Insights into sensitizing and eliciting capacity of gastric and gastrointestinal digestion products of shrimp (Penaeus vannamei) proteins in BALB/c mice
2024-02-16YoLiuSongyiLinKexinLiuShnWngWngLiSun
Yo Liu,Songyi Lin,b,Kexin Liu,Shn Wng,Wng Li,N Sun,b,*
a National Engineering Research Center of Seafood,School of Food Science and Technology,Dalian Polytechnic University,Dalian 116034,China
b Collaborative Innovation Center of Seafood Deep Processing,Dalian Polytechnic University,Dalian 116034,China
Keywords:Penaeus vannamei Allergenicity Digestion BALB/c mice model
ABSTRACT Shrimp (Penaeus vannamei) proteins have been shown an allergenic potential;however,little information is available on the sensitizing and eliciting capacity of shrimp protein digestion products.In this study,a BALB/c mice model was used to explore the allergenicity of shrimp protein sample (SPS) and their gastric and gastrointestinal digestion products (GDS/GIDS).As compared with the SPS groups,the GDS/GIDS groups caused lower specific immunoglobulins (IgE/IgG1) levels (P <0.05),but higher than the control groups,indicating that the digestion products sensitized the mice.Meanwhile,spleen index,mouse mast cell protease-1(mMCP-1) concentration and proportion of degranulated mast cells were significantly reduced in the GDS/GIDS groups (P <0.05);simultaneously,allergic symptoms,vascular permeability and histopathological changes of tissues were alleviated.Nevertheless,the allergenicity of digestion products cannot be eliminated and still cause systemic allergic reactions in mice.The study showed that the digestion products of shrimp still had high sensitizing and eliciting capacity.
1. Introduction
Allergy caused by food protein is a serious problem and a global health problem around the world[1,2].It has been found that IgE mediated type I hypersensitivity induced by food proteins might be accompanied by severe allergic symptoms such as urticaria,abdominal pain,diarrhea,dermatitis,and asthma,even life-threatening[3-6].However,allergic symptoms after seafood consumption are more common than other food allergens[7].Unfortunately,Penaeus vannameiis the most widely cultured shrimp globally and can also cause severe allergic symptoms[8,9].
Studies have found that food intake was the main way in which allergic reactions occur in humans[10,11].Food proteins can only be absorbed by the body after it is digested by the stomach and intestines[12].After food intake,shrimp proteins will be broken down into small peptides by pepsin,trypsin,and chymosin[13,14].These peptides may expose more IgE binding sites or mask binding sites and cause the changes of the allergenicity[15-17].Previous studies have considered that proteins resistant to the digestive enzymes have an allergenic potential.Toomer et al.[18]reported that shrimp proteins with molecular weights of 32 and 25 kDa were pepsin and/or pancreatin stable and had potential allergenicity.On the contrary,recent studies have shown that proteins with unstable digestive enzymes also have allergenic potential[19].Digestion of proteins might lead to an increase or decrease in the sensitizing capacities.Rao et al.[12]found that peanut proteins were digested by digestive enzymes which was accompanied by a loss of high molecular weight proteins,but the digestion products still had IgE-binding ability.Meanwhile,Korte et al.[20]characterized the digestion products of hazelnut and identified 130 peptides carrying IgE-binding epitopes and might represent sensitizers for hazelnut allergy.However,there are few studies on the sensitizing and allergenic capacity of digestion productsin vitro.
Most previous reports on the gastrointestinal digestion of allergens have focused on the fate of the purified proteins and their IgE binding potential[21-23].The main allergens of shrimp are tropomyosin (TM),sarcoplasmic calcium binding protein (SCP),arginine kinase (AK),myosin light chain (MLC) and myosin heavy chain (MHC)[18].Meanwhile,researchers found that these major allergens have sensitizing and allergenic potential.Chen et al.[24]purified the SCP of Crayfish and found that SCP had IgE binding activity.In addition,Zhang et al.[25]purified the MLC fromProcambarus clarkiimuscle,indicating that MLC had sensitizing capacities.However,the sensitizing and allergenic capacity of small peptides produced by digestion is not clear.In this study,the whole proteins ofP.vannameiand their digestion products were studied to systematically characterize the sensitizing and allergenic ability of gastric and gastrointestinal digestion products.
Currently,a BALB/c mice model has been widely used to assess the allergenicity of food proteins because of their ability to mimic the actual behavior of proteinsin vivo[26].In this study,all the BALB/c mice were divided into 5 groups,namely saline group,alum group,shrimp protein sample (SPS) group,gastric digested shrimp (GDS)group and gastrointestinal digested shrimp (GIDS) group.To prevent the repeated digestion of proteins and their digestion products in the mice,the mice were sensitized and challenged by intraperitoneal injection with SPS,GDS and GIDS,respectively.The present study aimed to evaluate the actual behavior and allergenic potential of shrimp proteins and their gastric and gastrointestinal digestion productsin vivoby a BALB/c mice model.The findings of this study will contribute to further studies on the allergenicity assessment of shrimp proteins.
2. Materials and methods
2.1 Materials
Shrimp (P.vannamei) was bought from the Shahekou seafood market (Dalian,China).Pepsin,pancreatin and Evan’s blue were obtained from Sigma Aldrich (St Louis,MO,USA).Rat monoclonal anti-mouse IgE epsilon chain (Biotin) antibody,goat anti-mouse IgG1(HRP) antibody,streptavidin (HRP) and 3,3’,5,5’-tetramethylbenzidine (TMB) enzyme-linked immunosorbent assay (ELISA) Substrate (High Sensitive) were bought from Abcam(Cambridge Science Park,UK).Toluidine blue solution was bought from Solarbio Science &Technology Co.,Ltd.(Beijing,China).Mouse HIS ELISA Kit and Mouse Mast Cell Protease 1 ELISA Kit were bought from Jianglai (Shanghai,China).
2.2 Shrimp (P.vannamei) protein extraction
Shrimp (P.vannamei) was boiled in boiling water for 5 min and the shell and head were removed.Total protein was extracted from boiled shrimp by alkali extraction and acid precipitation[27,28].Furthermore,the shrimp were stirred at 4 °C and pH 11.0 for 8 h and the supernatant was collected.Then,the supernatant was stirred at 4 °C and pH 5.5 for 1.5−2 h,and the precipitate was collected.Finally,SPS was freeze-dried and stored at −80 °C.
2.3 In vitro digestion
After lyophilization,SPS was digestedin vitroby combining the method of Minekus et al.[29]and Sun et al.[30],including simulated gastric and intestinal digestion.Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were prepared under the coordinated conditions.The detailed experimental procedure was as follows.
Gastric phase: The SPS was mixed with SGF and incubated for 30 min at 37 °C.Then,the mixture was set to pH 3.0 and the pepsin(2 000 U/mL of final enzymatic activity) was added to obtain an enzyme with a 1:100 substrate ratio (E:S).The incubation was performed at 37 °C for 120 min and NaOH was added to inhibit the pepsin.The GDS sample was centrifuged at 10 000 ×gand 4 °C for 10 min,and the sample was stored at −80 °C.
Intestinal phase: the GDS was mixed with SIF and the pH of the mixture was set to 7.0.Then,the pancreatin (100 U/mL of final enzymatic activity) was added to initiate the gastrointestinal digestion at 37 °C for another 120 min and the sample was heated for 10 min at 100 °C to inhibit the enzyme.The GIDS sample was centrifuged at 10 000 ×gand 4 °C for 10 min,and the sample was stored at −80 °C.
2.4 Experimental animals
Female BALB/c mice of 3−4 weeks old were received from Changsheng Biotechnology Co.,Ltd.(Benxi,Liaoning,China).All the mice were fed under a temperature of (22 ± 2) °C and a humidity of 50% ± 15% for 1 week before commencing the animal experiments.Then,the mice were kept in an air-conditioned atmosphere with a 12/12 h light/dark cycle and were fed with water and food ad libitum.All the studies of mice were approved by University of Dalian Polytechnic Animal Care Committee (protocol number: DLPU2021034).
2.5 Sensitization and challenge
All BALB/c mice were distributed to 5 groups (n=10 for each group) as follows: saline control group (Saline),mice injected with aluminum hydroxide (alum,Thermo Scientific,Cramlington,UK)(Alum),mice injected with Alum+SPS (SPS),mice injected with Alum+GDS (GDS),and mice injected with Alum+GIDS (GIDS).Alum as an adjuvant can stimulate the immune system and effectively enhance the immune response of the antigen[31].Moreover,in order to avoid the secondary digestion of the digestion products of shrimp protein extract in BALB/c mice,the methods of Misra et al.[32],Kumar et al.[33]and Morafo et al.[34]were referred to sensitize mice by intraperitoneal injection.Briefly,for the stage of basic sensitization,100 μg of SPS,GDS and GIDS were dissolved in 100 μL of saline solution.The mice were sensitized weekly by intraperitoneal injection with samples plus alum (3:1) per mice,and other two groups were injected with saline and alum,respectively.For the stage of challenge,the mice in the 5 groups were injected with 500 μg of SPS,GDS and GIDS and sacrificed on Day-43.All the experimental design of animal is shown in Fig.1.

Fig.1 Experimental design of animal sensitization and challenge.Fifty female BALB/c mice were distributed to 5 groups: saline control group(Saline),mice injected with Alum,mice injected with Alum+SPS,mice injected with Alum+GDS,and mice injected with Alum+GIDS.Alum:aluminum hydroxide;SPS: shrimp protein sample;GDS: gastric digested shrimp;GIDS: gastrointestinal digested shrimp.
2.6 Serum and plasma collection
Blood samples were collected by intraocular canthal vein puncture at 35,38,42 and 43 days.Serum samples were centrifuged at 4 000 r/min for 10 min at 4 °C.In addition,plasma samples were collected in anticoagulant tube (containing EDTA-K2) after 20 min of challenge and centrifuged at 4 000 r/min for 10 min at 4 °C.All serum and plasma samples were kept at −80 °C until analysis.
2.7 Monitoring of anaphylaxis
Anaphylactic reactions were characterized by the appearance of clinical symptoms and a decrease in ear temperature.The clinical symptoms were assessed by the scoring system as follows: 0=no reaction;1=puffiness around the eyes and mouth,puffier hair,diarrhea;3=no activity;4=wheezing,labored respiration;and 5=death[35].
Ear temperature was measured before challenge and 20−30 min following challenge using a handheld thermometer.The handheld thermometer was placed behind the ear of the mice and the ear temperature was recorded before and after challenge.The changes of ear temperature before and after were the changes of ear temperature for each mouse[36].
2.8 Analysis of spleen index
On day 43,the target organs (spleen) were removed and weighed.The spleen index was calculated according to the formula[37]:
2.9 Determination of vascular leakage
The mice were injected with 100 μL 0.5% Evan’s blue solution through the tail vein to assess vascular permeability before challenge.Then the mice were injected with 500 μg of different proteins.Visible blue color on the soles of the feet and small intestine was observed to sign of vascular leakage after 30−40 min of challenge[38].
The levels of albumin in the peritoneal fluid of BALB/c mice were determined 40 min after challenge.3 mL PBS (containing 10 mmol/L EDTA) solution was injected into the peritoneal membrane of mice.After abdominal massage for 1 min,PBS was extracted into EP tubes and maintained on the ice.Then,the extracted sample was centrifuged at 600 r/min for 6 min at 4 °C and the supernatant was stored at −80 °C[39].The content of albumin in supernatant was determined using a BCA Protein Assay Kit (Solarbio,Beijing,China),as outlined by the manufacturer’s instructions.
2.10 Measurement of specific IgE and IgG1 levels
The levels of serum specific IgE and IgG1in different groups of mice were measured using indirect ELISA.SPS,GDS and GIDS were diluted with 0.05 mol/L sodium carbonate buffer (pH 9.6) to prepare sample proteins at a concentration of 10 μg/mL.SPS,GDS and GIDS samples were added to 96-well ELISA plate and incubated at 4 °C overnight.Following incubation,the well protein samples were aspirated and washed 3 times with PBS-0.1% BSA-0.1% Tween 20.The plates were then blocked with PBS-1% BSA (150 μL/well) for 2 h at 37 °C.After washing the plates for 3 times,100 μL of serum was added to each well,and the plates were incubated at 37 °C for 2 h and washed for 5−6 times.Following this step,100 μL of rat monoclonal anti-mouse IgE epsilon chain (Biotin) antibody(1:500) was added for 1 h at 37 °C and washed for 5 times.After washing,100 μL of streptavidin (HRP) (1:500) was added and the plates were incubated at 37 °C for 1 h.In addition,serum specific IgG1was detected by addition of 100 μL of anti-mouse IgG1(HRP)pre-adsorbed antibody (1:4 000) and plates were incubated at 37 °C for 1 h.After washing 5 times with wash buffer as before,the reactions were developed with 100 μL of TMB chromogen solution in the dark for 10 min at 37 °C and the reaction was stopped with the addition of 50 μL of 2 mol/L H2SO4.Finally,the optical density (OD)in each well was read at 405 nm using a Microplate Reader (InfiniteTMM200,TECANE,Switzerland).
2.11 Measurement of plasma histamine and mouse mast cell protease-1 (mMCP-1) levels
The plasma from each mouse was collected,and histamine and mMCP-1 concentrations were detected by ELISA assay using a Mouse HIS ELISA Kit and Mouse Mast Cell Protease 1 ELISA Kit according to the manufacturer’s instructions.The results were reported as OD with a microplate reader.
2.12 Histopathology of lung,spleen and jejunum
Resected lung,spleen and jejunum tissues were removed after euthanasia and the jejunum was washed with normal saline.Then,the lung,spleen and jejunum tissues were fixed in 4% paraformaldehyde and embedded in paraffin.Serial paraffin sections with 4−6 μm thickness were made and prepared for staining of hematoxylin and eosin (H&E) to observe the histopathological[40].
To measure the mast cell degranulation,5 spleen sections in each group were stained with toluidine blue and the number of intact and degranulated mast cells in each section was counted under a microscope in 5 randomly chosen fields[41].
2.13 Statistical analysis
All the quantitative results were subjected to a one-way of analysis of variance using SPSS 18.0 software (IBM,Chicago,IL,USA).All data were expressed as mean ± standard error of the mean(SEM) of three biological replicates and statistical significance was set atP<0.05.
3. Results
3.1 Allergic responses of shrimp proteins and their digestion products
The effects of the shrimp (P.vannamei) proteins and their digestion products were studied in a mice model of food allergy following the experiment protocol (Fig.1).On Day 43,the sensitized mice were treated with different proteins (500 μg) by intraperitoneal injection during the challenge phase.As shown in Figs.2A-B,the food intake and water intake of all groups of mice did not change significantly during feeding,and there was no significant difference(P>0.05).Fig.2C illustrates the body weight measured in different groups of mice.Except for the saline-exposed mice,the body weight of all groups gradually decreased after 4 weeks.As shown in Fig.2D,the hypersensitivity scores were observed in different groups of mice.After challenge,mice in the SPS-challenged group developed statistically significant hypersensitivity scores of shrimp allergy when compared to those in the saline-and alum-exposed groups (P<0.05).As compared with SPS-challenged group,GDS-and GIDS-challenged groups showed a significant reduction in hypersensitivity scores(P<0.05).However,there were no significant effects from saline and alum injection on the hypersensitivity scores of allergy (P>0.05).The ear temperature of mice was measured before and after challenge with SPS,GDS and GIDS.The ear temperature of mice in the SPS-,GDS-and GIDS-challenged groups decreased significantly after challenge (P<0.05),which decreased by 1.62,1.35 and 1.11 °C,respectively,as shown in Fig.2E.However,there was no significant change in ear temperature in the saline-and alum-exposed groups,as compared with the SPS-,GDS-and GIDS-challenged groups.The spleen,as the most important largest immune tissue in the body,can be swollen by antigen stimulation.In comparison with the saline-and alum-exposed groups,the SPS-,GDS-,and GIDS-sensitized groups had a higher spleen index (P<0.05).However,the spleen index of the GDS and GIDS-sensitized groups was significantly lower than that of the SPS-sensitized group,but an increase in spleen size was still induced in mice in the GDS-and GIDS-sensitized groups compared with those in the saline-and alum-exposed groups (P<0.05) (Fig.2F).

Fig.2 Characteristics of the allergic response in BALB/c mice.(A) Food intake.(B) Water intake.(C) Body weight for individual mice.(D) Hypersensitivity scores for individual mice.(E) Ear temperature for individual mice.(F) Index of spleen for individual mice.Spleen was collected from each mouse and pooled within groups.Different letters indicate statistically significant differences (P <0.05).
3.2 Specific IgE and IgG1 levels of shrimp proteins and their digestion products
Most food allergic reactions are mediated by IgE and should therefore be taken into account when assessing the potential effect of food on allergens[42].The specific IgE concentrations in serum were detected in the serum of BALB/c mice to evaluate the allergenicity of SPS,GDS and GIDS.As shown in Fig.3A,the concentrations of serum specific IgE were lower in saline-and alum-exposed mice.The concentrations of specific IgE antibodies increased significantly in SPS-,GDS-and GIDS-sensitized mice,when compared with the saline-and alum-exposed mice (P<0.05).However,as compared with the SPS-sensitized mice,the specific IgE concentrations in GDS-and GIDS-sensitized mice decreased by 61.11% and 90.55%,respectively.But GDS-and GIDS-sensitized mice still had specific IgE binding ability.
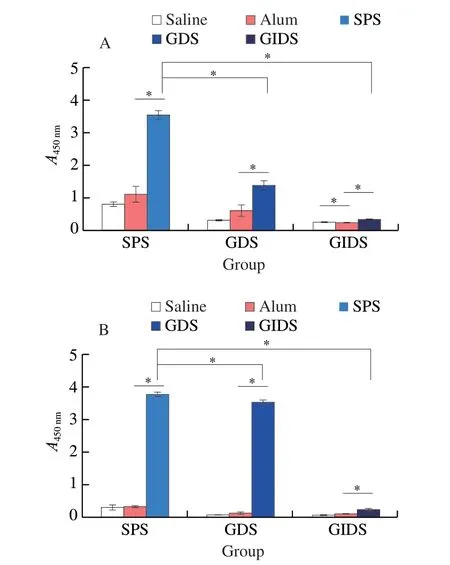
Fig.3 Serum specific IgE and IgG1 concentrations.IgE binding ability (A)and IgG1 binding ability (B) detected by ELISA.Serum samples were collected from mice by intraocular canthal vein puncture.* indicates a significant difference between two groups (P <0.05).
The changes of serum IgG1concentrations in the SPS-,GDS-and GIDS-sensitized mice were similar to that of the IgE concentrations.The serum IgG1concentrations were significantly higher in the SPS-sensitized mice,when compared with the saline-and alum-exposed mice (P<0.05) (Fig.3B).However,the treatment with GDS and GIDS significantly reduced the concentrations of serum specific IgG1(P<0.05).Interestingly,the specific IgG1concentrations were significantly higher in GDS-and GIDS-sensitized mice than those in saline and alum-exposed mice (P<0.05).
3.3 Systemic allergic symptoms induced by shrimp proteins and their digestion products
Systemic allergic symptoms were caused by the increase of vascular permeability and could be evaluated by measuring the vascular permeability in BALB/c mice after challenge[43].Vascular leakage induced by saline,alum,SPS,GDS and GIDS was determined,as shown in Fig.4.After Evan’s blue solution injection,the footpads and small intestine of mice were photographed.Bluish coloring of the footpads and small intestine in SPS-,GDS-and GIDS-challenged mice,indicating that SPS,GDS and GIDS induced allergic reactions in the mice.Furthermore,no vascular leakage was observed in footpads and small intestine of saline-exposed group and alum-exposed group (Figs.4A and B).
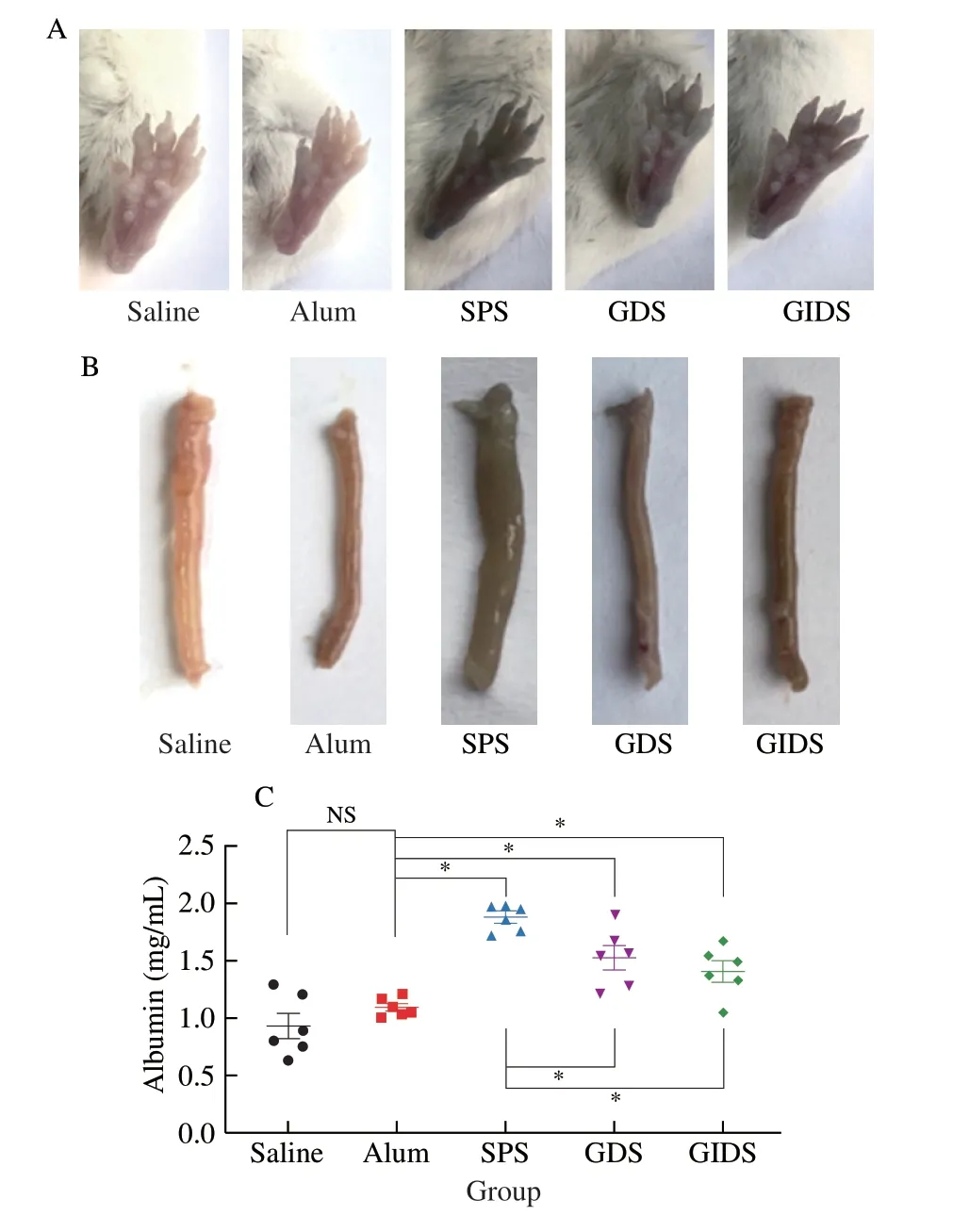
Fig.4 Detection of vascular permeability in BALB/c mice.The footpads (A)and intestines (B) photographed after Evan’s blue solution injection.(C) The levels of peritoneal albumin in the mice.NS indicates no significant difference between two groups (P >0.05) and * indicates a significant difference between two groups (P <0.05).
Since the difference in vascular permeability among SPS-,GDS-and GIDS-challenged groups could not be distinguished by observation of Evan’s blue solution,and the quantitative analysis could be performed by measuring the level of albumin in the peritoneal fluid.The albumin concentration in peritoneal lavage fluid was detected by BCA binding and the results were shown in Fig.4C.The levels of albumin in SPS-challenged mice were higher and the levels of albumin in SPS-,GDS-and GIDS-challenged mice were 1.87,1.52,and 1.40 mg/mL,respectively.As compared with SPS-challenged mice,the levels of albumin in the GDS-and GIDS-challenged mice were significantly decreased (P<0.05),indicating that the allergic symptoms in the SPS-challenged group were more severe.In addition,the levels of albumin were lower in the saline-and alum-exposed groups,and there was no significant difference between the two groups (P>0.05).The results showed that SPS,GDS and GIDS could induce allergic reaction in the mice.
3.4 Histamine and mMCP-1 levels of shrimp proteins and their digestion products
Histamine,a primary amine synthesized from histidine in mast cell Golgi apparatus,has been recognized as an important mediator of allergic reactions[44].As shown in the Fig.5A,the histamine concentrations significantly increased in the SPS-challenged mice(27.71 ng/mL) when compared with the saline-exposed mice(24.13 ng/mL) and alum-exposed mice (25.06 ng/mL) (P<0.05).Furthermore,the histamine concentrations in GDS-challenged mice(27.06 ng/mL) and GIDS-challenged mice (26.50 ng/mL) were significantly higher than those in saline and alum-exposed mice(P<0.05).Interestingly,all mice treated with SPS,GDS and GIDS did not differ significantly (P>0.05).At the same time,there was no significant difference between saline-exposed mice and alum-exposed mice (P>0.05).

Fig.5 Plasma histamine and mouse mast cell protease-1 (mMCP-1) levels.(A) Plasma histamine concentrations and (B) mMCP-1 concentrations.The histamine and mMCP-1 concentrations were measured by ELISA (n=5 individual plasma).NS indicates no significant difference between two groups(P >0.05) and * indicates a significant difference between two groups (P <0.05).
In addition,the degranulation of mast cell leads to the increased concentrations of mMCP-1,which also indicates the occurrence of allergic reactions[45].Representative images for mMCP-1 concentrations were shown in Fig.5B.The concentrations of mMCP-1 in the saline-exposed mice (29.75 ng/mL) and alum-exposed mice (29.12 ng/mL) were lower (P<0.05).However,the SPS-challenged mice (34.18 ng/mL) had higher mMCP-1 concentrations as compared with those in the alum-exposed mice (P<0.05).Meanwhile,the mMCP-1 concentrations in GDS-challenged mice and GIDS-challenged mice were 32.46 and 31.61 ng/mL,respectively,which were significantly higher than those in the alum-exposed mice(P<0.05).The results showed that SPS,GDS and GIDS treatment significantly increased the concentrations of plasma histamine and mMCP-1,and resulted in more severe anaphylactic symptoms.
3.5 Degranulation of mast cells after challenge with shrimp proteins or their digestion products
To further investigate the allergenicity of shrimp (P.vannamei)and its digestion products,the effects of SPS,GDS and GIDS on the degranulation of mast cells were evaluated.For selected fields,the intact and degranulated mast cells were counted.The intact mast cells had intact contours,while the degranulated mast cells had broken cell membranes with unclear contours and expelled mediators[46].There were a large number of mast cells in the SPS-challenged group,most of which were degranulated,and only a few intact mast cells were observed (Fig.6A).The number of mast cells was significantly reduced in the GDS-and GIDS-challenged groups when compared to the SPS-challenged group.In contrast,only a few mast cells were observed in the saline-and alum-exposed groups and most were not degranulated.The counting results of degranulated mast cells were shown in Fig.6B.As compared with the saline-exposed group (2.50%)and alum-exposed group (8.99%),the proportion of degranulated mast cells was significantly increased in the SPS-challenged group(61.28%) (P<0.05).Additionally,the proportion of degranulated mast cells in the GDS-challenged group and GIDS-challenged group was 18.10% and 24.22% lower than that in the SPS-challenged group,but still significantly higher than that in the saline-and alum-exposed groups (P<0.05).
The results showed that SPS,GDS and GIDS could induce the degranulation of mast cells in the spleen of BALB/c mice.Thus,the shrimp (P.vannamei) and its digestion products have high allergenic potential.Similarly,the allergenic potential is not eliminated during gastric digestion and gastrointestinal digestion.
3.6 Histopathological evaluation of lung,spleen and jejunum after challenge with shrimp proteins or their digestion products
Representative images of spleen,lung and jejunum sections of each injection group are shown in Fig.7.The histopathological studies of spleen revealed megakaryocyte proliferation.Large-sized megakaryocytes were present in the SPS-,GDS-and GIDS-challenged groups,indicating chronic inflammation within immune organs.However,there were significantly fewer inflammatory cells and megakaryocytes in the GDS-and GIDS-challenged groups than those in the SPS-challenged group.Also,saline-and alum-exposed groups retained normal structure of spleen.
Obvious histopathological changes in lung tissues were observed in the SPS-challenged group.The lung in the SPS-challenged group showed marked hyperplasia of bronchial lining epithelial,thickening of alveolar septum,and infiltration of inflammatory cell (black arrows indicate inflammatory cells) (Fig.7).It is of interest to note that although alveolar septa were thickened in the lung tissues of the GDS-and GIDS-challenged groups,no inflammatory cell infiltration occurred.Furthermore,the lung in saline-and alum-exposed groups contained neat alveolar septa and showed no pathological changes.
As shown in Fig.7,significant allergic symptoms were observed in jejunum in the SPS-,GDS-,and GIDS-challenged groups,such as the production of inflammatory cells in jejunum (black arrows indicate inflammatory cells),while the histopathology of saline-and alum-exposed groups was normal,with neatly arranged jejunum villi and intact mucosa.
4. Discussion
P.vannamei,as a crustacean,can cause severe allergic reactions.However,studies on the sensitizing and allergenic potential ofP.vannameiafter digestion are limited.Therefore,we designed animal experiments to explore thein vivoallergic reactions ofP.vannameiand its digestion products in BALB/c mice.In this study,mice were sensitized by intraperitoneal injection of saline,alum,SPS,GDS,and GIDS.The sensitizing and allergenic effect ofP.vannameiand its digestion products were systematically evaluated by the hypersensitivity symptoms,serum specific IgE/IgG1concentrations,vascular permeability,histamine/mMCP-1 concentrations,mast cell degranulation and histopathological sections of the animal models.
During feeding,the food intake and water intake of mice were basically unchanged (Figs.2A-B).However,except for the saline-exposed group,the body weight of mice in other groups decreased in the 4thweek,which may be caused by allergic reaction of mice (Fig.2C).Increased in scratching frequency,puffiness around the eyes,drowsiness,gastrointestinal symptoms,diarrhea and shock are the main clinical signs ofP.vannameiallergy[47].SPS-,GDS-and GIDS-challenged groups showed obvious clinical signs,such as scratching,hair explosion,facial edema and decreased activity.However,as compared with the SPS-challenged group,the clinical signs of mice were significantly improved in the GDS-and GIDS-challenged groups (Fig.2D).Meanwhile,the ear temperature of SPS-,GDS-and GIDS-challenged groups was significantly lower than that of the saline-and alum-exposed groups,which was consistent with changes in hypersensitivity scores (Fig.2E).In this study,digestive processing could relieve allergic symptoms in the mice.However,gastric and gastrointestinal digestion products of shrimp proteins might still cause severe allergic reactions.
An increase in allergen-specific IgE,a key biomarker,is adistinct feature of type 1 hypersensitivity[1].Thus,the severity of food allergy is related to changes in specific IgE concentrations[48].In comparison with the saline-and alum-exposed groups,mice in the SPS-,GDS-and GIDS-challenged groups significantly increased specific IgE concentrations,and gradually significantly reduced with the progress of digestion (Fig.3A).At the same time,similar to IgE,IgG1can also induce systemic anaphylaxis[49].The concentrations of IgG1were found to be parallel to the concentrations of IgE(Fig.3B).Shrimp proteins were digested into small peptides,resulting in lower IgE/IgG1-binding capacity than shrimp proteins.Although shrimp proteins were digested gradually,there were still great deal of complete and and/or newly formed even after gastric and gastrointestinal digestion.These allergens could still cause allergic symptoms in the mice.
Numerous studies have shown that the increase of vascular permeability was caused by the vasoactive mediators in response of IgE-mediated hypersensitivity,which was a mark of systemic allergic reaction[38,43].In our model,bluish coloring of the footpads and small intestine were observed in the SPS-,GDS-and GIDS-challenged groups (Fig.4A).Then,the vascular permeability was quantified by the determination of albumin concentrations in the peritoneal lavage fluid[39].As compared to the SPS-challenged group,the concentrations of albumin significantly decreased in the GDSand GIDS-challenged groups.However,the concentrations of albumin significantly increased in the GDS-and GIDS-challenged groups,as compared to the saline-and alum-exposed groups (Fig.4B).
Mast cell degranulation plays a key role in the allergic reaction induced by immediate allergic food mediated by IgE[42,50].As the key allergic mediator released by mast cells,the increase of histamine concentrations was also a sign of allergic reaction[51].In addition,mMCP-1 was mainly expressed in the mucosal mast cells[52].The degranulation of mast cell leads to the increased concentrations of mMCP-1 and enhances the permeability of intestinal epithelial cells,leading to food allergic disease[53,54].Histamine and mMCP-1 concentrations were significantly increased after SPS,GDS and GIDS injection (Fig.5).These results were consistent with those regarding the production of the above mentioned specific antibodies.These results showed that the digestion products ofP.vannameistill contained allergens.Moreover,these allergens increased the intestinal vascular permeability and the occurrence of food allergic diseases.Histopathological sections of the jejunum showed the rupture of intestinal villi and production of inflammatory cells (Fig.7).These results can support the view that allergenicity ofP.vannameiand its digestion products increased the intestinal permeability,which made antigens enter the systemic circulation quickly and further induced mast cells to release allergic mediators.
Mast cells are widely distributed in the whole body and play a key role in allergic diseases[55].The mast cells in spleen sections were stained with toluidine blue solution and the morphology and degranulation levels of mast cells in main immune organs could be observe.A large number of degranulated mast cells were found in the spleen of SPS-challenged group,and the production of degranulated mast cells was also found in the GDS-and GIDS-challenged groups(Fig.6).On the other hand,the spleen acts as the largest and the most important immune tissue in the human body and antigen stimulation can cause immune responses and phagocytosis of exogenous substances in spleen macrophages and lymphocytes,resulting in the swelling of the spleen[31,34].SPS and its digestion products could significantly induce higher spleen index in the mice and cause the immune tissue swelling (Fig.2F).In addition,the histopathological studies of spleen showed lymphoid hyperplasia.There were a large number of large-sized megakaryocytes in SPS-challenged group,which was due to the proliferation and differentiation of T lymphocytes,indicating chronic inflammation within lymphoid organs.Meanwhile,a small number of megakaryocytes were also produced in the GDS-and GIDS-challenged groups (Fig.7).The histological sections of the lung of SPS-,GDS-and GIDS-challenged groups showed infiltration of inflammatory cells and thickening of alveolar septum,resulting in airway blockade and more severe anaphylactic symptoms in the mice (Fig.7).The above results suggest thatP.vannameiand its digestion products might still cause clinical signs in the BALB/c mice.After gastric and gastrointestinal digestion,the small peptides produced byP.vannameiprotein still have a large number of allergens,and these allergens still have allergenic potential.
In conclusion,we explored the sensitizing and eliciting capacity ofP.vannameiand its gastric and gastrointestinal digestion products in the BALB/c mice model and illustrated the changes of the allergenicity in shrimp proteins during digestion.The shrimp proteins were digested into small peptides by pepsin,trypsin and chymosin and the allergenic ability ofP.vannameiwas altered.The results showed that the gastric and gastrointestinal digestion products groups had lower specific IgE/IgG1levels as compared with the shrimp proteins group.However,the specific IgE/IgG1levels in the digestion products groups was higher than that in the saline and alum groups,indicating that the digestion products made the mice sensitized.At the same time,the digestion products caused lower spleen index,mMCP-1 concentration and the proportion of degranulated mast cells.In addition,the digestion products significantly reduced the frequency of allergic symptoms;notably,the digestion products mitigated the vascular permeability and the histopathological changes in spleen,lung,and jejunum in the mice.Nevertheless,the allergenicity of gastric digestion and gastrointestinal digestion products cannot be completely eliminated and still could elicit severe allergic reaction in the mice.Since shrimp proteins could be hydrolyzed by enzymes,future studies should focus on the relationship between the changes of allergenicity and structure of shrimp (P.vannamei) proteins during digestion,as well as the study of the immunodominant allergen epitopes in the digestion products.
Declaration of competing interests
The authors state no conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (32022067) and the Dalian Sci-Tech Talent Innovation Support Program (2022RY04).
杂志排行
食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing
