Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
2024-02-16RenyouGnYiLiuHngLiYuXiHunGuoFngGengQiguoZhungHuinLiDingtoWu
Renyou Gn*,Yi Liu,Hng Li,Yu Xi,Hun Guo,Fng Geng,Qiguo Zhung,Huin Li,Dingto Wu,*
a Research Center for Plants and Human Health,Institute of Urban Agriculture,Chinese Academy of Agricultural Sciences,Chengdu National Agricultural Science and Technology Center,Chengdu 610213,China
b Key Laboratory of Coarse Cereal Processing,Ministry of Agriculture and Rural Affairs,Sichuan Engineering and Technology Research Centre of Coarse Cereal Industralization,School of Food and Biological Engineering,Chengdu University,Chengdu 610106,China
c China-New Zealand Belt and Road Joint Laboratory on Kiwifruit,Sichuan Provincial Academy of Natural Resource Sciences,Chengdu 610213,China
d Guangdong Provincial Key Laboratory of Food,Nutrition and Health,School of Public Health,Sun Yat-sen University,Guangzhou 510080,China
Keywords:Nobiletin O-Methyltransferases Gut microbiota Bioactivities Molecular mechanism
ABSTRACT Polymethoxyflavones (PMFs) are a type of uncommon dietary flavonoids,characterized by more than one methoxy group,which exist in limited plant species,like Citrus species and Kaempferia parviflora.In addition,different PMFs,such as nobiletin,sinensetin,tangeretin,and casticin,have been isolated from these natural sources.PMFs have received increasing attention due to their multiple bioactivities,such as antioxidant,anti-inflammatory,anti-cancer,metabolic regulatory,immunoregulatory,neuroprotective,and skin protective effects.These bioactivities of PMFs should be associated with the regulation of critical molecular targets and the interaction with gut microbiota. In order to provide a comprehensive and updated review of PMFs,their natural sources,refined extraction,biosynthesis,metabolism,and bioactivities are summarised and discussed,with the emphasis on the molecular mechanisms of PMFs on regulating different chronic diseases.Overall,PMFs may be promising flavonoids to the forefront of nutraceuticals for the prevention and/or treatment of certain human chronic diseases.
1. Introduction
Polymethoxyflavones (PMFs) are a special category of flavonoids.The difference of PMFs with other flavonoids is that the former possesses more than o ne methoxy group (-CH3O),which has been suggested to significantly influence the bioactivities of PMFs.Most PMFs are hydrophobic,leading to their poor bioavailability when consumed orally.In nature,PMFs are not widely distributed,but restricted in certain plant species.C itrusspecies are the most rich dietary sources of PMFs,and recent studies suggest that several other plants,likeKaempferiaparvifloraandFructusviticis,also contain abundant of PMFs.B esides,many advanced extraction,separation,purification,and identification technologies have been maturely applied to PMFs,significantly promoting their downstream research,such as studying their interaction with gut microbiota and bioactivities by using specific pure PMF compounds.As a result,increasing evidence supports that PMFs exhibit multiple bioactivities,such as antioxidant,anti-inflammatory,anti-cancer,regulation of metabolic syndrome and immune system,neuroprotective,and skin protective effects.
Compared to common flavonoids,PMFs have received limited attention,while they may be also important for human health due to their diverse bioactivities.In order to highlight the importance of PMFs and also provide a better understanding of them,literature search was conducted in Web of Science,Scopus,and PubMed databases with the search term “polymethoxyflavone” in either titles or abstracts published up to 19thJuly,2021.The relevant full-text articles were retrieved to assess their eligibility,and additional articles were retrieved by checking their references and manual searching.The included articles were mainly published in the last 5 years to reflect the recent situation.In this review paper,the main natural sources,refined extraction technologies,and metabolism of PMFs by gut microbiota are briefly summarized and discussed.Then,the main bioactivities of PMFs are emphasized,with related molecular mechanisms intensively discussed.It is believed that this review can promote the application of PMFs and their natural sources in the prevention and treatment of certain human chronic diseases.
2. Main natural sources of PMFs
PMFs are not widely distributed in the plant kingdom,but restricted in a few plants,mainly including theCitrusspecies(Rutaceae),K.parviflora(Zingiberaceae),Artemisia annuaL.(Compositae),Artemisia indica(Compositae),Lantana ukambensis(Verbenaceae),F.viticis(Verbenaceae),Leucosidea sericea(Rosaceae),andNicotiana plumbaginifolia(Solanaceae),and the chemical structures of representative PMFs are summarised in Fig.1.

Fig.1 Chemical structures of representative polymethoxyflavones: (1) casticin;(2) nobiletin;(3) sinensetin;(4) sudachitin;(5) tangeretin;(6) 3,5,6,7,8,3’,4’-heptamethoxyflavone;(7) 5,7-dimethoxyflavone;(8) 5-hydroxy-3,7,3’,4’-tetramethoxyflavone.
Citrusspecies are the most rich dietary sources of diverse PMFs and their representative PMFs are nobiletin and tangeretin[1].DifferentCitrusspecies have been reported to contain diverse PMFs,mainly existing in the citrus peel and citrus leaves.The main PMFs inCitrusspecies are summarised in Table 1.BesidesCitrusspecies,a few other plants also contain PMFs.For example,black ginger(K.parviflora) is rich in PMFs,and 3,5,7,3’,4’-pentamethoxyflavone,5,7,4’-trimethoxyflavone,and 5,7-dimethoxyflavone were identified as the main PMFs in it[2].In addition,Qinghao (Artemisia annuaL.),a first-line antimalarial drug,also contained PMFs,such as artemetin,in its leaves and inflorescences[3,4].Indian wormwood(Artemisia indica) is also a medicinal herb,and several PMFs,such as artemetin and casticin,were found in its leaves[5].Lantana ukambensisis an African food and medicinal herb,and two PMFs,including 5,6,7,3,4,5-hexamethoxyflavone and 5-hydroxy-6,7,3,4,5-pentamethoxyflavone,were isolated from the whole plant[6].Casticin,a main PMF,was found inF.viticis[7].Besides,several other plant species were also reported to contain PMFs,including Eau de cologne mint (Mentha×piperita citrata) (Lamiaceae)[8],Helichrysum cassianum(Compositae)[9],Laggera pterodonta(Compositae)[10],Leucosidea sericea(Rosaceae)[11],Murraya paniculate(Rutaceae)[8],andNicotiana plumbaginifolia(Solanaceae)[12],and detailed information is presented in Table 1.According to Table 1,relatively high levels of naringin (18.3 mg/g),nobiletin (8.15 mg/g),and 3,6,7,4’-tetramethoxyflavone (10.13 mg/g) were detected inCitrus aurantiumfruits,Ougan peels,andKiyomi tangorpeels,respectively.As the representative PMFs,the content of tangeretin and nobiletin inCitrus sunkipeels varied significantly due to different extraction solvents.For example,tangeretin had the yield of 9.8 and 540.4 mg/g by then-butanol andn-hexane extraction,respectively,and nobiletin had the yield of 10.4 and 259.4 mg/g by then-butanol and chloroform extraction,respectively.

Table 1 Main natural sources of polymethoxyflavones (PMFs).
Overall,Citrusspecies in Rutaceae family are the most rich dietary sources of diverse PMFs.In addition,several plants in the Compositae,Labiatae,Rosaceae,Solanaceae,Verbenaceae,and Zingiberaceae families,can also be good sources of certain PMFs.In the future,it is suggested to pay more attention to other plant species in the Compositae,Rutaceae,and Verbenaceae families to discover additional sources of PMFs.
3. Refined extraction of PMFs
Efficient extraction,separation,purification,and identification of natural products are essential for their further research and application,which is defined as “refined extraction” herein.The detailed information of the refined extraction technologies for PMFs in various plants are summarised in Table 2.In general,non-polar or low-polar organic solvents can be more appropriate for PMF extraction,and ethanol and methanol solutions are the most common solvents.Maceration extraction,reflux extraction,ultrasonic-assisted extraction,and supercritical CO2extraction,are commonly used to extract PMFs.
After the extraction,thin layer chromatography (TLC) coupled with silica gel,column chromatography,medium-pressure liquid chromatography (MPLC),and preparative high-performance liquid chromatography (HPLC) are widely applied to further separate and purify PMFs from crude extracts.Finally,the identification of PMFs can be carried out using different detection techniques,such as analytic HPLC coupled with diode array detector (DAD) or mass spectrometer (MS),gas chromatography (GC) coupled with MS,high-resolution electrospray ionization mass spectrometry (HRMS),and nuclear magnetic resonance (NMR).
In the future,more green and advanced extraction technologies applied for other phytochemical extraction,such as tea catechins and hemp bioactive compounds[13-16],may be further utilized to extract plant PMFs.
4. Biosynthesis of PMFs
The biosynthesis of citrus flavonoids is intensively reviewed by a recent study[17],however,the biosynthesis of PMFs in citrus or other species has not been summarized.Taken citrus PMFs as an example,their biosynthesis also stems from a precursor molecule phenylalanine,like the biosynthesis of other flavones in citrus via different enzymatic reactions[17].Afterwards,O-methyltransferases(OMTs) play a critical role in the methylation of flavone hydroxyl groups (-OH),but the genetic basis for the methylation process has not been fully understood in citrus.

Recent studies indicate that different OMTs can be cooperatively involved in PMF methylation with different substrate specificities and regioselectivities.For example,5 genes encoding flavonoidO-methyltransferases (CdFOMT1,3,4,5,and 6) were isolated fromCitrusdepressa,and theCdFOMT5encodes anO-methyltransferase enzyme possessing a wide range of substrate specificity and regioselectivity for flavonoids[18].Liu et al.[19]characterized a caffeoyl-CoA OMT-like enzyme (CrOMT1) in theCitrusreticulata,and the recombinant CrOMT1 could efficiently methylate flavones with neighboring OH groups,and it exhibited high catalytic efficiencies for 6-OH-and 8-OH-containing compoundsin vitro.In addition,transient overexpression ofCrOMT1gene resulted in the accumulation of three major PMFs in the citrus fruit,suggesting thatCrOMT1is probably involved in the biosynthesis of PMFs in citrus[19].Moreover,Liu et al.[20]again isolated anotherOMTgene,CrOMT2,from the fruit peel ofCitrusreticulata.Its recombinant protein was able to efficiently catalyze the methylation of 3’-,5’-,and 7-OH of flavonoids with vicinal hydroxyl substitutions,similar toCrOMT1.In addition,CrOMT2 recombinant enzyme preferred PMF-type substrates in the substrate preference assayin vitro[20].Zohra et al.[21]also identified two novel OMT candidate genes,CreOMT1andCreOMT4in citrus,and the expression ofCreOMT1gene had a positive correlation with the content of nobiletin in the flavedo of 10 citrus cultivars.Seoka et al.[22]showed that the recombinant protein of a novelOMTgene (CitOMT) had the methylation activity to transfer a methyl group to the 3’-hydroxy group of flavones.In a recent study,Ma et al.[23]also showed thatO-methyltransferase (CitOMT2) was highly expressed in the citrus fruit varieties rich in nobiletin.Thus,CitOMTis a key gene for nobiletin biosynthesis in citrus fruits.
Based on the above results,OMTs has been seldom revealed in other plants besides certainCitrusspecies,and understanding the methylation process and related molecular basis is essential to better characterize the biosynthesis of PMFs in them.Moreover,discovering OMTs with high enzymatic activities and substrate specificities can promote the large-scale biosynthesis of specific PMFs with excellent bioactivities using advanced metabolic engineering techniques.
5. Metabolism of PMFs involving in gut microbiota
The bioavailability of natural products is a general concept of their absorption,distribution,metabolism,and excretion.A previous article reviewed the relationship between the side chains (e.g.,hydroxyl and methoxy) of PMFs and their solubility as well as the permeability of PMFs[24].Another review discussed the actions of different enzymes on PMFs and their metabolites[25].Herein,we updated some recent progress on the metabolism of PMFs involving in gut microbiota.
Gut microbiota play an important role in the metabolism of diverse plant components.Recent studies demonstrate that gut microbiota are also involved in the metabolism of certain PMFs.An activity-guided screening strategy was used to discover PMF-metabolizing gut microbiota under anaerobic conditions,and a strict anaerobic bacterium,Blautiasp.MRG-PMF1,was isolated,which exhibited the demethylation activity and could metabolize different PMFs into the corresponding demethylated flavones[26].For example,5,7,4’-trimethoxyflavone could be completely metabolized to 5,7,4’-trihydroxyflavone (apigenin) by this strain.In addition,it was suggested that gut microbiota exhibited a stronger activity on the biotransformation of nobiletin than the host when analyzing its demethylated metabolite profiles in the urine and faeces of rats[27].Moreover,the oral administration of PMF-rich extract from Ougan (Citrusreticulatacv.Suavissima) in mice led to the occurrence of 21 PMF metabolites in the intestine,mostly through the effects of demethylation,demethoxylation,hydroxylation,and glucuronidation,and gut microbiota should play an important role in metabolizing PMFs[28].
On the other hand,PMFs can also regulate the composition of gut microbiota.It was found that citrus peel extracts rich in PMFs could significantly increase the abundance ofPrevotella,but reduce the abundance of rc4-4 bacteria in high-fat diet (HFD)-induced obese mice[29].In a recent study,treatment of aged citrus peels (Chenpi) extract significantly decreased the abundance ofProteobacteriaand the ratio ofFirmicutestoBacteroidetesin HFD-induced obese mice,while dose-dependently increased two beneficial bacteria,Akkermansiaspp.andAllobaculumspp.[30].Similarly,the relative abundance of two probiotics,LactobacillusandBifidobacterium,dramatically increased after oral administration of PMF-rich extract from Ougan (Citrusreticulatacv.Suavissima)in mice[28].Therefore,PMF can overall ameliorate gut microbial dysbiosis by inhibiting pathogenic microbes and increasing beneficial or probiotic microbes in the gut,which can be important for the gut barrier and overall health[31].Falduto et al.[32]showed that the treatment of a PMF-enriched Chenpi extract significantly increased the numbers ofRoseburia,Blautia,Subdoligranulum,Eubacterium,andFirmicutesin the human gut.Chen et al.[33]showed that after oral administration of PMF-rich fraction from Ougan (Citrusreticulatacv.Suavissima),the relative abundance ofLactobacillusandBifidobacteriumwas found to be increased significantly in the gut of mice.Additionally,Wu et al.[34]proved that the consumption of PMF extracts also altered the composition of gut microbiota by increasing butyrate-producing probiotics and decreasing colorectal cancer(CRC)-related bacteria.
However,there is still lack of enough evidence to fully explain the interaction of diverse PMFs with gut microbiota,and the relationships among gut microbiota,PMFs,and their bioactivities remain not very clear.Currently,most studies still focus on the interaction of PMF-enriched extracts with gut microbiota.In the future,more specific PMF compounds prepared by refined extraction should be explored about their influences on the regulation of gut microbiota,and more gut microbiota capable of metabolizing PMFs should be discovered and isolated,with intensive clarification of their interactions and related influences on gut and overall health.Besides,whether gut microbiota can influence the bioavailability of PMFs and whether the metabolites of PMFs transformed by gut microbiota have enhanced bioactivities than PMFsperseremain to be verified in the future.
6. Bioactivities of PMFs
PMFs have been reported to exhibit plenty of bioactivities,such as antioxidant,anti-inflammatory,anti-cancer,anti-obesity,and neuroprotective effects.In the following section,their main bioactivities and related molecular mechanisms are discussed,and the detailed information of thesein vitroandin vivostudies are summarised in Tables 3,4,and S1,respectively.
6.1 Antioxidant effect
Flavonoids are one of the main antioxidants existing in plant-based foods,such as fruits,vegetables,and teas[35-38].PMFs as a type of uncommon flavonoids also exhibit good antioxidant effectsin vitroandin vivo.

6.1.1 Antioxidant effect in vitro
PMFs have been demonstrated to possess antioxidant effects in cell models.K.parvifloraextracts and its two main PMFs,5,7-dimethoxyflavone and 5-hydroxy-3,7,3’,4’-tetramethoxyflavone,exhibited good antioxidant effects through inhibiting the cellular reactive oxygen species (ROS)[39].Since nuclear factor (erythroidderived 2)-like 2 (Nrf2) is a major activator of a series of endogenous antioxidant enzymes such as heme oxygenase 1 (HO-1),superoxide dismutase (SOD),glutathioneS-transferase (GST),nicotinamide adenine dinucleotide phosphate (NADPH),quinone oxidoreductase 1(NQO1),and catalase (CAT),suppression of the ubiquitination of Nrf2 can stabilize it,contributing to its translocation to the nucleus and subsequently binding to antioxidant response element (ARE)to upregulate the expression of these related antioxidant enzymes to neutralize cellular ROS[40].Cell-based assays suggested that PMFs,such as (2S)-5,6,7,3’,4’-pentamethoxyflavanone,tangeretin,and nobiletin from citrus,were novel Nrf2 activators with the potential molecular mechanism of enhancing the stabilization of Nrf2 by blocking its ubiquitination and degradation[41-43].In addition,PMFs may have different antioxidant mechanisms compared to other common citrus flavonoids.For example,both tangeretin and neohesperidin increased the expression of Nrf2 via the inhibition of its partner protein Kelch-like ECH-associated protein 1 (KEAP1),while only tangeretin could suppress ubiquitin ligase Cullin 3 to decrease the ubiquitination of Nrf2[43].
6.1.2 Antioxidant effect in vivo
Limited studies have demonstrated the antioxidant effects of PMFsin vivo.PMFs from citrus peels,including nobiletin,5-demethylnobiletin,tangeretin,and 5-demethyltangeretin,were reported to alleviate oxidative damages inSaccharomyces cerevisiaeby reducing intracellular ROS and lipid peroxidation[44].
6.1.3 Structure-antioxidant effect relationship
For the structure-antioxidant effect relationship of PMFs,5-demethylnobiletin and 5-demethyltangeretin were found with stronger effects to reduce the lipid peroxidation damage than their parental compounds nobiletin and tangeretin,respectively[45].These results suggest that C-5 position demethylation of PMFs may enhance their antioxidant effects,probably due to the hydroxyl group at the C-5 position,which exhibits a higher antioxidant effect than the methoxy group.
Generally,PMFs possess antioxidant effects by reducing intracellular ROS and lipid peroxidation,and stabilizing Nrf2 via blocking its ubiquitination and degradation.However,few studies investigated the antioxidant effects of PMFs based onin vitrospectroscopic assays,which can be easy and useful to investigate their structure-antioxidant effect relationships.Moreover,PMFs seem to be a novel type of Nrf2 agonists,and theirin vivoantioxidant mechanisms still need further investigation.
6.2 Anti-inflammatory effect
Inflammation is involved in the pathogenesis and progression of many chronic diseases.Several studies demonstrated that PMFs can inhibit inflammation in differentin vitroandin vivomodels.
6.2.1 Anti-inflammatory effect in vitro
The anti-inflammatory effects of PMFs are partially attributed to their ability to decrease the production of pro-inflammatory mediators.Decreases in certain pro-inflammatory mediators,including IL-1,IL-6,tumour necrosis factor-α (TNF-α),high-mobility group box 1(HMGB1),prostaglandin E2 (PGE2),and matrix metalloproteinase(MMP) 3 and 13,were observed after the administration of PMFs (e.g.,tetramethyl-O-scutellarin,nobiletin,tangeretin,and 5-demethylnobiletin) to various cell lines[45-47].IL-21 is an upstream inflammation-related mediator of certain inflammatory cytokines like IL-6,TNF-α,and HMGB1,the inhibition of which might be partially responsible for the anti-inflammatory effect of PMFs.For example,it was proved that nobiletinit could significantly downregulate the expression of IL-21 receptor and subsequently decrease the downstream inflammation-related mediators of IL-21[48].In addition,inflammatory processes can upregulate inducible nitric oxide synthase (iNOS) to produce excessive nitric oxide (NO).Cell-based assays found thatK.parvifloraextracts,5,7-dimethoxyflavone,nobiletin,tangeretin,and 5-demethylnobiletin could decrease the nitrite levels in various cell lines[39,46].Furthermore,a study indicated that the phosphorylation of both Janus kinase 2(JAK2) and signal transducer and activator of transcription 3 (STAT3)could be suppressed by tangeretin and 5-demethylnobiletin,but not by nobiletin[47].However,a different study indicated that nobiletin exerted an inhibitory effect on the phosphorylation of JAK1 and STAT3[46].These results suggest that the JAK-STAT signaling can be a key pathway involved in the anti-inflammatory effect of certain PMFs,but different PMFs may target different molecules in the JAK-STAT signaling.Cytokines have effects on bone cells and are linked to the development of osteoporosis[48].Sudachitin could inhibit inflammatory bone destruction via the direct inhibition of osteoclastogenesis in the osteoclast cells,probably associated with inhibiting the gene expression of osteoclast differentiation-related signaling molecules,such as c-fos,nuclear factor of activated T-cells,cytoplasmic 1 (NFATc1),cathepsin K,and osteoclast fusion proteins,as well as blocking the activation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK),and the ROS production[49].
6.2.2 Anti-inflammatory effect in vivo
PMFs also exhibited anti-inflammatory effects of somein vivostudies.Nobiletin was reported to alleviateL-arginine-induced acute pancreatitis in C57BL/6 mice by blocking the phosphorylation and activation of p38MAPK and AKT[50].
Overall,PMFs exhibit anti-inflammatory effects mainly by blocking the production of inflammation-related factors,such as NO,ROS,MMPs,PGE,and pro-inflammatory cytokines (e.g.,IL-1,IL-6,IL-21,and TNF-α),and targeting different signaling molecules in inflammation-related pathways,such as the MAPK,NF-κB,and JAK-STAT,and AKT signaling.In the future,more animal-basedin vivostudies are needed to further verify the anti-inflammatory effect and related mechanism of PMFs.
6.3 Anti-cancer effect
Many dietary polyphenols possess anti-cancer effects,such as epigallocatechin gallate[51],curcumin[52],resveratrol[53],rutin[54],and dihydrochalcones[55],are commonly used as dietary supplements to prevent cancer.PMFs have also been widely demonstrated to exhibit anti-cancer effects bothin vitroandin vivo[56],which are illustrated in Fig.2 and discussed below,highlighting related molecular mechanisms.
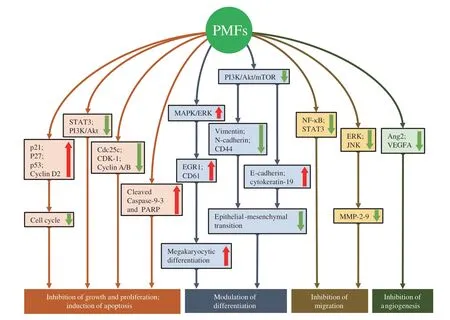
Fig.2 Molecular mechanisms of anti-cancer effects of PMFs by inhibiting cancer cell growth,inducing cancer cell apoptosis,modulating cancer cell differentiation,suppressing cancer cell migration,and blocking cancer angiogenesis.Ang2,Angiopoietin 2;Cdc25c,cell division cycle 25c;CDK,cyclin-dependent kinase;EGR1,early growth response 1;ERK,extracellular-signal-regulated kinase;JNK,c-Jun N-terminal kinase;MAPK,mitogen activated protein kinase;MMP,matrix metalloproteinase;mTOR,mechanistic target of rapamycin kinase;NF-κB,nuclear factor-kappa B;PARP,poly (ADP-ribose)polymerase;PI3K,phosphatidylinositol 3-kinase;STAT3,signal transducer and activator of transcription 3;VEGFA,vascular endothelial growth factor A.Red arrows: up-regulation;green arrows: down-regulation.
6.3.1 Inhibition of cancer cell growth and induction of cancer cell apoptosis
Recent studies demonstrate that PMFs can inhibit cancer cell growth and/or induce cancer cell apoptosisin vitro.The induction of G0/G1 phase arrest and reduction of G2/M phase can be caused by the inhibition of diverse cyclins by upregulating the expression of p53,p21,and p27.In a study,5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone[57],nobiletin[58-60],casticin[61-63],and 5-acetyloxy-6,7,8,4’-tetramethoxyflavone (5-AcTMF,a acetylated derivative of tangeretin)[64]have been evidenced that they can induce cell cycle arrest in various cancer cell lines,which was partially attributed to the suppression of cell proliferation caused by blocking the PI3K/Akt signaling pathway[57].Cell-based assays also suggested that PMFs could induce cancer cell apoptosis via both intrinsic and extrinsic apoptosis pathways[6,54,57,59,61,62,64].The intrinsic apoptosis pathway was mediated by inhibiting the phosphorylation of STAT3 at the site of tyrosine 705,leading to the blockade of JAK2/STAT3/BCL-2/BCL-xL signaling[64].The suppression of BCL-2 and BCL-xL could increase the expression of cleaved Caspase-9/-3 and poly (ADP-ribose)polymerase (PARP) and subsequently induce apoptosis[59].The induction of pro-apoptotic proteins,such as p53,p21,and checkpoint kinase 1 (CHK-1),and the inhibition of anti-apoptotic proteins,such as Cdc25c,were also involved in the pro-apoptotic effects[63].Despite existing cytotoxic effects on some cancer cell lines,5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone did not exhibit cytotoxicity against peripheral blood mononuclear cells (PBMCs)from healthy human donors[6].On the other hand,it was found that nobiletin could promote or inhibit autophagy in different cancer cells.In a study,nobiletin was reported to induce protective autophagy in SNU-16 cells,suggesting that combination of nobiletin with autophagy inhibitors,such as chloroquine,might be promising for gastric cancer treatment[58].Also,nobiletin was found to mediate autophagic flux inhibition by activating the Akt signaling in SKOV3/TAX cells[59].These effects were probably dependent on the different genetic background of different cancer cells as well as the specific experimental context.
The animal models,including murine gastric cancer and colon cancer models,have been used to study the ability of PMFs to inhibit tumor formation and carcinogenesis.In a murine gastric cancer model,the tumor growth in xenograft models was inhibited by 5-hydroxy-3,6,7,8,3,4’-hexamethoxyflavone and the mechanism might be associated with targeting PI3K/Akt[57].In a murine colon cancer model,the colitis-associated colon carcinogenesis in the colonic tissue of azoxymethane (AOM)/dextran sulfate sodium (DSS)-treated mice was inhibited by nobiletin and the mechanism might be associated with blocking inflammation (e.g.,iNOS),inducing antioxidative enzymes (e.g.,HO-1 and NQOI) and Nrf2 signaling,and arresting cancer cell cycle progression[65].
6.3.2 Modulation of cancer cell differentiation
Recent studies find that PMFs,such as tangeretin and nobiletin,can modulate the differentiation of cancer cellsin vitro.Tangeretin blocked the epithelial-mesenchymal transition (EMT) through blocking the PI3K/Akt/mTOR signaling pathway,leading to downregulating the expression of the mesenchymal proteins,including vimentin,cluster of differentiation (CD)44,and N-cadherin,but upregulating the expression of epithelial proteins,including E-cadherin and cytokeratin-19[61].In addition,nobiletin promoted megakaryocytic differentiation through upregulation of EGR1 and CD61 gene expression by activating MAPK/ERK signaling pathway[55].
6.3.3 Suppression of cancer cell migration,invasion,and metastasis
Certain PMFs,such as nobiletin[67,68]and casticin[7,69],have also been reported to inhibit the migration,invasion,and metastasis of cancer cellsin vitro.These effects of PMFs could be mediated by downregulating the expression of MMPs (e.g.,MMP-2andMMP-9)genes and proteins via blocking the ERK and JNK pathways and their downstream NF-κB,cAMP response element-binding protein(CREB),and specificity protein 1 (SP-1) transcription factor activities[67].MMPs could also be inhibited via suppressing the Ras/Akt/NF-κB signaling pathway[69].PMFs could also upregulate the gene expression of cell adhesion molecule 1 B (SCN1B) and metallopeptidase inhibitor 2TIMP2 (TIMP),downregulate the gene expression of NADH dehydrogenase (ubiquinone) Fe-S protein 4 (NDUFS4),vascular endothelial growth factor A (VEGFA),and DNA-damage-inducible transcript 3 (DDIT3),and block the PI3K/AKT and NF-κB/STAT3 signaling pathways[7,68].
6.3.4 Other anti-cancer effects
Several other anti-cancer effects,such as blocking the cancer angiogenesis and cancer-stem cells,have also been reported.For example,nobiletin was reported to block the angiogenesis in human colorectal cancer cells by suppressing the expression of VEGFA and angiopoietin 2 (Ang2)[68].VEGFA and Ang2 are capable of sustaining tumor angiogenesis and limiting antitumor immunity[70].In addition,casticin could inhibit the sphere-and colony-formation in lung cancer stem-like cells (LCSLCs) from H446 small cell lung cancer cells by activating the AMPK/FoxO3a signaling[71].
6.3.5 Synergistic anti-cancer effects with other agents
Many natural products have been reported to combine with current anti-cancer therapies to improve cancer outcomes.PMFs,especially nobiletin,have been combined with other anti-cancer agents to fight against cancerin vitroandin vivo.Cell-based assays suggested that nobiletin enhanced the anti-cancer abilities of imatinib and bicalutamide against chronic myeloid leukaemia cells[60]and prostate cancer cells[72],respectively.An animal study reported that nobiletin and atorvastatin could also synergistically inhibit colonic tumor incidence and multiplicity in an azoxymethane-induced colon cancer rat model,accompanied with dramatically modulating key cellular signaling regulators associated with inflammation,cell proliferation,cell cycle progression,apoptosis,angiogenesis,and metastasis[73].In addition,the combined administration of atorvastatin and nobiletin increased the population of the cancer cells at G0/G1 phase by 30.4%[73].Taken together,PMFs,especially nobiletin,have the potential to be combined with current anti-cancer therapies to reduce the side effects of anti-cancer drugs,and the synergistic anti-cancer effects of other PMFs should also be investigated in the future.
In general,PMFs exert their anti-cancer effects through multiple actions,including inhibiting cancer cell growth,inducing cancer cell apoptosis,modulating cancer cell differentiation,suppressing cancer cell migration,invasion,and metastasis,blocking cancer angiogenesis and cancer-stem cells,and strengthing anti-cancer effects of other agents.Several cancer-related signaling pathways,such as the PI3K-Akt,JAK-STAT,MAPK,NF-κB,and AMPK-FoxO3a signaling pathways,have been demonstrated to be regulated by PMFs and played critical roles in the anti-cancer effect of PMFs,which is briefly illustrated in Fig.2.
6.4 Regulation of metabolic syndrome
Metabolic syndrome is a pathogenic condition featured by a constellation of risk factors,including obesity,dyslipidaemia,insulin resistance,hypertension,and low-grade inflammation,and can progress to more severe metabolic complications,such as type 2 diabetes and non-alcoholic fatty liver disease.Many natural products have been demonstrated to regulate metabolic syndrome and its complications[74-77].Recent studies indicate that PMFs and PMF-rich natural products can also regulate the risk factors and complications of metabolic syndrome (Fig.3),which are discussed below.
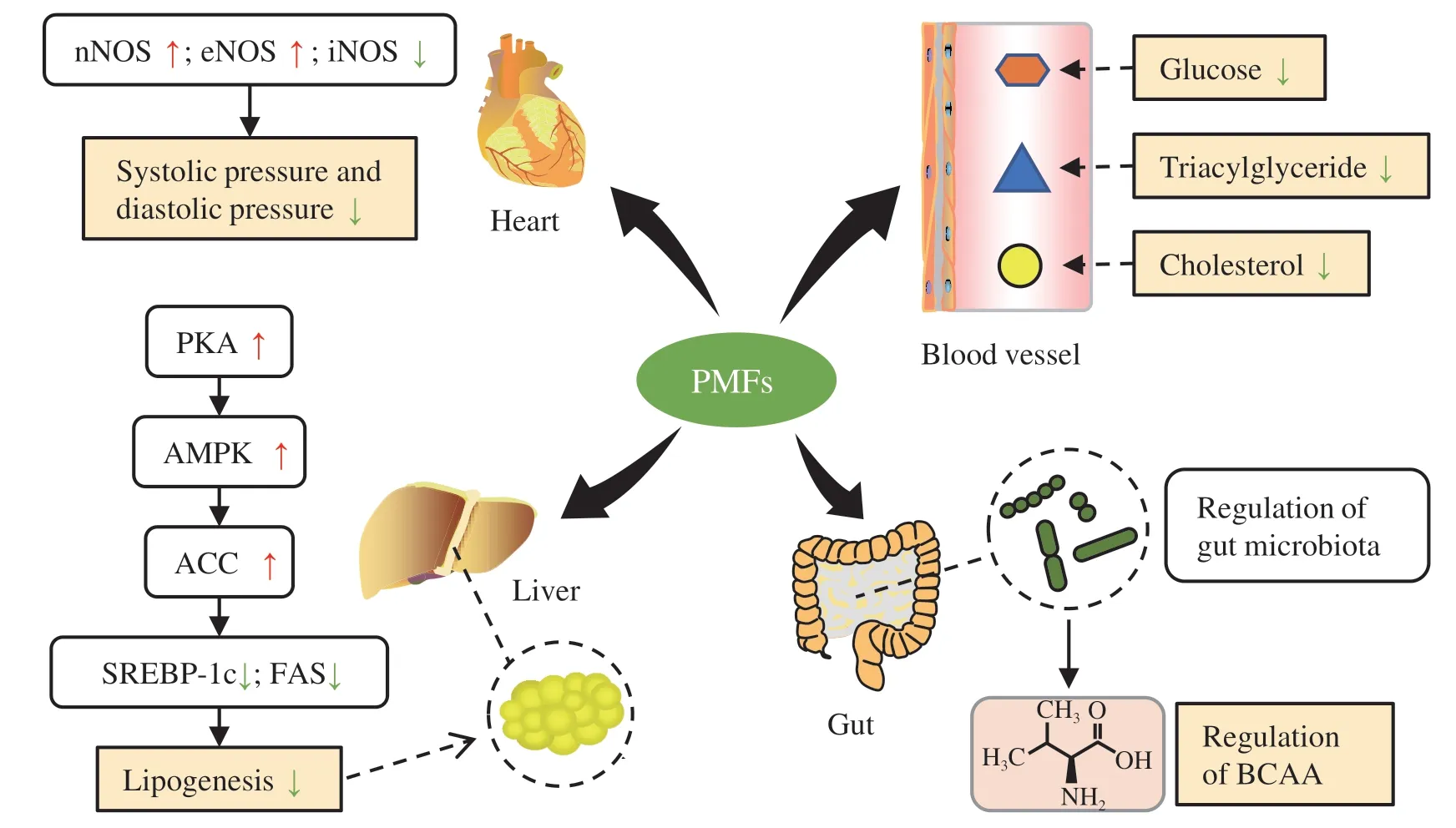
Fig.3 Molecular mechanisms of metabolic syndrome regulatory effects of PMFs by lowering blood pressure and sugar,ameliorating dyslipidaemia,inhibiting oxidative stress and inflammation,and modulating gut homeostasis.ACC,acetyl-CoA carboxylase;AMPK,adenosine monophosphate-activated protein kinase;BCAA,branched-chain amino acid;eNOS,endothelial nitric oxide synthase;FAS,fatty acid synthase;iNOS,inducible nitric oxide synthase;nNOS,neuronal nitric oxide synthase;PKA,protein kinase A;PMFs,polymethoxyflavones;SREBP-1c,sterol regulatory element-binding protein 1c (SREBP-1c).Red arrows: up-regulation;green arrows: down-regulation.
6.4.1 Regulation of metabolic syndrome in vitro
Several PMFs have been reported to inhibit the risk factors of metabolic syndromein vitro.Cell-based assays suggested that nobiletin and 3,5,6,7,8,3’,4’-heptamethoxyflavone could inhibit lipid accumulation and adipogenesis by downregulating the protein expression of lipogenic factors,including sterol regulatory element-binding protein 1c (SREBP-1c) and fatty acid synthase (FAS)[78,79].
6.4.2 Regulation of metabolic syndrome in vivo
Moreover,pure PMFs can also block the risk factors of metabolic syndromein vivo.An animal study reported that PMF-rich orange peel oil reduced the systolic pressure and diastolic pressure in hypertensive rats,probably associated with upregulating the protein expression of neuronal (nNOS) and endothelial (eNOS) NO synthase and downregulating the protein expression of iNOS[80].A reduction in the activity of eNOS is known to be mainly responsible for the elevation of blood pressure,while eNOS/nNOS-derived NO is protective against the development of atherosclerosis[81].Another animal study supported the inhibitory effect of PMFs on the fat accumulation in adipose tissues[82].Gut microbiota also plays a vital role in PMF-mediated regulation of metabolic diseases.For example,a purified citrus PMF-rich extract could significantly alleviate HFD-induced metabolic syndrome in mice,accompanied with ameliorating gut dysbiosis and regulating branched-chain amino acid(BCAA) metabolism[83].These effects were mainly associated with the regulation of gut microbiota by PMFs,especially the commensal bacteriumBacteroides ovatus,since its intragastric administration reduced BCAA concentrations and alleviated metabolic syndrome in HFD mice[83].These studies suggest that PMFs may be applied as potential prebiotic agents to manage metabolic syndrome.Additionally,cardiovascular dysfunction,such as the hemodynamic parameters and vascular reactivity,could be ameliorated by nobiletin through inhibiting oxidative stress,MMP-2,and MMP-9[84].Also,nobiletin could inhibit the proliferation of rat pulmonary artery smooth muscle cells and protect against pulmonary arterial hypertension,probably via regulating the Src/STAT3 signaling pathway[85].High levels of cholesterol,triacylglyceride,and glucose are risk factors for metabolic disorders,which could be alleviated by the ingestion of citrus fruit peels that were rich in nobiletin and 4’,5,7,8-tetramethoxy flavone[86].
Overall,PMFs exhibit excellent effects on managing metabolic syndrome by lowering blood pressure and sugar,ameliorating dyslipidaemia,inhibiting oxidative stress and inflammation,and modulating gut homeostasis.Several key molecules,like eNOS,SREBP-1c,FAS,MMPs,and signaling pathways,such as the AMPK/ACC and Src/STAT3 signaling,can be potential molecular targets of PMFs to fight against metabolic syndrome.
6.5 Neuroprotection
Recent studies indicate that PMFs exhibit different neuroprotective effects (Fig.4).They can protect from neurodegenerative diseasesin vitroandin vivo.

Fig.4 Neuroprotective effects of PMFs and related molecular mechanisms by inhibiting the activities of BACE1,promoting ChAT gene transcription,enhancing the level of ACh and the activity of nAChR.AcCoA,acetyl coenzyme A;Ach,acetylcholine;APP,amyloid precursor protein;Aβ,amyloid β peptide;BACE1, β-site amyloid precursor protein cleaving enzyme 1;ChAT,choline acetyltransferase;nAChR,nicotinic acetylcholine receptor.
6.5.1 Neuroprotection in vitro
β-Site amyloid precursor protein cleaving enzyme 1 (BACE1) is the rate-limiting enzyme for the abnormal production ofβ-amyloid peptides involving in Alzheimer’s disease.PMFs,including 5,7-dimethoxyflavone,5,7,4’-trimethoxyflavone,and 3,5,7,3’,4’-pentamethoxyflavone,exhibited strong inhibitory effects on BACE1 without significantly inhibiting the activities ofα-secretase or other serine protease,suggesting that they were relatively specific and selective BACE1 inhibitors[87].Acetylcholine (ACh) is the neurotransmitter synthesized by choline and acetyl coenzyme A(AcCoA) with the presence of acetyl coenzyme acetyltransferase(ChAT)[88].TheChATgene transcription could be promoted by theAspergillus kawachii-fermentedCitrus reticulata(Ponkan) fruit squeezed draffs extracts that were rich in 4’-demethylnobiletin[89].In addition,the level of synaptic ACh and the activity of nicotinic acetylcholine receptor (nAChR) could be enhanced by 5-desmethylnobiletin[90].Tangeretin could improve the cognitive impairments in transgenicDrosophilamodel of Parkinson’s disease,and the molecular docking study suggested that it could bind with the α synuclein molecule[91].
6.5.2 Neuroprotection in vivo
It was reported that 3,5,6,7,8,3’,4’-heptamethoxyflavone could alleviate locomotive hyperactivity in mice by activating extracellular signal-regulated kinases 1/2 (ERK1/2) in the hippocampus,and it had a much higher permeability to the brain tissues than other citrus PMFs,such as nobiletin,tangeretin,and natsudaidain[92].The depressive-like behaviour and hippocampal neurochemical changes in chronic unpredictable mild stressed mice could be ameliorated by 3,5,6,7,8,3’,4’-heptamethoxyflavone,partially owing to increasing brain-derived neurotrophic factor (BDNF) in the hippocampus via activating the ERK1/2/MAPK signaling[93].
Therefore,PMFs may be promising agents for the prevention and management of neurodegenerative diseases,such as the Alzheimer’s disease,Parkinson’s disease,and depression,and several molecules and signaling,like BACE1,ChAT,nAChR,BDNF,and the ERK signaling,should be their main molecular targets.
6.6 Immune regulation
PMFs can regulate immune functions.Antigen presentation process is pivotal for T cells to recognize antigenic epitopes[94].Cell-based assays reported that the antigen presentation capability in bone marrow-derived dendritic cells could be enhanced by nobiletin[95]and sudachitin[96].Animal studies also demonstrated that PMFs could modulate the production of cytokines and antibodies.The production of IL-4 could be reduced by 3,5,6,7,8,3’,4’-heptamethoxyflavone via inhibiting the activities of phosphodiesterase (PDE) and increasing the content of cyclic AMP (cAMP)[97,98].In ovalbumin(OVA)-immunized mice,nobiletin and sudachitin were reported to increase OVA-specific IL-4,IL-10,as well as IgE,IgG,and IgG1 production[95,96].In generally,these studies suggest that PMFs can facilitate antigen presentation,modulate inflammatory factor production,and activate antibody response to regulate immune functions.
6.7 Skin protection
Several studies support that PMFs can protect skin from ultraviolet (UV) B irradiation (Fig.5).The exposure to UVB can trigger the expression of MMPs in the skin to degrade collagen,leading to the occurrence of deep wrinkles.
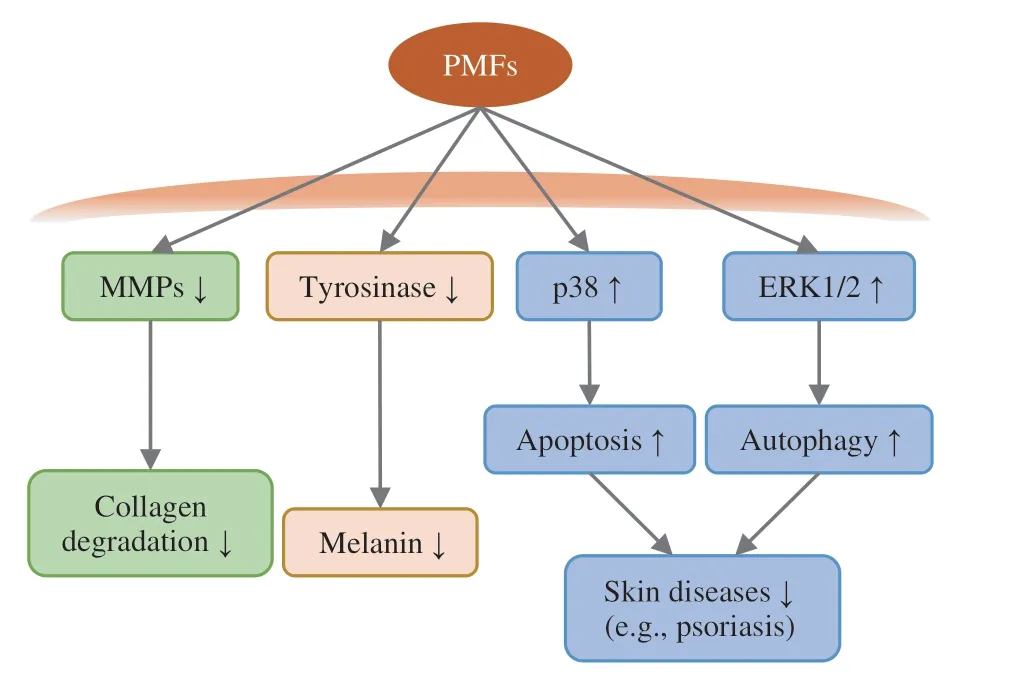
Fig.5 Skin protective effects of PMFs and related molecular mechanisms by inhibiting the activities of MMPs and tyrosinase and activating the p38 MAPK and ERK1/2 pathway.ERK,extracellular signal-regulated kinase;MMPs,matrix metalloproteinases;PMFs,polymethoxyflavones.“↓”: down-regulation;“ ↑ ”: up-regulation.
First,PMFs were potential candidates of anti-photoaging agents.The UVB-induced expression of MMP-1 could be suppressed by PMFs extracted from orange peels through inhibiting JNK phosphorylation and its activity[99].In addition,the orange peel coldpressed oil rich in PMFs could maintain the dermal structure,enhance serum and skin tissue antioxidant defence system,and block serum and skin inflammation[100].Intriguingly,these protective effects led to the partial decomposition of PMFs into 5-hydroxy-PMFs[100].
Second,PMFs have whitening effects on the skin (Fig.5).Tyrosinase is a copper-containing oxidase enzyme that catalyzes the first step in the formation of melanin in the melanocytes,and PMFs can inhibit the tyrosinase,leading to the reduction of melanin production.Previous studies suggested that nobiletin,chrysosplenetin,and PMF mixtures (nobiletin,3,3’,4’,5,6,7,8-heptamethoxyflavone,and tangeretin),could induce the degradation of tyrosinase in lysosomes[101,102].The possible mechanism was that PMFs could acidify cell organelles,especially the melanosomes,finally resulting in the suppression of melanogenesis[101].In addition,in vitrodocking studies suggested that the methoxy groups on the B-ring of PMFs faced the catalytic site in the tyrosinase,and they caused steric hindrance,preventing alternative binding modes in the enzyme[102].
Third,PMFs have the potential to treat certain skin diseases,such as psoriasis (Fig.5).For example,sudachitin,a PMF isolated from the peel ofCitrussudachi,was found to induce cell apoptosis via activating the p38 MAPK pathway in human keratinocyte HaCaT cells[103,104].In addition,sudachitin could also inhibit EGF-induced cell migration and proliferation in HaCaT cells by blocking the activation of Raf-1-ERK1/2 signaling[103].However,another common PMF,nobiletin,was reported to promote autophagy but not apoptosis in HaCaT cells[104],probably by activating ERK1/2[103],and 3’-demethoxysudachitin,on the other hand,failed to induce apoptosis or autophagy[104].These studies suggest that different PMFs may lead to different cellular responses,probably by regulating different signaling pathways.
As a summary,PMFs can protect skins from UV irradiation,whiten skins,and treat certain skin disorders,and these effects should be attributed to antioxidant,anti-inflammatory,anti-tyrosinase,and regulation of different cellular responses.In the future,more deep insights about the skin protection mechanism of PMFs should be revealed to facilitate their applications in the cosmeceuticals.
6.8 Other bioactivities
PMFs also exhibit many other bioactivitiesin vitroandin vivo,such as antimicrobial effects[105,106],antitrypanosomal effects[107],antinociceptive effects[12],anxiolytic effects[12],antimutagenic effects[108],bone protective effects[109],intestinal protective effects[110,111],regulation of circadian rhythm[2,112],improving male sexual functions[113],and anti-aging effects[114],with detailed information summarized in Tables 3,4,and S1.
The human beneficial dose equivalence of PMFs was calculated based on the body surface area (BSA) normalization method according to a previous study[115].According to Table 4,most beneficial doses of PMFs ranged from 2 mg/(kg·day) to 50 mg/(kg·day)in mouse models.To convert the dose used in a mouse (20 g) to an equivalent dose for humans,the corresponding doses of PMFs for adults (60 kg) should be 9.6−122 mg/day.According to Table 1,the concentrations of PMFs in different plant sources ranged from 0.9−20 mg/g,therefore,the suggested level of daily PMF-rich food consumption can be 0.5−136 g for adults (60 kg)with potential health benefits.This suggests that the potential health benefits of PMFs in humans should be achieved by the consumption of plant-based foods rich in PMFs,while it still needs further verificationin by clinical trials.
7. Conclusions and perspectives
In conclusion,this review updated recent advances on the natural sources,refined extraction technologies,biosynthesis,metabolism,as well as main bioactivities and related molecular mechanisms of PMFs.Since flavonoids andOMTgenes are widely existed in the plant kingdom,it is speculated that PMFs should exist in more plants besides the reported species,so,it is possible to discover novel PMFs and their natural sources to enrich the PMF pool.In addition,increasing evidence supports that gut microbiota play a critical role in the metabolism and bioactivities of PMFs,while the studies about the interaction between PMFs and gut microbiota are still limited,and how gut microbiota connect PMFs and their bioactivities is still not clear.Besides,whether the metabolites of PMFs transformed by gut microbiota have enhanced bioactivities than PMFsperseremains to be verified in more studies.Moreover,encapsulation may be an effective method to increase their bioaccessibility and bioavailabilityin vivo,and should also be explored in the future.Finally,although PMFs exhibit promising bioactivitiesin vitroandin vivo,especially on cancer and metabolic syndrome,the health benefits of them in humans have been much less investigated.This review still have some limitations.For example,most bioactivities summarized in this review are based onin vitroand animal-based studies due to the lack of human-based studies,therefore,it is recommended to verify the health benefits of PMFs in humans based on well-designed clinical trials in the future,which can promote the large-scale refined extraction of PMFs in the industries,and the PMFs with specific health benefits can be developed into nutraceuticals and pharmaceuticals to prevent and manage certain chronic diseases.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Local Financial Funds of National Agricultural Science and Technology Center,Chengdu,China (NASC2021KR01) and the Agricultural Science and Technology Innovation Program (ASTIP-IUA-2022002).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250003.
杂志排行
食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing
- Food nutrition and toxicology targeting on specific organs in the era ofsingle-cell sequencing
