and filtered through a 0.22 μm membrane(Merck,USA).The monosaccharide composition was analyzed using a Dionex ICS-5000 ion chromatography equipped with a DionexTMCarboPacTMPA10 column (250 mm × 4.0 mm,10 μm) at 30 °C.Distilled water (A) and 100 mmol/L NaOH (B) constituted the mobile phase at a flow rate of 0.5 mL/min.The elution gradient was as follows: 2.5%–20% B for 0–30 min,40% B for 30–45 min,and 2.5% B for 45–60 min.
2.5.3 Fourier transform infrared (FT-IR) spectrum
The sample (2 mg) was mixed with KBr powder (200 mg) and pressed into a pellet.A FT-IR (Vetex70,Germany) was used to acquire the signal in a vibration zone of 4 000 to 400 cm-1.
2.5.4 Nuclear magnetic resonance (NMR) spectrum
The lentinan fraction (30 mg) was dissolved in 0.5 mL of D2O and subjected to1H and13C NMR experiments.The 1D (1H and13C)and 2D (HMBC,COSY and HSQC) NMR spectra were recorded on the Bruker AVANCE HD III 600 MHz Spectrometer (Bruker Technologies,Germany).
2.5.5 Methylation and GC-MS analysis
Methylation analysis was performed using a previously reported method with slight modifications[25].Polysaccharide samples (10 mg) were dissolved in 1.0 mL ultrapure water,then 1.0 mL of carbodiimide (100 mg/mL) was added and allowed to react for 2 h at room temperature.After 1.0 mL imidazole was added to the reactants,the samples were divided into two equal volumes,and 1.0 mL of NaBH4(30 mg/mL) and 1.0 mL of NaBD4(30 mg/mL) were added respectively and reacted for 3 h.Subsequently,100 μL of glacial acetic acid was added to terminate the reaction.The above reactants were dialyzed and lyophilized for further analysis.The lyophilized samples were dissolved in 500 μL dimethylsulphoxide (DMSO),1 mg of NaOH dry powder was added and incubated for 30 min,and then methyliodide (50 μL) was added slowly and allowed to react for 1 h.Then the reactants were extracted with dichloromethane (2 mL)and washed three times with distilled water.The dichloromethane phase was collected and evaporated.TFA (2 mol/L) was added to the residues for hydrolysis at 121 °C for 1.5 h and excess TFA was removed by decompression evaporation at 30 °C.Ammonia (2 mol/L,50 μL) and NaBD4(1 mol/L,50 μL) were added to the mixture and incubated at room temperature for 2.5 h.Glacial acetic acid (20 μL)was added to terminate the reaction.After drying the samples with nitrogen,the reactants were dissolved in 250 μL of methanol and dried with nitrogen.Next,acetic anhydride (250 μL) was added and the mixture was allowed to react at 100 °C for 2.5 h.The reactants were added with 1 mL of distilled water and incubated for 10 min.500 μL dichloromethane was added to the reactant and the sample was washed three times with distilled water.Finally,the dichloromethane phase was collected and analyzed via gas chromatography-mass spectrometry (GC-MS,7890A/5977B,Agilent Co.,USA).
2.6 Immunomodulatory activity of the NF-LEP and F-LEP
2.6.1 Animals and treatments
BALB/c mice (Male,6–7 weeks old,weight (18 ± 2) g) were obtained from Hunan SJA Laboratory Animal Co.,Ltd.(Hunan,China).All animal experiments were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the U.S.National Institutes of Health (NIH Publications No.8023,1978),and approved by Animal Ethics Committee of Northwest A&F University.After a week of acclimatization,all mice were randomly assigned to nine groups (n=6): the normal control group(Normal),model group (Model),positive control group (Positive),NF-LEP low dose group (NF-LEPL),NF-LEP medium dose group(NF-LEPM),NF-LEP high dose group (NF-LEPH),F-LEP low dose group (F-LEPL),F-LEP medium dose group (F-LEPM) and F-LEP high dose group (F-LEPH).Normal control group was administered with normal saline,and the other eight groups were intraperitoneally injected with CTX at 80 mg/kg body weight (BW)/d for 4 consecutive days.The polysaccharide groups were respectively administered with different levels of NF-LEP and F-LEP (100,200 and 400 mg/kg).The mice of positive group were treated with 20 mg/kg levamisole hydrochloride by gavage,and the normal and model group were administered with 0.9% normal saline solution.Intragastric administration was conducted once per day in each group for 8 days.
2.6.2 Determination of immune organ index
The mice were sacrificed by cervical dislocation,meanwhile the thymus and spleen were immediately removed after dissection,washed with pre-cold saline and weighed.The spleen or thymus index was calculated by the following formula: spleen or thymus index=weight of spleen or thymus (g)/BW (g).
2.6.3 Determination of immunoglobulin contents in serum
Blood samples of all mice were collected and centrifuged at 4 °C (860 ×g,15 min),and the upper layer containing the serum was acquired.The levels of IgG and IgM in the serum were measured using ELISA kits according to the manufacturer’s protocols.
2.6.4 Histological characterization
The colon tissues of mice were fixed in 4% paraformaldehyde for 48 h and paraffin-embedded.After division into 3 μm sections,hematoxylin-eosin (H&E) staining was used and then dehydration was carried out.The sections were observed under a microscope and the scanning images were generated to discover histological differences.The villus length was measured by Image Pro Plus software (v6.0;Media Cybernetics).
2.6.5 Determination of liver SOD,CAT and MDA levels
The liver homogenates were prepared according to the previous methods[26].The supernatant of liver homogenates was collected by centrifugation (21 380 ×g,10 min) at 4 °C.SOD,CAT and MDA activities were determined by kits based on the manufacturer’s protocols.
2.6.6 16S rDNA sequencing of gut microbiota
Genomic DNA was extracted from the fecal samples stored in sterile microtubes using the DNA Kit (Omega Bio-tek,Norcross,GA,USA) based on the manufacturer’s directions.The bacterial community composition in fecal samples was analyzed by 16S rRNA gene amplicon sequencing.PCR targeting the V3-V4 region of the 16S rRNA gene with primers 338F and 806R,and subsequent amplicon sequencing were performed by Shanghai Majorbio Biopharm Technology Co.,Ltd.Sequences from all samples were quality filtered and paired using the merge-illumina-pairs application and Fastp (v0.19.6).Operational taxonomic units (OTUs) clustered at 97%identity and an OTU table was generated using Usearch v.7.0.Nonchimeric sequences were submitted to Ribosomal Database Project Classifier for taxonomic assignment.
2.7 Statistical analysis
All results were expressed as the mean ± standard deviation.Oneway analysis of variance (ANOVA) with Tukey’s test from SPSS 20.0 software was applied to analyze the differences between different groups,and theP<0.05 was deemed as significant.
3. Results and discussion
3.1 Extraction and purification of the polysaccharides from NFL and FL
The crude polysaccharides were obtained from fermentedL.edodes(FL) and non-fermentedL.edodes(NFL) by water extraction,ethanol precipitation,deproteination,decolorization,dialysis and freeze-drying,which were named as F-LEP and NF-LEP,respectively.F-LEP and NF-LEP were separated by the DEAE-52 cellulose column with 0,0.1,0.3 and 0.5 mol/L NaCl solutions (Fig.1A–B).Three fractions were obtained from NF-LEP and F-LEP,and NF-LEP-2 and F-LEP-2 were then filtered through a Sephadex G-100 column due to high yields.As shown in Fig.1C and D,each fraction had a single and symmetric peak,indicating that they were homogeneous fractions,designated as NF-LEP-2a and F-LEP-2a,respectively.The chemical compositions of NF-LEP-2a and F-LEP-2a were presented in Table 1.The contents of total polysaccharides in NF-LEP-2a and F-LEP-2a were 75.72% and 77.68% with the yields of 2.25% and 2.79%,respectively,implying that polysaccharide was the major component in NF-LEP-2a and F-LEP-2a.The protein contents of these two polysaccharides were barely detected.Total polysaccharides content of F-LEP was higher than that in NF-LEP,consistent with previous findings[13].
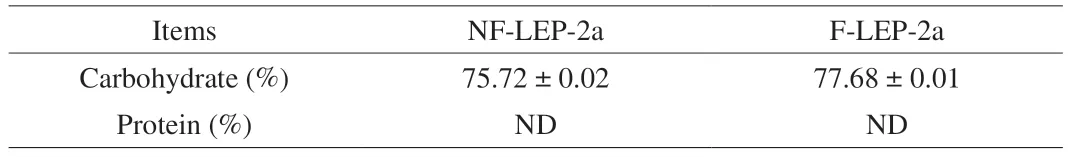
Table 1 Chemical compositions of NF-LEP-2a and F-LEP-2a.

Fig.1 Elution curves of NF-LEP (A) and F-LEP (B) on DEAE-52 column,and elution curves of NF-LEP-2 (C) and F-LEP-2 (D) on Sephadex G-100 column.
3.2 Physicochemical properties of NF-LEP-2a and F-LEP-2a
FT-IR is extensively used to analyze the functional groups in organic molecules.The FT-IR spectra of NF-LEP-2a and F-LEP-2a were separately analyzed (Fig.2A).NF-LEP-2a and F-LEP-2a presented characteristic peaks of polysaccharides with bands of 3 500-3 300,3 000-2 800,1 700-1 200 and 1 100-500 cm−1.The intense peaks at approximately 3 400 cm-1corresponded to the O–H stretching vibrations.The peaks at approximately 2 925 cm−1were attributed to the stretching vibrations of the C–H bonds.The absorption peak at 1 643 cm−1might correspond to–OH bending vibration of water[27,28].Absorption peaks in the range of 1 160–1 000 cm−1were due to the vibrations of C–O–C and C–O–H[29].The peak at 890-900 cm−1could be identified as theβ-type glycosidic linkages[30].The absorption band at 914.26 cm−1was assigned to the ring vibrations of pyranoses.In summary,the FT-IR spectra of NF-LEP-2a and F-LEP-2a exhibited high similarity except for the absorption peak at 881 cm−1.The weak peak in F-LEP-2a may be due to the hydrolysis ofβglycosidic bonds byL.fermentumfermentation.

Fig.2 FT-IR spectra (A) and HPGPC chromatograms (B) of NF-LEP-2a and F-LEP-2a.
NF-LEP-2a and F-LEP-2a showed a single peak on highperformance gel permeation chromatography (HPGPC)chromatograms (Fig.2B),demonstrating that they were all homogeneous.According to the fitted curve of standard dextrans,the molecular weights of NF-LEP-2a and F-LEP-2a were calculated to be 1.16 × 104and 1.87 × 104Da,respectively,indicating that LAB fermentation altered the molecular weight of lentinan.Similarly,Bacillussp.DU-106 fermentation increased themwofD.officinalepolysaccharides (from 4.92 × 105to 5.21 × 105Da)[15].Fermentation withMonascus ankaaltered the proportions of oat polysaccharides fractions with differentmw.After fermentation,the fraction with 104
The monosaccharide compositions of NF-LEP-2a and F-LEP-2a analyzed by high performance anion exchange chromatography(HPAEC) were shown in Table 2.NF-LEP-2a and F-LEP-2a were composed of the identical monosaccharide profiles and chiefly contained glucose,galactose,mannose and fucose.According to the literature,glucose,galactose and mannose commonly appeared in lentinan[33].The proportion of glucose in NF-LEP-2a increased from 45.94% to 48.16% afterL.fermentumfermentation,while galactose and mannose were dropped from 29.58% to 28.77% and from 13.00% to 12.02%,respectively.The results suggested that probiotic fermentation slightly altered the monosaccharide compositions of polysaccharides,which were in line with previous reports[15].
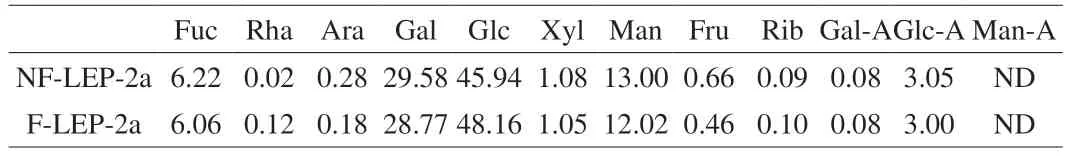
Table 2 Monosaccharide compositions (%) of NF-LEP-2a and F-LEP-2a.
Scanning electron microscope (SEM) was commonly applied to confirm the morphology of polysaccharides.The surface morphologies for NF-LEP-2a and F-LEP-2a under SEM at magnifications of 5 000 × are presented in Fig.3.NF-LEP-2a has a smooth surface with a flaky appearance,consisting of a few protrusions and attached fragments.By contrast,F-LEP-2a had an uneven surface and there were many irregular pores.The differences in surface morphologies suggested thatL.fermentumfermentation may change the structural features of NF-LEP-2a.
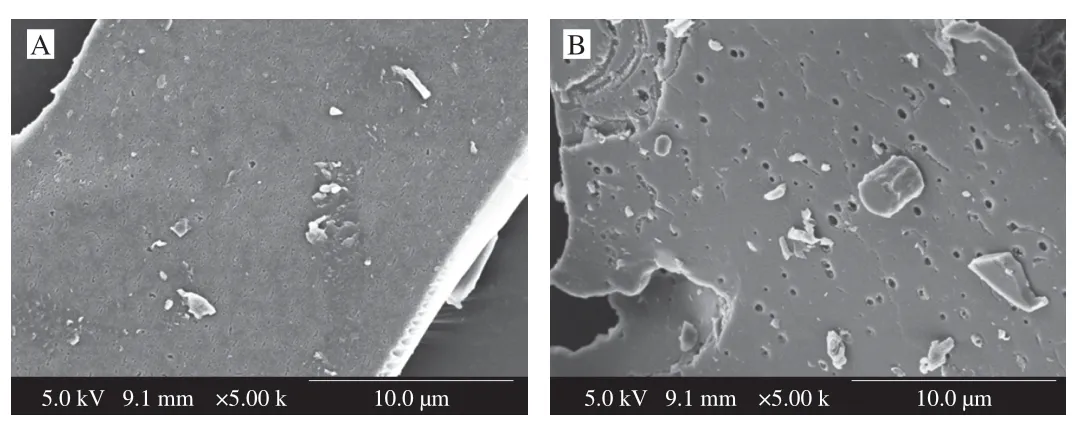
Fig.3 Scanning electron micrographs (5 000 ×) of NF-LEP-2a (A) and F-LEP-2a (B).
3.3 Linkage patterns of NF-LEP-2a and F-LEP-2a
The methylation results revealed that the major linkage patterns of NF-LEP-2a and F-LEP-2a were similar,although some differences were detected in the molar ratios of glycosidic bonds (Table 3).The terminal residues of NF-LEP-2a consisted of T-Fucp,T-Manp,T-Glcpand T-Galpwith the corresponding molar ratios of 4.248:8.463:12.538:0.988.The backbone of NF-LEP-2a mainly contained 6-Glcpand 6-Galp,while the branches of NF-LEP-2a contained 3,6-Glcpand 2,6-Galp.Similarly,a novel polysaccharide fromLentinusedodesconsisted of aβ-1,6-linked glucan and branched at C-4 with side chains[34].Further studies proved that (1 → 6)-β-Dglucan isolated fromL.edodesexerts important biological activity[35].By contrast,F-LEP-2a was accompanied with decreased 2,6-Galpand 3,6-Glcpafter fermentation treatment,and it did not contain 3,6-Manpor 2,6-Glcp.Based on the contents of branching,linear and terminal residues,the degree of branching (DB) of NF-LEP-2a and F-LEP-2a was calculated.The DB values of NF-LEP-2a and F-LEP-2a were 0.45 and 0.43,respectively,which was consistent with the view that the polysaccharides with highermwpossess smaller DB values[36].Hyperbranched NF-LEP-2a and F-LEP-2a may possess features such as good solubility,low viscosity and abundant end-group functional groups,making them potential resources for biological applications.In short,the methylation results revealed thatL.fermentumfermentation did not markedly change the linkage patterns of the NF-LEP-2a.Both NF-LEP-2a and F-LEP-2a contained glucose and galactose units in the backbone,and glucose and traces of mannose and fucose residues in the branched chains.
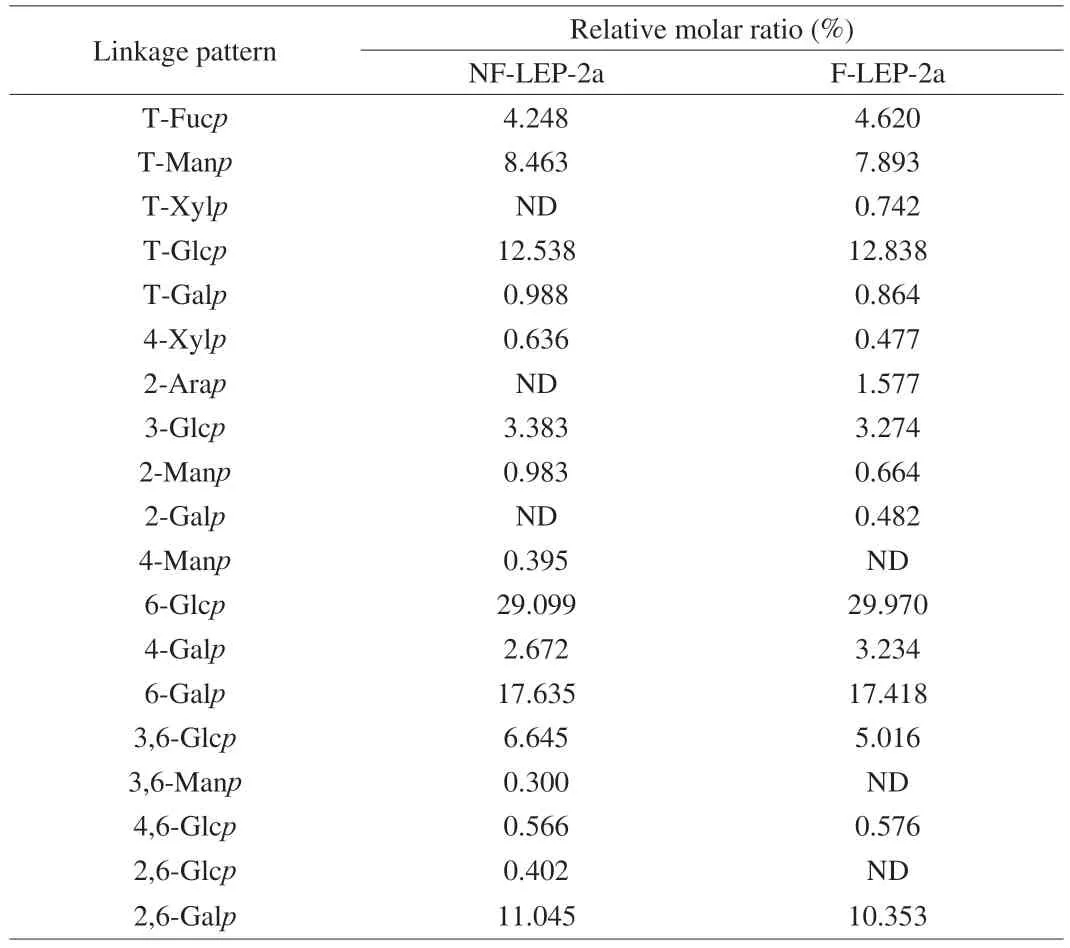
Table 3 Linkage analysis of NF-LEP-2a and F-LEP-2a.
3.4 NMR spectra analysis of NF-LEP-2a and F-LEP-2a
As shown in Fig.4 and 5,NF-LEP-2a and F-LEP-2a were further analyzed by 1D and 2D NMR.The main chemical shifts of sugar residues were assigned with reference to the methylation results and the literature values[37,38].The main chemical shifts of NF-LEP-2a concentrated in the region ofδ3.0–5.5 in the1H NMR spectrum(Fig.4A),which is a typical polysaccharide signal mode.The13C NMR spectrum (Fig.6A) showed carbon signals mainly appeared betweenδ60 and 110.From the1H-NMR and13C-NMR spectra,some characteristic signals were observed.Multiple anomeric hydrogen(H-1) signals in the anomeric regionδ4.5-5.5 and anomeric signals atδ90-110 indicated that bothαandβconfigurations were present in the NF-LEP-2a.Combined with monosaccharide compositions and methylation results,it was found that there were some anomeric proton and carbon regions that could be used for structural analysis.The chemical shifts of these regions wereδ4.45/105.57,δ4.47/105.57,δ4.92/100.52,δ5.07/101.15,δ5.02/104.20,δ4.99/100.66,δ4.67/105.43 andδ4.73/104.45,respectively.We compared our results with HSQC (Fig.4B),1H–1H COSY spectra (Fig.4C),and chemical shifts published in the literature[39,40],all glycosidic bond signals were assigned (Table 4).The linkage sequence of NF-LEP-2a was identified by HMBC (Fig.4D).For instance,the cross signals atδ4.45/71.61 andδ4.14/105.57 of sugar residue A (Table 4) indicated the presence of glycosidic bond→6)-β-D-Glcp-(1→6)-β-D-Glcp-(1→;the cross signal atδ4.92/71.61 demonstrated the connection between H-1 of→6)-α-D-Galp-(1→ and the C-6 of→6)-β-D-Glcp-(1→,implying glycosidic bond →6)-α-DGalp-(1→6)-β-D-Glcp-(1→;and the cross signal atδ3.84/100.52 indicated a correlation between H-6b and C-1 of→6)-α-D-Galp-(1→,implying glycosidic bond→6)-α-D-Galp-(1→6)-α-D-Galp-(1→.Other cross signals atδ5.07/69.43,δ4.47/69.43,δ4.73/79.70 andδ4.67/87.17 indicated the presence of→2,6)-α-D-Galp-(1→and →6)-α-D-Galp-(1→,β-D-Glcp-(1→,→3)-β-D-Glcp-(1→and→2,6)-α-D-Galp-(1→,and →3,6)-β-D-Glcp-(1→and →3,6)-β-DGlcp-(1→ linkages.Based on the methylation and NMR results,the backbone of NF-LEP-2a was inferred to beβ-D-Glcp-(1 → 2)-α-DGalp-(1 → 6)-β-D-Glcp-(1 → 6)-β-D-Glcp-(1 →.The branched chain was composed ofβ-D-Glcp-(1→,α-D-Manp-(1→,andα-L-Fucp-(1→.We analyzed the spectrum of F-LEP-2a in the same way and observed that there were eight significant signal peaks in the anomeric proton and carbon regions (Fig.5,Fig.6 and Table 5),indicating that the structure of F-LEP-2a was similar to that of NF-LEP-2a.The results further revealed that LAB fermentation may affect the connection between the repeating units in the polysaccharide without significant effects on the structure.

Table 4 1H and 13C NMR chemical shifts of NF-LEP-2a.
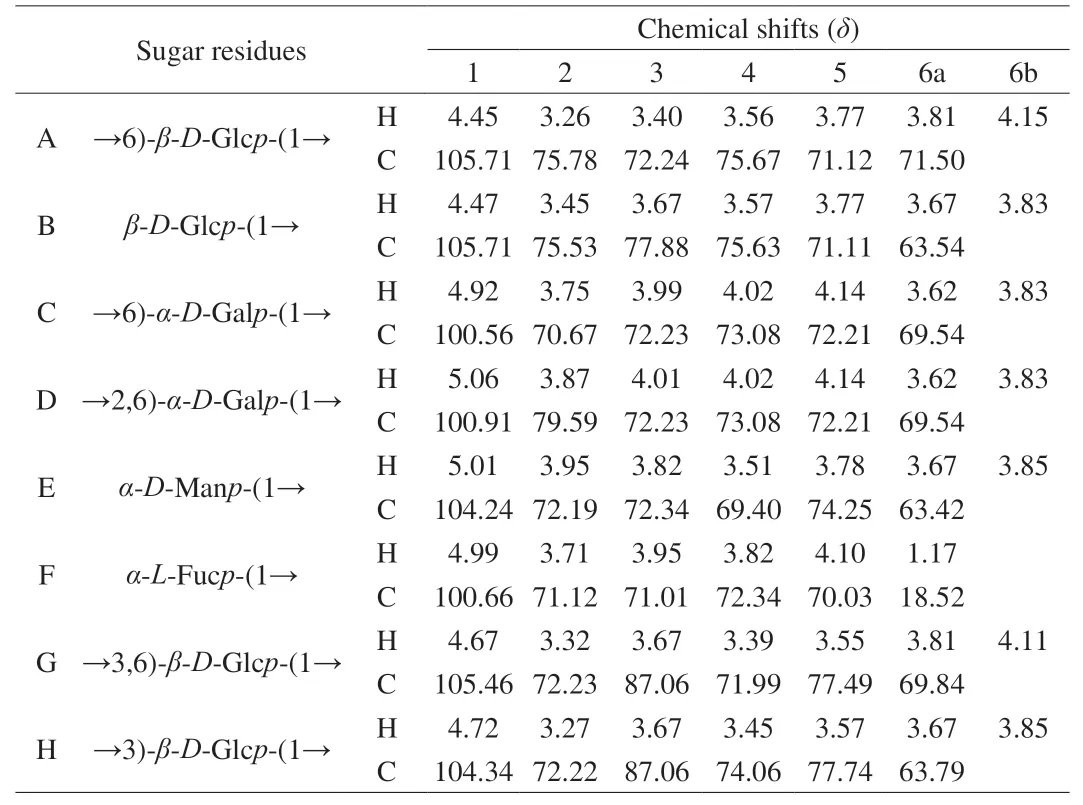
Table 5 1H and 13C NMR chemical shifts of F-LEP-2a.

Fig.4 NMR spectra of NF-LEP-2a.A: 1H;B: HSQC;C: COSY;and D: HMBC.
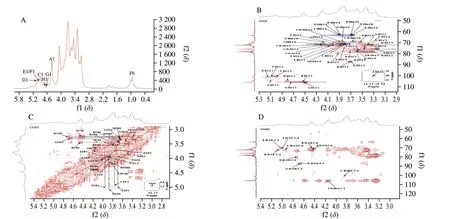
Fig.5 NMR spectra of F-LEP-2a.A: 1H;B: HSQC;C: COSY;and D: HMBC.
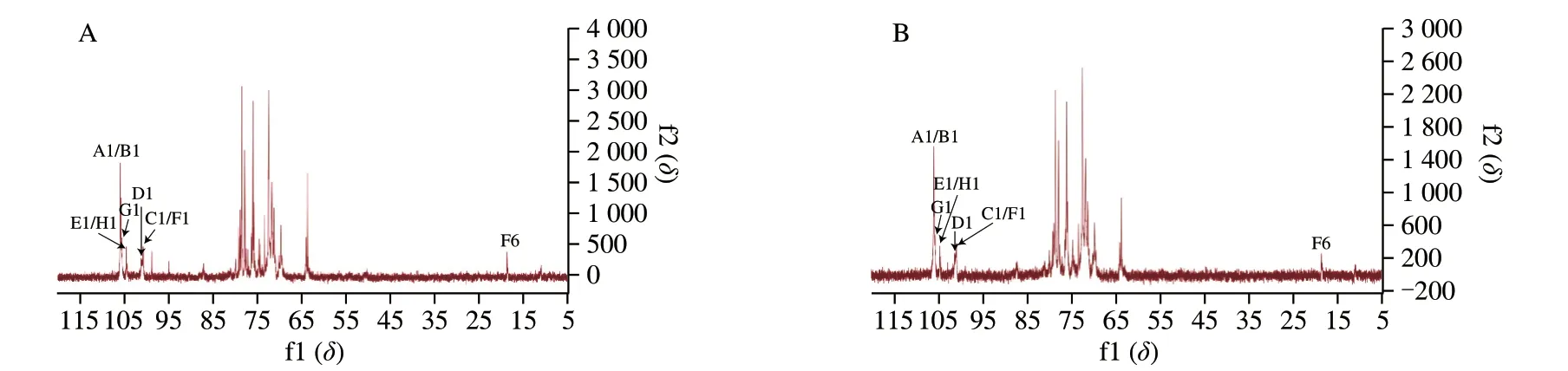
Fig.6 13C NMR of NF-LEP-2a (A) and F-LEP-2a (B).
3.5 Immunomodulatory activity
3.5.1 Immune organ index
Immune organs can protect the body from infection,and thymus and spleen are the main organs for the distribution of immune cells.The immunoregulatory activity is closely associated with changes in the immune organ index[41].As shown in Table 6,there was no significant difference in thymus index among groups,however,the spleen index of the Model group markedly decreased compared with the Normal group (P<0.05),indicating that the immunosuppression model was established.Levamisole hydrochloride treatment remarkably improved the spleen index of immunosuppressed mice compared with the Model group (P<0.01).Meanwhile,this decline was recovered by the administration of NF-LEP and F-LEP.Previous studies have demonstrated thatL.edodespolysaccharides exert an immune-enhancing effect by promoting immune organ indices and levels of serum antibodies[42].Most notably,the ameliorative effect of F-LEP on the spleen index was stronger compared to the NF-LEP at the same dose.
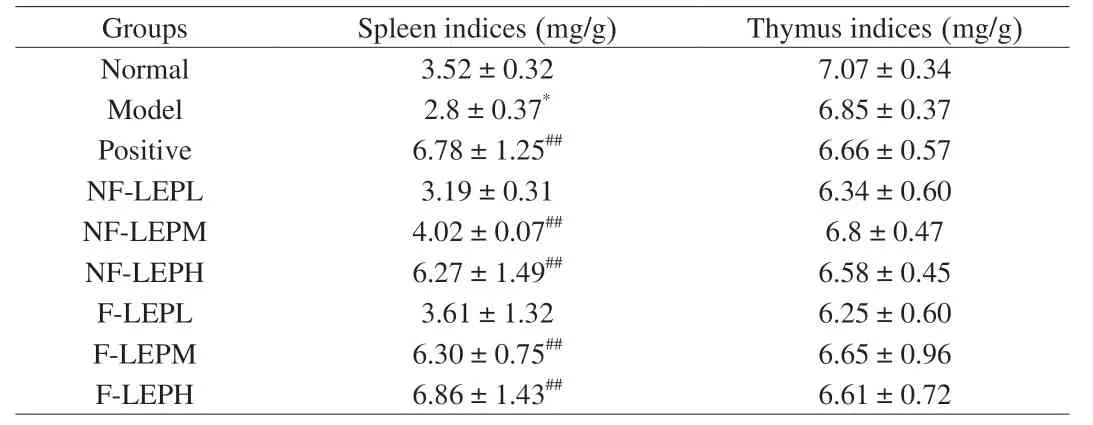
Table 6 Effects of NF-LEP and F-LEP on immune organs in mice (n=6).
3.5.2 Serum IgG and IgM levels
Immunoglobulins (Ig) are proteins secreted by B cells and are responsible for humoral immunity by interacting with foreign material or antigens.Specific categories of immunoglobulins have been demonstrated to be associated with the development of different diseases[43].As shown in Fig.7A and 7B,the immunoglobulin contents in serum significantly declined in the Model group compared with the Normal group (P<0.05),indicating that CTX inhibited the immunomodulatory function of mice.After NF-LEP and F-LEP treatment,IgG and IgM levels were improved in varying degrees.Both NF-LEP and F-LEP increased the IgG content,but a significant difference was only observed in the high-dose NF-LEP and F-LEP groups (P<0.05).The F-LEP group displayed better moderating effects than the NF-LEP group,especially at the concentration of 400 mg/kg.Thus,L.edodespolysaccharides could more effectively regulate the immune function of the body by promoting the secretion of IgG and IgM through LAB fermentation.
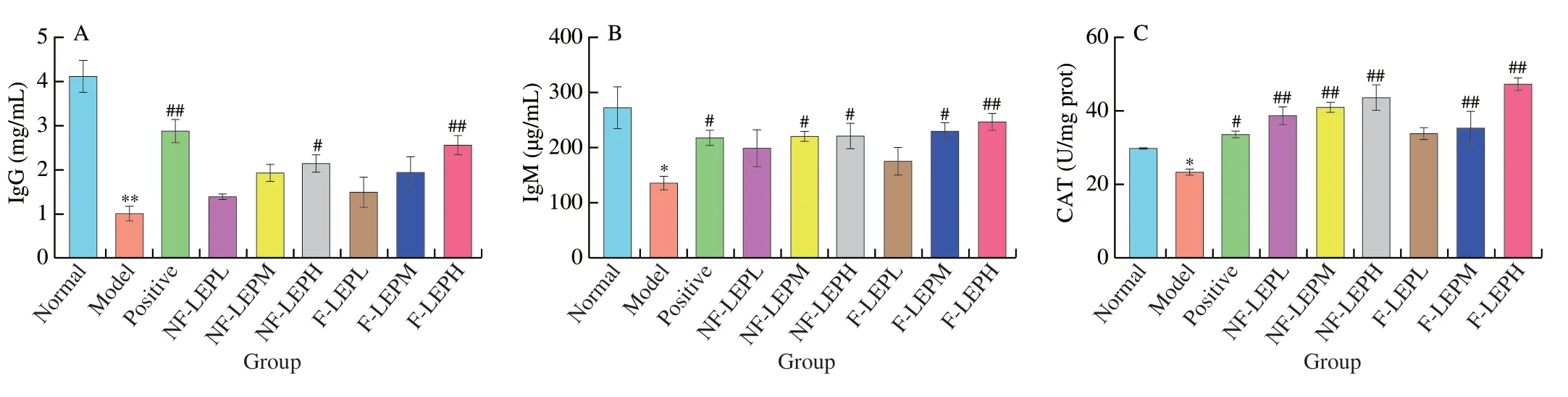
Fig.7 Amelioration of NF-LEP and F-LEP against CTX-treated mice.The IgG (A),IgM (B),CAT activity (C),SOD activity (D),MDA level (E),villus length (F),IL-2 level (G) and IL-6 level in spleen (H) were improved by NF-LEP and F-LEP treatments.Compared with normal group: *P <0.05,**P <0.01;compared with model group: #P <0.05,##P <0.01.(I) Hematoxylin-eosin stained colonic tissues;scale bars,200 μm.
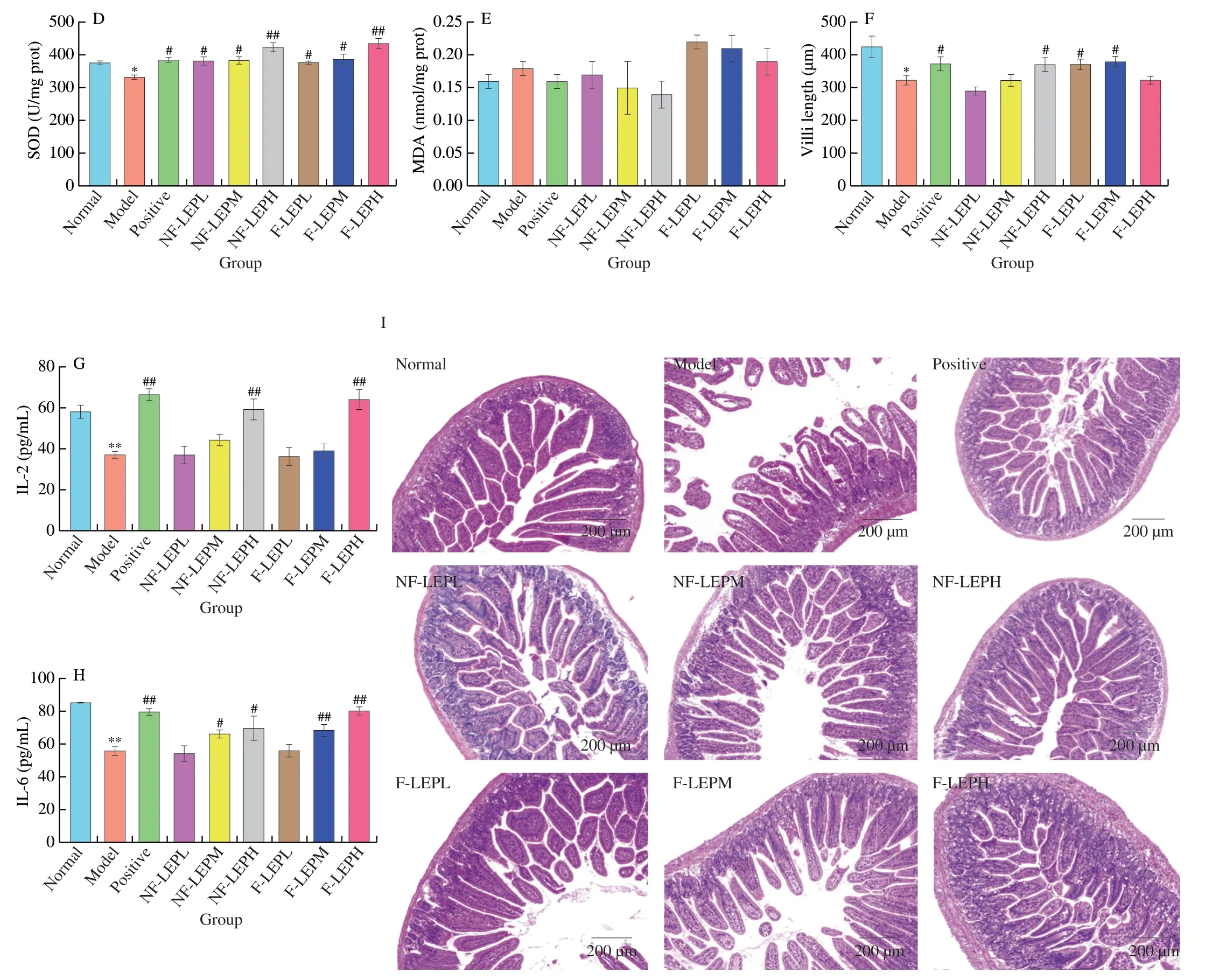
Fig.7 (Continued)
3.5.3 Antioxidant activity in liver and cytokine levels in spleen
Oxidative stress is believed to be one of the major contributors to CTX-induced toxicity,and the production of free radicals leads to the disruption of multiple signaling pathways[44].Excessive reactive oxygen species (ROS) activate nuclear factor kappa B(NF-κB),which induces the expression of inflammatory cytokines such as TNF-α,IL-1β and IL-6 and triggers inflammation[45].In addition,the immune function of cells decreased after being stimulated by ROS.Antioxidant enzymes can effectively inhibit oxidative stress by transforming free radicals into stable forms.Therefore,we investigated the immunoregulatory activities of NFLEP and F-LEP on CTX-induced immunosuppressive mice by detecting their effects on liver antioxidant enzymes.As shown in Fig.7C–E,the catalase (CAT),superoxide dismutase (SOD) and malondialdehyde (MDA) levels of liver were detected.CAT and SOD levels were significantly lower (P<0.05) and MDA levels were higher in the model group compared with the normal group,revealing that intraperitoneal injection of CTX reduced the antioxidant ability of mice.Compared with the Model group,the CAT levels in the NF-LEP groups were noticeably increased (P<0.01) regardless of polysaccharides concentration,however,F-LEP achieved a significant increase in CAT (P<0.01) at the concentration of 200 and 400 mg/kg.SOD levels were significantly increased after NF-LEP and F-LEP administration treatment (P<0.01,P<0.05).Xu et al.demonstrated that different concentrations ofL.edodespolysaccharides (100,200 and 300 mg/kg) treatment led to a significant decrease in antioxidant enzyme activities and had definite antioxidative activity in high-fat-diet rats[46].MDA is the main product of the peroxidation of polyunsaturated fatty acids and has strong toxic effects on the body[47].Compared with the Model group,a decrease in MDA levels was observed in the Positive and NF-LEP groups.NF-LEP and F-LEP treatment significantly increased the activities of SOD and CAT in the liver,and the restoration of redox balance could protect the liver,spleen and other tissues from damage.These findings supported the results that NF-LEP and F-LEP remarkably improved the spleen index of immunosuppressed mice.In short,NF-LEP and F-LEP were able to scavenge free radicals and corresponding products,or promote antioxidant enzyme activity,thereby exerting antioxidant activity.
Cytokines play many critical roles in host defense against pathogens by connecting the acquired and congenital arms of the immune system.Extensive data from cellular and animal studies have indicated that cell-mediated immune abnormalities are involved in the pathogenesis of various diseases,and the levels of certain cytokines,such as IL-2,IL-6 and TNF-α,are assessed as parameters of disease severity[48,49].In the present study,two key inflammatory cytokines (IL-2 and IL-6) were assayed to evaluate the effects of NFLEP and F-LEP on CTX-induced immunosuppressed mice.As shown in Fig.7G-H,the levels of IL-2 and IL-6 in spleen were markedly decreased by CTX treatment,while NF-LEP and F-LEP reversed the decline in these cytokines in a dose-dependent manner.IL-2,as a T cell-stimulatory factor,enhances the cytolytic activity of nature killer (NK) cells and promotes the production of immunoglobulins by B cells[50].The concentration of IL-2 in NF-LEP and F-LEP high-dose groups showed a significant increase compared with the model group.However,both the middle and high doses of NF-LEP and F-LEP exerted a significant improvement effect on IL-6.Molecular weight is closely related to the biological activity of polysaccharides.L.edodespolysaccharides fractions (LST1 and LJT1) with highmwshowed stronger immunomodulatory effects,compared with the lowmwpolysaccharides (LST2,LJT2 and LJT3)[51].In our study,F-LEP displayed better moderating effects on the spleen index,immunoglobulin and cytokines than NF-LEP(400 mg/kg),possibly related to theirmwand degrees of branching(DB) values.As previously calculated,compared with NF-LEP-2a,F-LEP-2a had a higher DB value.Researchers have noticed that the highmwpolysaccharides fractions had smaller DB values,and the suitable DB contributed to the formation of a stable triple helix structure[36].The triple-helical conformation of (1 → 3)-β-glucan is considered to be the structural feature of its immunomodulatory activity.These results suggested that the alleviating effects of NF-LEP and F-LEP on CTX-induced immunosuppression were partially due to the promotion of cytokine secretion.
3.5.4 Histologic changes of intestine tissue
To examine the effects of NF-LEP and F-LEP on CTX-induced intestine tissue damage,the jejunum tissues were prepared for histopathological analysis (Fig.7I).The jejunum histological morphology in the Model group exhibited shorter villus,shallower crypt,epithelial cell injury and a loose and irregular structure,which revealed that CTX-treated mice suffered intestinal barrier damage.However,the intervention of NF-LEP and F-LEP reversed these pathological changes.Moreover,the villi length of Model group mice significantly declined compared to that of the Normal group(P<0.05,Fig.7F).The CTX-induced decrease of villi length was notably ameliorated by NF-LEP and F-LEP administrations.We observed a significant increase in villus length in the mid-dose,lowdose F-LEP groups (P<0.05) and the high-dose NF-LEP group.Together,the intervention of NF-LEP and F-LEP could attenuate CTX-induced colon tissue damage.
3.5.5 Gut microbiota composition
Gut microbiota interacts with the host and provides crucial signals for the function of the immune system[52].Disruption of the balanced relationship is accompanied by gut dysbiosis and immune system disruption,making the host more susceptible to pathogen infection.Evidence from animal models suggests that a common mechanism in autoimmune diseases may be impaired intestinal barrier function[53].The gut microbiota influences multiple aspects of host homeostasis through its impacts on the innate immune system,which in turn plays an important role in shaping the native microbial community and regulating its metabolic activity.To determine whether the alleviating effect ofL.edodespolysaccharides on immunosuppression was related to changes in the gut microbiota,we performed 16S rRNA gene sequencing analysis.Theα-diversity including ACE,Chao,Shannon and Simpson indexes was applied to evaluate the diversity and richness of gut microbiota (Table 7).NF-LEP and F-LEP increased theα-diversity (ACE and Chao indexes) of the bacterial community (P<0.01,P<0.05) at the concentration of 200 and 400 mg/kg.However,only the Shannon index in the F-LEPH group was notably higher (P<0.01) than that of the Model group (Table 7).The results revealed that F-LEP treatment could improve the richness and diversity of the fecal bacterial community,relative to NF-LEP treatment.
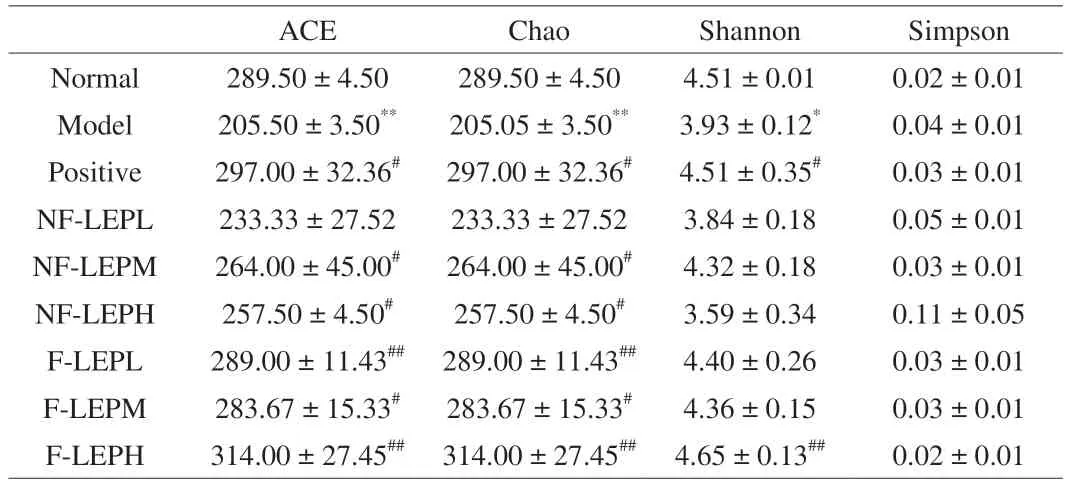
Table 7 The a-diversity of the fecal bacterial community in all groups.
In line with the changes in α-diversity,NF-LEP and F-LEP altered the relative abundance of bacterial taxa (Fig.8).At the phylum level,the NF-LEP and F-LEP groups mainly consisted of Bacteroidota,Firmicutes and Campilobacterota (Fig.8A).Specifically,NF-LEP and F-LEP treatments reversed the decreased level of Firmicutes and Campilobacterota in CTX-treated mice.Compared with the NF-LEPH group,F-LEP (400 mg/kg) notably increased the level of Bacteroidota.Bacteroidetes,enriched by NF-LEP and F-LEP treatments,is considered to be the primary bacteria to degrade polysaccharides[54].At the genus level,Lactobacillus,norank_f__Muribaculaceae,Alistipes,unclassified_f__Lachnospiraceae,Bacteroidesand Lachnospiraceae_NK4A136_group were identified as major genera in the NF-LEP and F-LEP groups (Fig.8B).The expansion of Muribaculaceae_unclassified was also observed in theA.cristatuspolysaccharides-treated mice,which was associated with the gut immunity[55].Bacteroidesencoding glycoside hydrolase genes is related to the metabolism of glycans,and could regulate the immune dysfunction by inducing TNF-α production and T-cell proliferation response[56].Furthermore,Bacteroidespromotes the synthesis of SCFAs to impact on immune response[57].However,inBacteroides,Bacteroidesfragilishas been reported to secrete enterotoxin that could lead to the formation of a pro-inflammatory microenvironment and increase the permeability of colonic epithelial cells[58].Compared with the Model group,Lactobacillus,unclassified_f__Lachnospiraceae and Lachnospiraceae_NK4A136_group were enriched in NF-LEPH group;Alistipes,Odoribacter,unclassified_f__Lachnospiraceae and Lachnospiraceae_NK4A136_group were improved in F-LEPH group(Fig.8B).Lachnospiraceae greatly reduced radiation damage to the hematopoietic and intestinal,and the tissue structure and cell death of the bone marrow and spleen were also preserved.In addition,Lachnospiraceae can produce large amounts of SCFAs,which are important substances that regulate the immune response and inflammatory response of the body[59].The innate immune system and microbes interact to coordinate the physiology of the entire organism.Most studies reported that gut microbiota is implicated in regulating immune cell activation and inflammation development through NF-κB and inflammasome activation,and triggers local immune responses by interacting with immune cells that express pattern recognition receptors[60].In addition,gut microbiota produces diverse metabolites that enter and interact with host cells through the epithelial monolayer to influence immune responses.For example,SCFAs affect peripheral T cells,especially Treg cells,by inhibiting histone deacetylases[61].Many polysaccharides can be fermented by gut microbiota to produce SCFAs with potential health benefits.Collectively,NF-LEP and F-LEP could maintain microbial ecological balance related to gut barrier and host immune function.

Fig.8 Heatmap of the mean log2-transformed fold change of phyla and genera in all groups.
4. Conclusion
The purpose of this study was to explore the impacts of LAB fermentation on the structure and immunoregulatory activity ofL.edodespolysaccharide.We usedL.fermentum21828 to fermentL.edodes,then isolated and purified water-solubleL.edodespolysaccharide (NF-LEP-2a and F-LEP-2a) from non-fermented and fermented liquid.Although NF-LEP-2a and F-LEP-2a had similar functional groups,LAB fermentation changed the monosaccharide composition of NF-LEP-2a.Further analysis revealed that the linkage patterns and NMR spectra were similar between NF-LEP-2a and F-LEP-2a.Examination of the immunomodulatory activityin vivoshowed that fermented lentinan had a better protective effect on immunosuppressed mice.Our study provides a basis for understanding the biological activity of F-LEP-2a and taps an interesting source of polysaccharides with stronger biological activities by probiotic fermentation.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by grants from the National Key R&D Program of China (2019YFC1606701).
杂志排行
食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing
