Microbial phenolic metabolites 3-(3’,4’-dihydroxyphenyl)propanoic acid and 3’,4’-dihydroxyphenylacetic acid prevent obesity in mice fed high-fat diet
2024-02-16WanbingChnRuonanLiuXiaolingZhuQunLuHongYangRuiLiu
Wanbing Chn,Ruonan Liu,Xiaoling Zhu,Qun Lu,Hong Yang,Rui Liu,*
a College of Food Science and Technology,Huazhong Agricultural University,Wuhan 430000,China
b Key Laboratory of Environment Correlative Dietology (Huazhong Agricultural University),Ministry of education,Wuhan 430000,China
c Wuhan Engineering Research Center of Bee Products on Quality and Safety Control,Wuhan 430000,China
d Key Laboratory of Urban Agriculture in Central China,Ministry of Agriculture and Rural Affairs,Wuhan 430000,China
e Hubei Provincial Institute for Food Supervision and Test,Wuhan 430070,China
Keywords:Obesity Dietary polyphenol metabolites Gut microbiota Metabonomics
ABSTRACT Obesity is associated with numerous metabolic disorders,and dietary polyphenols have been confirmed to have beneficial effects on the metabolism in obesity.However,the effect of 3-(3’,4’-dihydroxyphenyl)propanoic acid (DHPA) and 3’,4’-dihydroxyphenylacetic acid (DHAA),two main metabolites of dietary polyphenols,on obesity remains poorly understood.In this study,DHPA and DHAA were found to alleviate obesity,as well as regulate insulin resistance,lipid metabolism,and oxidative stress response in high-fat diet (HFD)mice.Surprisingly,the 16S rRNA sequencing and UHPLC-Q-TOF/MS demonstrated that DHPA and DHAA only slightly disturbed the intestinal microbiome,but significantly altered the urine metabolome of HFD mice mainly by regulating pentose and glucuronate interconversion,tyrosine metabolism,pentose phosphate and tricarboxylic acid (TCA) cycle as indicated by metabolic pathway analysis based on Kyoto Encyclopedia of Genes and Genomes (KEGG) database.Correlation analysis revealed that the differential metabolites are strongly associated with body weight,blood glucose,insulin level,and superoxide dismutase (SOD) enzyme activity.Our results revealed that DHPA and DHAA exert their anti-obesity effect by regulating important metabolites in the glucose,lipid and tyrosine metabolism pathways.
1. Introduction
Obesity has been rapidly increasing at the global level,posing a major challenge to the health of the public[1].It is known as a major risk factor of cardiovascular diseases and numerous complications,such as type II diabetes,dyslipidemia and hypertension[2].Accumulating evidence has indicated that the development of obesity is closely related to the gut microbiota[3,4].Moreover,a recent study has demonstrated that oxidative stress plays a crucial role in the deleterious effects of obesity,and revealed an imbalance between oxidants and antioxidants in obesity models[5].In addition,obesity is considered as a metabolic disease,and its deterioration will trigger metabolic changes and dysregulation[6],particularly glucose and lipid metabolism.Obese individuals generally have significantly higher intestinal glucose absorption and gluconeogenesis in intestinal stem cells[7].Metabolomic studies of obesity have contributed to the discovery of many biomarkers and better understanding of the disease progression at the metabolic level.These biomarkers include fatty acids,tricarboxylic acid (TCA) cycle intermediates,carbohydrates,amino acids,choline and bile acids[8-10].
According to existing research,dietary polyphenols can ameliorate the obesity induced by high-fat diet (HFD)[11,12].However,only a very small proportion of these ingested dietary polyphenols are directly absorbed in the small intestine or enter into the circulatory system.Hence,these polyphenolic compounds have very limited direct biological activities on tissues and organs[13].Subsequently,it was found that dietary polyphenols are mostly transformed into metabolites by gut microbiota and/or host tissues.These metabolites are transported through the portal vein into the liver and then into the circulatory system to all organs and tissues of the body,which eventually regulate oxidative stress response and other health effects[14].Some studies in animals and humans have shown that 3-(3’,4’-dihydroxyphenyl)propanoic acid (DHPA)and 3’,4’-dihydroxyphenylacetic acid (DHAA) are produced by gut microbiota from dietary polyphenols.These dietary polyphenols include catechin[15],epicatechin[16],epigallocatechin-3-gallate[17],procyanidins (procyanidin dimers: B1,B2,B3,B4,A1;procyanidin trimers: C1) and procyanidin extracts[18],anthocyanin[19],phenolic extract such as blueberry[20],and grape pomace phenolic compounds[18,21].
DHPA and DHAA have been demonstrated to have healthy effects in a cell model of high-glucose condition,which is always associated with diabetes and obesity.These two metabolites can relieve oxidative stress by decreasing the reactive oxygen species (ROS)level,increasing superoxide dismutase (SOD) activity,and altering the expression of the genes encoding Cu/ZnSOD,MnSOD,catalase,glutathione peroxidase (GPx) and heme oxygenase-1 (HO-1)[22].DHPA has the potential of preventing some chronic diseases such as cardiovascular diseases and cancer,because it can reduce the possibility of increase in C-reactive protein,which is known as an inflammatory biomarker[23].
According to the above review,there has been very limitedin vitroresearch on the healthy activity of DHPA and DHAA,and almost no research on how DHPA and DHAA directly exert their impactsin vivo,or whether they are as effective as dietary polyphenols in relieving obesity.In this study,we investigated the effects of DHPA and DHAA on gut microbiota and metabolomic profile to further determine their effects on obesity in mice fed with HFD.Elucidation of thein vivobeneficial effect of DHPA and DHAA as the metabolites of dietary polyphenols may be of great significance for the better utilization of these two compounds.
2. Materials and methods
2.1 Chemicals
Methanol,acetonitrile and formic acid were of chromatographic purity (Merck.Co.Inc.,Darmstadt,Germany).Distilled water was purified using a Milli-Q system (Millipore,Billerica,MA,USA).DHPA and DHAA were purchased from Yuanye Biological Technology Institute (Shanghai,China).The assay kits for the determination of insulin,malondialdehyde (MDA),SOD and glutathione (GSH) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,China).Triglycerides (TG)and total cholesterol (TC) were purchased from Dongou Diagnose Institute (Zhejiang,China).
2.2 Animals and experimental design
Eight-week-old male C57BL mice were provided by the Experimental Animal Center of Huazhong Agricultural University(Wuhan,China) and subsequently housed under a specific pathogen free (SPF) environment in a light/dark cycle of 12 h/12 h,with 4 mice in a cage.All mice received tap water and a standard rodent chow during a one-week acclimatization period.
After 2 weeks of adaptation period,the mice were randomly divided into 4 groups (n=8 per group),and then started to accept different experimental feeds prepared by Xietong Pharmaceutical Bio-engineering Co.Ltd.(Nanjing,China).In the normal diet group (N group),the mice were allowed free access to the purified rodent chow diet (10% calories from fat,Product No.XTCON50J).In the HFD model group (M group),the mice were fed only with a purified HFD(60% calories from fat,Product No.XTHF60).In the DHPA group,the mice were fed with purified HFD supplemented with DHPA(0.5 g/kg in feed).In the DHAA group,the mice were fed with HFD supplemented with DHPA (0.5 g/kg in feed).
During the 16-week experiment,the food intake and body weights of mice were measured weekly.The animal experiments were approved by the Animal Care and Use Committee of the Huazhong Agricultural University (SCXK 2017-0012) (Wuhan,China).
2.3 Oral glucose tolerance test (OGTT) and insulin resistance
OGTT was implemented two days before euthanasia on mice after a 12 h fast.The blood glucose concentration of each mouse was measured via the tail snip method after oral administration of glucose at 2 g/kg body weight,and the test was conducted at 0,30,60,90 and 120 min using the Roche Blood Glucose Monitoring System (Roche,Switzerland).
The blood samples were centrifuged at 3 000 ×gfor 10 min,and then the serum was portioned in 200 μL tubes,and used for the test of insulin with commercial kits.The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the formula below.
2.4 Sample collection and determination of biochemical indicators
At the end of the experiment,fresh feces of mice were collected and rapidly placed in a liquid nitrogen tank,and then store at −80 °C for microbiome analysis.Then,the mice were placed in a metabolic cage with only access to distilled water,and urine samples were collected within 24 h and stored at −80 °C for metabolic analysis.
After obtaining feces and urine samples,all mice were fasted but allowed with free access to distilled water for 12 h,and then blood samples were collected from mice anesthetized with diethyl ether,and the liver and the epididymal adipose tissues were respectively sampled.
2.5 Biochemical assay
Adipose tissues were fixed in 4% formalin for 24 h,and then treated with paraformaldehyde and processed to be embedded in paraffin for sectioning.hematoxylin and eosin (H&E) solution was used to stain the paraffin sections,followed by observation under an optical microscopy.
Liver tissues from mice were fixed in 10% buffered formaldehyde,processed for histological examination by conventional methods,and stained with H&E.Finally,observation and photography were performed under a 200× optical microscope.
Half of the liver tissues were weighed and homogenized in 9-fold(m/V) cold physiological saline,and then centrifuged at 12 000 ×g.The liver homogenate supernatant was collected and used for liver antioxidant analysis.The other half liver tissues were homogenized in anhydrous ethanol,and then centrifuged at 12 000 ×g.The liver homogenate supernatants were collected and used for liver lipid profiling.SOD,GSH,TG,TC and MDA in the liver homogenate supernatants were measured with commercial kits according to the instructions.
2.6 Urine metabolomic analysis by UHPLC-Q-TOF/MS
Urine samples were thawed,adjusted the pH by the addition of 25 μL 1 mol/L HCL,then vortexed,diluted in water (1:4,V/V)and centrifuged at 12 000 ×gfor 10 min.Finally,the urine sample supernatants were filtered (0.22 μm nylon filter) before analysis.To monitor the stability of the ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry(UHPLC-Q-TOF/MS) system,the quality control (QC) sample was analyzed for every 10 test samples.The QC sample was prepared by mixing equal volumes (10 μL) of each sample.
The analysis was performed using a TripleTOF 5600+System(AB SCIEX,Concord,Ontario,Canada).The liquid chromatographic system was Thermo Scientific UltiMate 3000 UHPLC.The column employed was a Thermo Hypersil GOLD C18(100 mm × 2.1 mm,1.9μm).The mobile phases were 0.13% formic acid in water (A) and acetonitrile (B).The gradient program was as follows: 0 min,95% A,5% B;3 min,95% A,5% B;20 min,70% A,30% B;25 min,40% A,60% B;and 26 min,95% A,5% B;with the flow rate of 0.3 mL/min and the injection volume of 2 μL.The mass spectrometer (MS)was operated in an electrospray ionisation mode (ESI−).Data were acquired under the conditions of an ion spray voltage of −4 500 mV,declustering potential of −60 V,and temperature of 550 °C with N2as a curtain (35 arbitrary units) and nebulizer (55 arbitrary units) gas.One TOF/MS survey scan (250 ms) and eight MS/MS scans (70 ms)were performed.MS was performed in the negative ionization mode with a mass-to-charge ratio (m/z) of 100–1 500[24].The data were processed using Progenesis QI software (Waters,Milford,MA,USA).
2.7 DHPA or DHAA and the metabolites in cecal contents
Mouse cecal contents were weighed and added to ultrapure water at a mass/volume ratio of 100 mg/1 mL,vortexed at high speed for 30 min,and then centrifuged at 16 000 ×gfor 10 min at 4 °C.The supernatant (500 μL) was collected,mixed with 1 500 μL acetonitrile(containing 2% formic acid),and filtered with a 0.22 μm filter.The method of UHPLC-Q-TOF/MS was as described in section 2.6.
2.8 Gut microbiota analysis
Microbial DNA was extracted from faeces using the Fast DNA SPIN extraction kit (MP Biomedicals,Santa Ana,CA,USA).The V3-V4 region of bacterial 16S rRNA gene was subjected to PCR amplification using the forward primer 5’-ACTCCTACGGGAGGCAGCAG-3’ and the reverse primer 5’-GGACTACHVGGGTWTCTAAT-3’.General primers (341F,805R) were used to amplify the V3-V4 hypervariable regions of the bacterial 16S rDNA gene for an Illumina MiSeq platform (Illumina,San Diego,CA,USA)[25].
The sequence data were clustered into operational taxonomic units (OTUs) defined at 97% similarity.These OTUs were applied for analysis of diversity and richness.Taxonomic assignment of OTUs reaching the 97% similarity level was carried out using the QIIME (quantitative insights into microbial ecology) package through comparison with the SILVA,Greengene and RDP databases[26].All analyses were performed on the online platform of Majorbio Institute.
2.9 Statistical analysis
Data were expressed as the mean ± standard error (SEM) of eight mice in each group.Statistical significance was tested by oneway analysis of variation SPSS 16.0 (SPSS,Chicago,IL,USA).The differences between means were compared using the Duncan’s test for multiple comparisons,and the significance was set atP<0.05.
3. Results
3.1 Body weight,glucose profile and liver lipid
At the end of treatments,the mean body weights of DHPA,DHAA,M and N groups were 27.64,28.40,32.87 and 26.68 g,respectively.The DHPA and DHAA groups showed lower body weights than the M group (P<0.05) (Fig.1C).Moreover,the daily food intake and calorie intake of mice in DHPA and DHAA groups were similar to those in the M group (Figs.1A,B).Obviously,both of the two compounds had a weight-sparing effect.
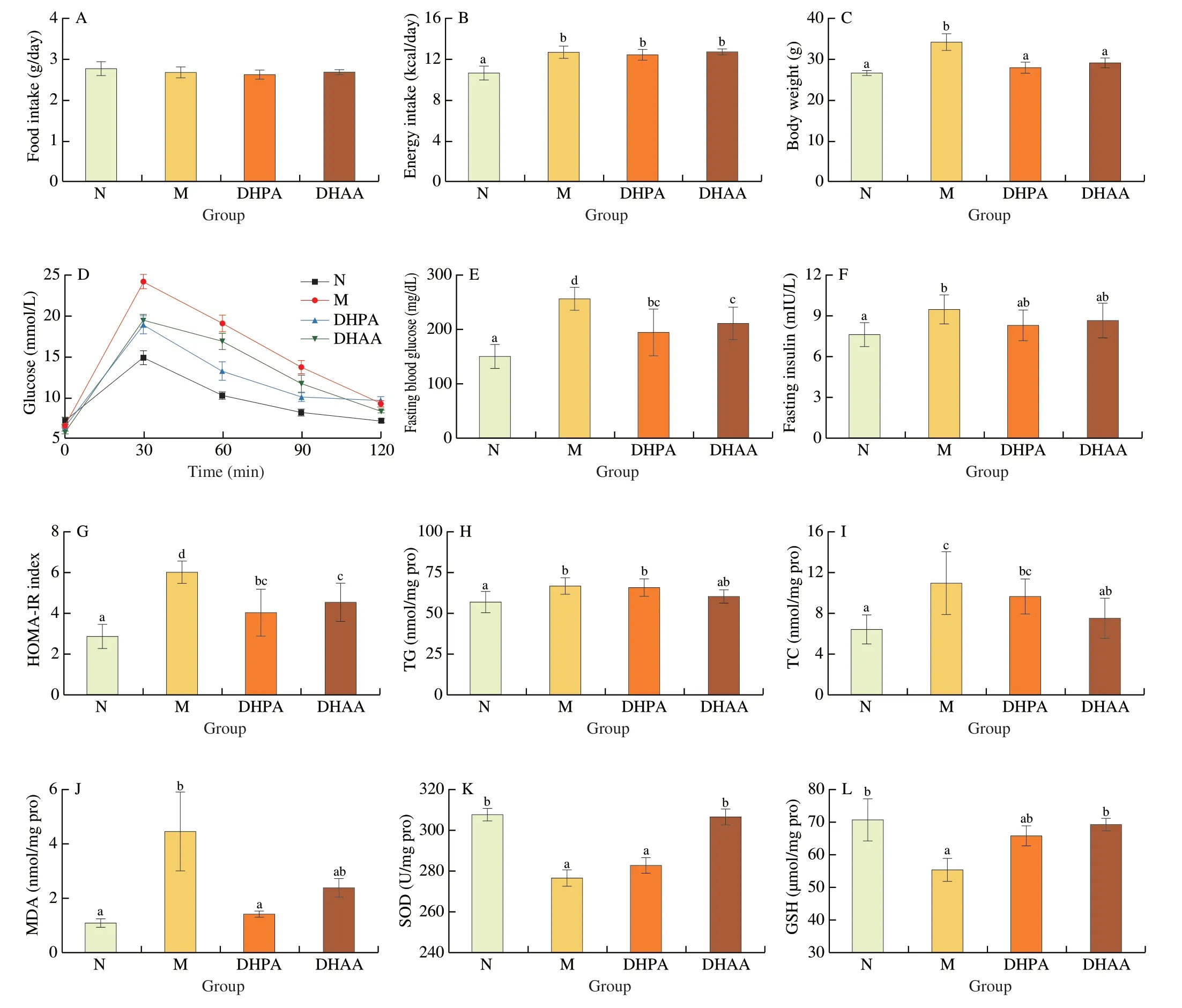
Fig.1 Effects of DHPA and DHAA on glucose metabolism,lipid metabolism and oxidative stress response.(A) Food intake;(B) Energy intake;(C) Body weights;(D) OGTT;(E) Fasting blood glucose;(F) Fasting insulin in serum;(G) HOMA-IR level;(H) TG content in hepatic tissue;(I) TC content in hepatic tissue;(J) MDA content in hepatic tissue;(K) SOD activity in hepatic tissue;(L) GSH content in hepatic tissue.Data are presented as mean ± standard error (SEM),and different letters mean significant differences between the groups (n=8,one-way ANOVA followed by Duncan’s test,P <0.05).The same below.
In the OGTT,the M group showed lower glucose tolerance compared with the N group as indicated by the highest glucose concentration (24.22 mmol/L) (Fig.1D).Both DHPA and DHAA could reduce the blood glucose level by nearly 20% at each time point after glucose administration compared with the M group (Fig.1D).Mice in the M group developed fasting hyperglycemia (Fig.1E) and hyperinsulinemia (Fig.1F),while DHPA and DHAA intervention significantly reduced fasting blood glucose (Fig.1E) and HOMA-IR(Fig.1G) (P<0.05).The DHPA and DHAA groups exhibited lower levels of fasting insulin than the M group,but the differences were not significant (P=0.18 for DHPA,andP=0.51 for DHAA relative to the M group).In addition,the DHPA and DHAA groups showed significantly lower TC content in the liver than the M group (P<0.05)(Fig.1I),but exhibited no significant difference in TG level (Fig.1H).
3.2 Evaluation of oxidative stress in liver
Relative to those in the N group,the mice in the M group showed an increase in MDA level and a decrease in SOD enzyme activity and GSH level,indicating the development of oxidative stress in their liver (Figs.1J-L).In addition,the DHPA group had a significantly lower level of MDA (Fig.1J),and the DHAA group exhibited higher SOD activity than the M group (P<0.05) (Fig.1K).However,the levels of GSH in the DHPA and DHAA groups were not significantly different from those in the M group (Fig.1L).
3.3 Histological analysis of hepatic and epididymal adipose tissues
The M group exhibited the development of hepatic steatosis,which was featured by microvesicular fat accumulation in the liver and the presence of a large number of fat vacuoles.Intervention with DHPA and DHAA visibly alleviated these histological alterations and even led to the disappearance of fat vacuoles (Fig.2A).Histological and quantitative analysis of adipose tissue slices revealed that the size of epididymal adipocyte in the DHAA and DHPA groups was similar to that in the N group and significantly smaller than that in the M group (P<0.05) (Figs.2B,C).

Fig.2 Histological changes after 16 weeks of treatment.(A) Liver histology;(B) Epididymal adipose tissue.H&E stain,200× magnification for liver and 100×magnification for adipocyte.(C) Average adipocyte size of epididymis adipose.Three fields were randomly selected for each slice to analyze the size of adipocytes by using Image J software.Results were obtained from 8 mice in each experimental group and expressed as mean ± standard error (SEM).
3.4 Faecal microbiology
Principal coordinate analysis (PCoA) showed that the M group was clearly separated from the N group.The faecal microbiota in DHPA and DHAA groups appeared to be clustered together,and was close to those in the M group (Fig.3A),indicating that DHPA or DHAA could not improve faecal microbiota.
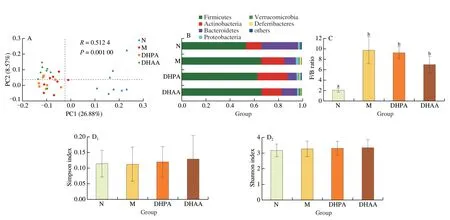
Fig.3 Effects of DHPA and DHAA on fecal microbiome.(A) Principal co-ordinates analysis of unweighted-unifrac distances at the OUT level.(B) Taxonomic composition analysis at the phylum level.(C) Ratio of Firmicutes/Bacteroidetes (F/B).(D) α-Diversity analysis at the OTU level.(E) Top 50 taxonomic composition analysis at the genus level.
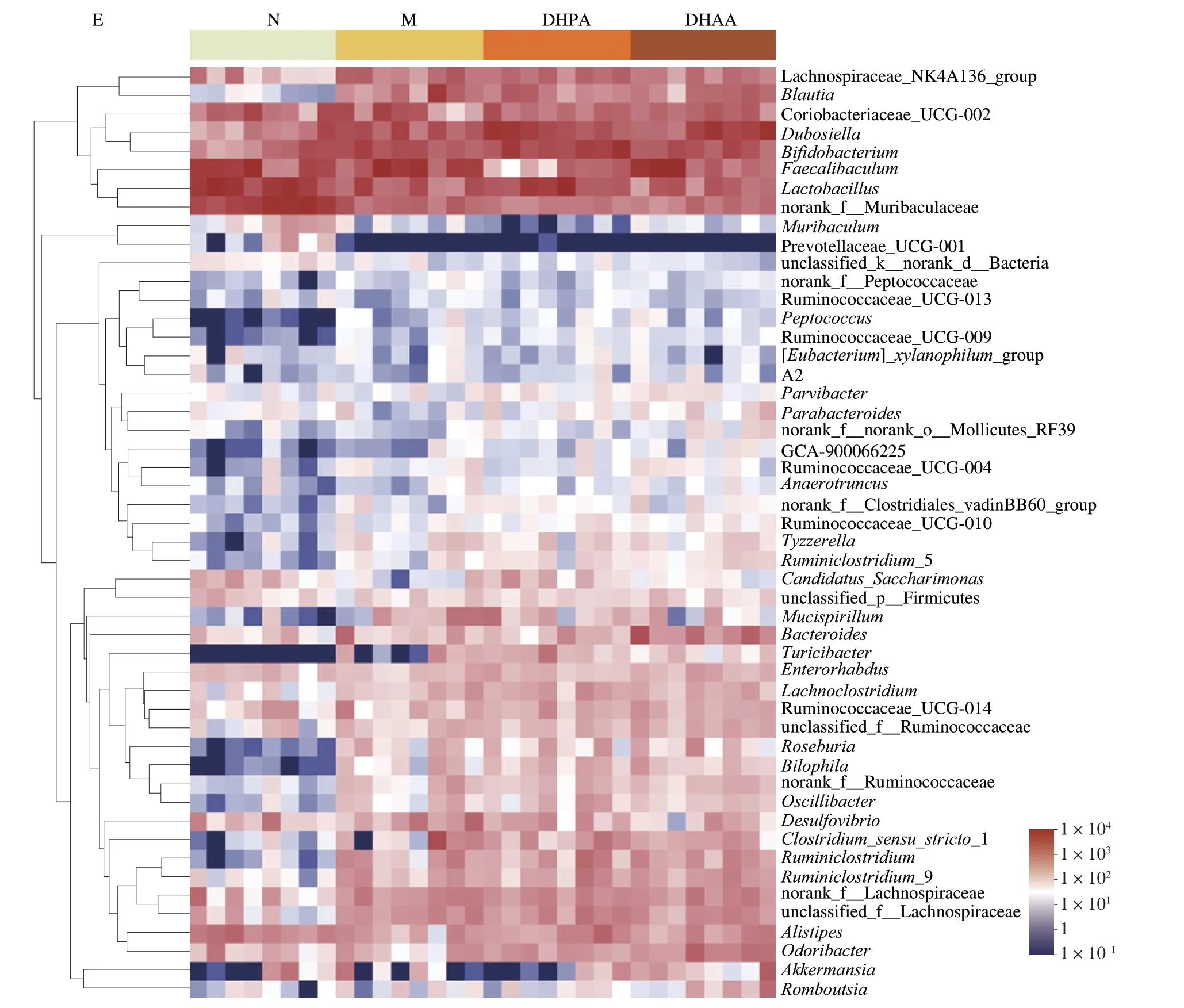
Fig.3 (Continued)
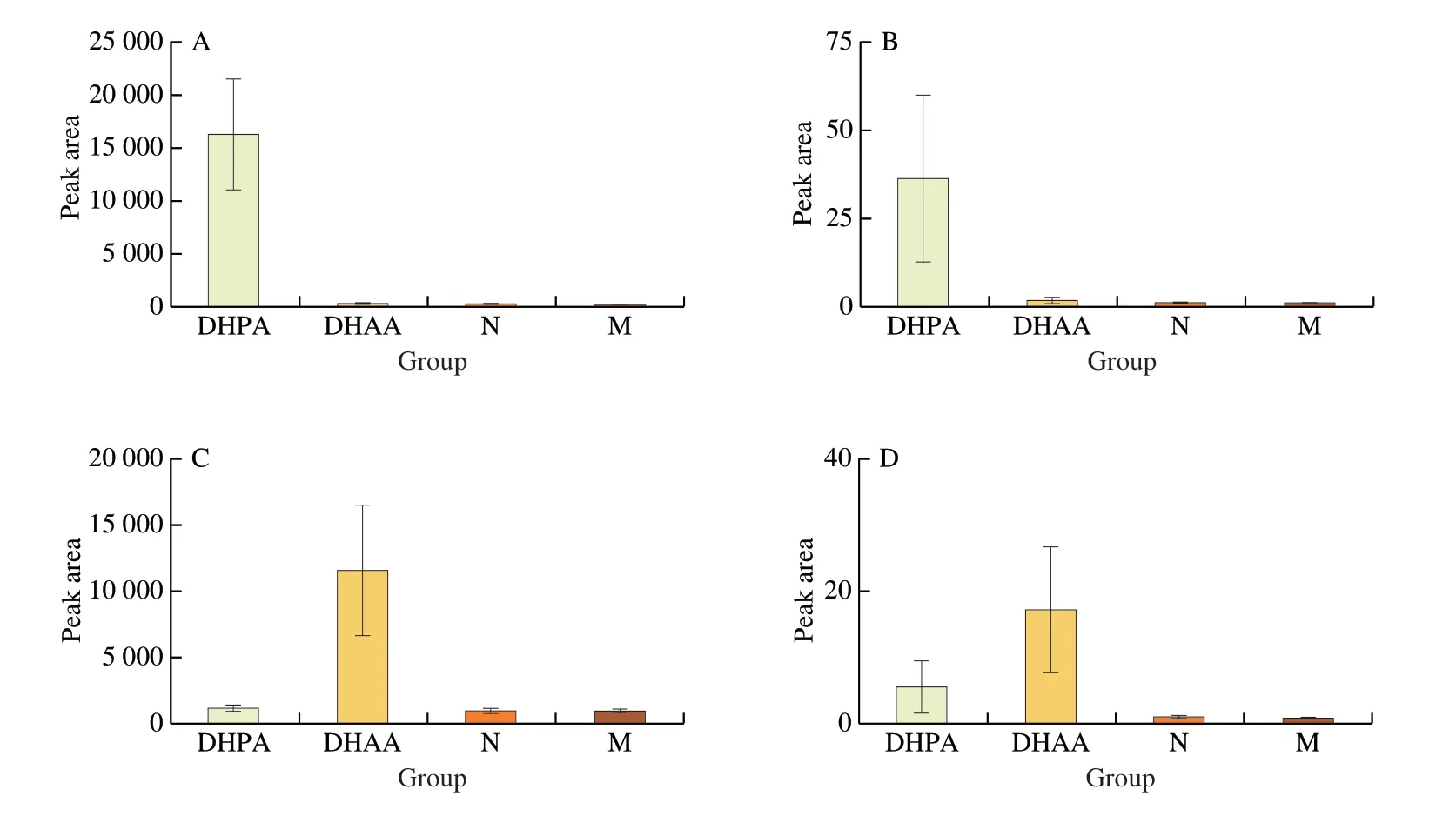
Fig.4 Levels of sulfated DHPA and DHAA in urine and cecal contents.(A) DHPA-sulfate in urine;(B) DHPA-sulfate in cecal contents;(C) DHAA-sulfate in urine;(D) DHAA-sulfate in cecal contents.
To determine whether structural changes occurred within the intestinal microbial environment,we compared the relative proportions of predominant taxa identified in the four groups.The results demonstrated that there were six phyla in the gut microbiota of N group,with Firmicutes being the most dominant phylum,followed by Bacteroidetes;while in the M group,the gut microbiota comprised seven phyla.It should be noted that the relative abundance of Firmicutes and Actinobacteria in the M group was significantly higher than that in the N group,while that of Bacteroidetes was significantly lower.However,after DHPA and DHAA treatments,the two groups showed no significant difference in microbial composition or proportion from the M group(Fig.3B).The Firmicutes/Bacteroidetes (F/B) ratio was considered as an important index to evaluate the risk of diabetes and obesity in previous studies[27,28].Thus,we calculated the F/B ratio of all groups.Consistent with the results of PcoA,the F/B ratio of the M group was higher than that of the N group,but showed no remarkable difference from that of the DHPA and DHAA groups(Fig.3C).HFD had a weak effect on the Simpson index and Shannon index,which can reflect the diversity of intestinal microbiota,while intake of DHPA and DHAA also led to no change in these indices (Fig.3D).
Heatmap analysis of the gut microbiota at the genus level revealed that the M group had a distinct microbial profile relative to the N group.HFD mainly induced increases inRoseburia,Bilophila,OscillibacterandRuminiclostriduim.However,DHPA and DHAA treatment for 16 weeks did not result in much change in the microbial profile,and only DHPA treatment slightly altered the abundance of Akkermansia (Fig.3E).
We further analyzed the level of DHPA (or DHAA) and its metabolites in mouse urine and cecal contents by UHPLC-Q-TOF/MS.It was found that the urine contained higher levels of sulfated metabolites without the prototype DHPA or DHAA (Figs.4A,C).Neither the prototype nor the sulfated metabolites of DHPA or DHAA were found in the cecal contents (Figs.4B,D).Therefore,it can be speculated that DHPA or DHAA may be almost completely absorbed in the small intestine,and thus have a minor impact on the gut microbiota.
3.5 Urine metabolomics
The metabolomic profiles of mouse urine were analyzed using UHPLC-Q-TOF/MS and the differential metabolites between those groups were screened by metabonomics.Fig.5A shows the PCA results of the four groups.The M group and N group were completely separated from each other,and the urine metabolic pattern of both DHPA and DHAA groups was significantly different from that of the M group.Besides,the urine metabolomic profiles of the DHPA and DHAA treatment groups were largely overlapped,indicating that both compounds could alter the metabolism of HFD-fed mice,and might influence the metabolic process through similar mechanisms.The heatmap in Fig.5C presents the differential metabolites among the four groups,and the results are consistent with the PCA results.These results confirmed that DHPA and DHAA could alter the metabolism of HFD-fed mice,and restore it to nearly the level of the N group.A total of 54 differential metabolites were identified,and the potential biomarkers were imported into the MetaboAnalyst database for the analysis of pathways (Fig.5B).The results indicated that DHPA and DHAA alleviated obesity mainly through four pathways,including pentose and glucuronate interconversion,tyrosine metabolism,pentose phosphate and TCA cycle.In addition,the DHPA and DHAA groups also had their unique metabolites.The intake of DHPA induced the production of homovanillic acid sulfate,4-HPAA-sulfate,DHPA-sulfate,methyl-DHPA-sulfate,4-acetybutyrate,and 4-HPAA sulfate;and that of DHAA stimulated the production of DHAA sulfate,caftaric acid,and 3-HPAA sulfate.
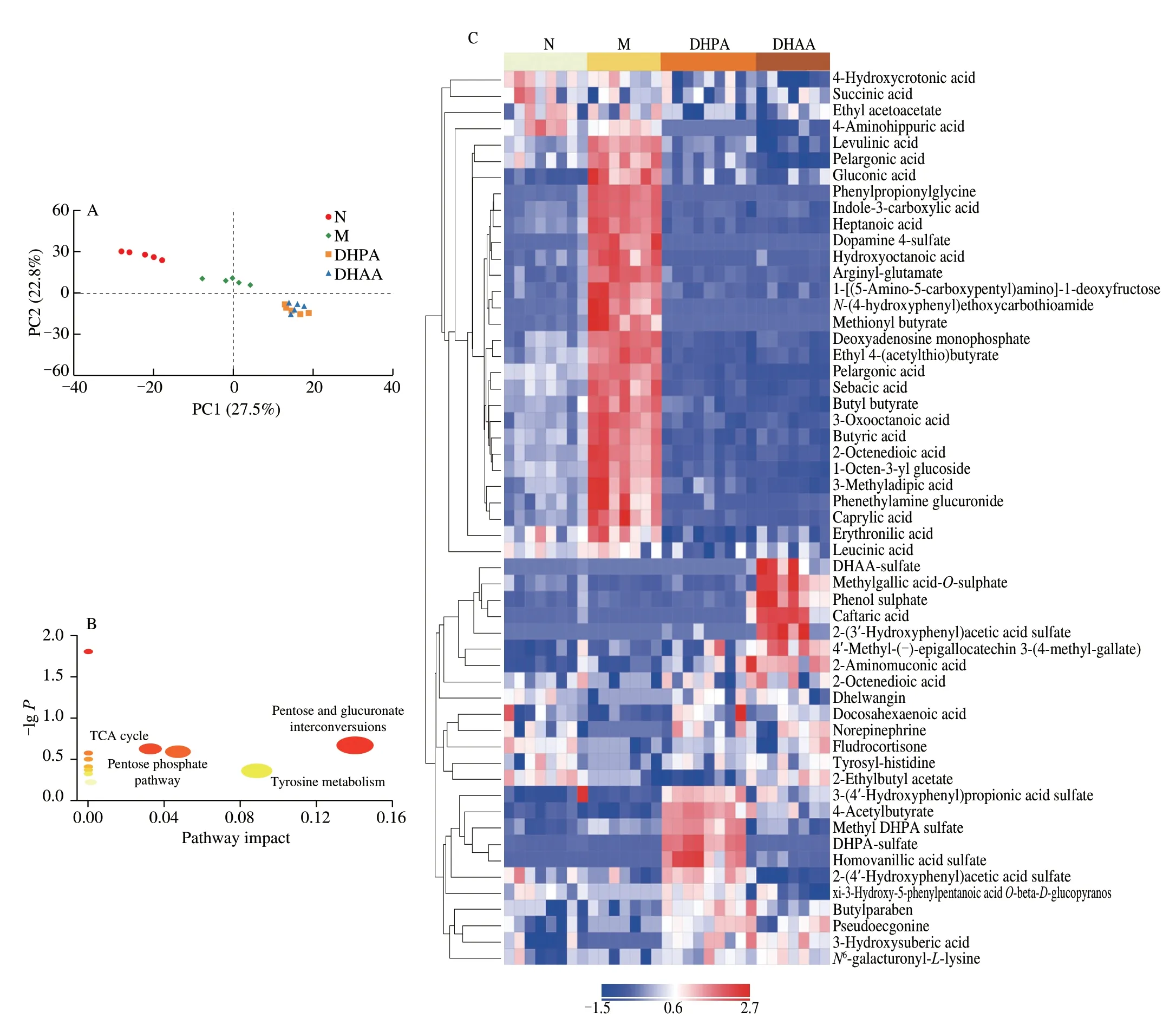
Fig.5 Effects of DHPA and DHAA on urine metabolite profiles.(A) Score plots of principle component analysis (PCA).(B) Bubble map of KEGG pathway analysis.(C) Heatmap of differential metabolites screened by orthogonal projection in latent structure-discriminant analysis (OPLS-DA).
3.6 Comprehensive omics analysis
A correlation analysis was performed between intestinal microbiome and urine metabolome in mice.Bacteroides,Odoribacter,andMuribaculumhad strong correlations with the differential metabolites in urine (Fig.6).Fatty acids and their metabolites in urine were strongly correlated with different bacterial taxa,such as pelargonic acid,caprylic acid,heptanonic acid,levulinic acid,sebacic acid,and 4-hydroxycrotonic acid (Fig.6).However,consistent with the result that intestinal microbiota was little affected by DHPA and DHAA,the metabolites of DHPA and DHAA also exhibited weak correlations with intestinal microbiota.
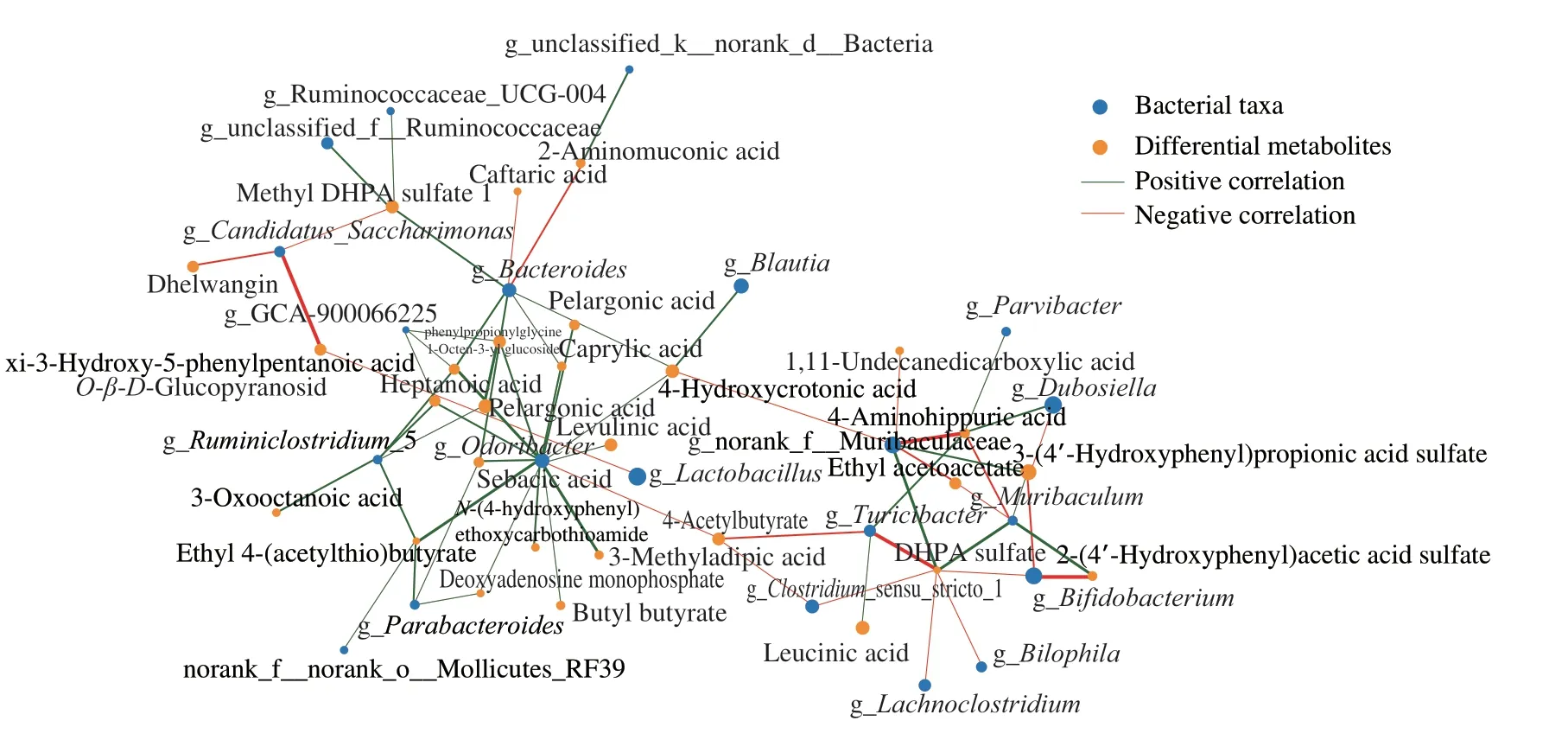
Fig.6 Spearman’s correlation network between differential metabolites in urine and bacterial taxa at the genus level.The size of nodes indicates the abundance of species or metabolites.The red and green color of lines represent positive and negative correlation (R >0.5,P <0.05),respectively.The thickness of the line indicates the correlation coefficient.The thicker the line,the higher the correlation.
A correlation analysis was performed between the metabolites in urine and the metabolic phenotype,including the body weight,area under the curve (AUC) of OGTT,fasting insulin,TG,TC,MDA,GSH and SOD in the liver (Fig.7).The results demonstrated that the changes in urine metabolome had strong correlations with the body weight and SOD level.Succinic acid,4-hydroxycrotonic acid,fludrocortisone,and 2-ethylbutyl acetate were negatively correlated with the levels of OGTT and fasting insulin in plasma.Fludrocortisone and 2-ethylbutyl acetate showed negative correlations with the level of TC in the liver.4-Hydroxycrotonic acid was negatively correlated with the MDA level in the liver.Fludrocortisone and 2-ethylbutyl acetate were positively correlated with SOD activity.Methionyl butyrate had positive correlations with body weight and fasting insulin level.DHPA and DHAA may exert their fat-reducing effect by affecting these metabolites.
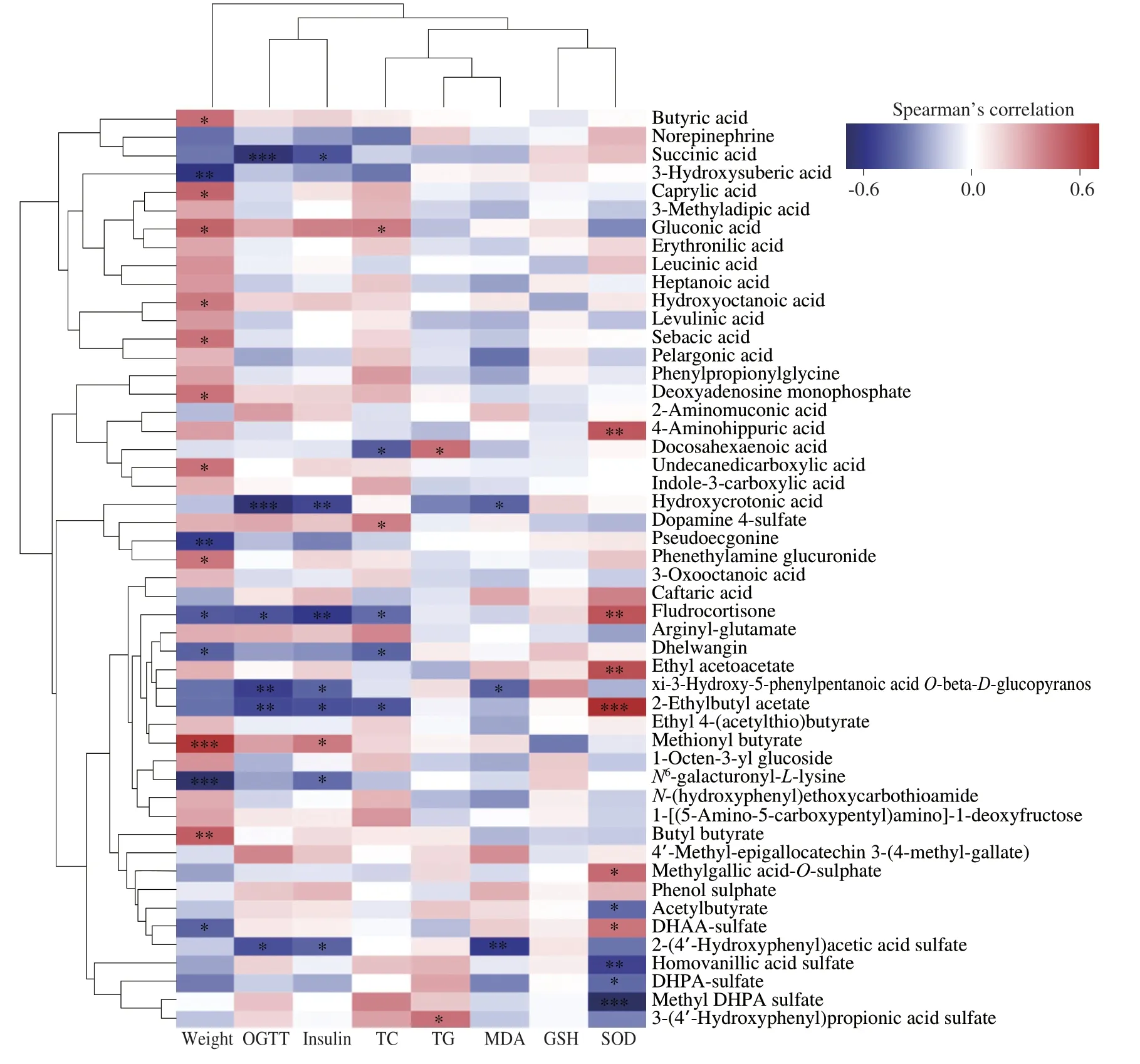
Fig.7 Heatmap of the Spearman’s correlation coefficients between bacterial taxa and metabolic parameters.*P <0.05,**P <0.01,***P <0.001.OGTT,oral glucose tolerance test;TC,total cholesterol;TG,triglyceride;MDA,malondialdehyde;GSH,glutathione;SOD,superoxide dismutase.
4. Discussion
DHPA and DHAA are common degradation products of dietary polyphenols by intestinal microbiota,and have been found in the metabolic process of flavan-3-ols,flavonols,flavanones,and anthocyanins[29-31].These two compounds may be highly active metabolites of dietary polyphenols and play an important role in their health promoting effect.The physiological functions of phenylpropionic acid metabolites (including DHPA) and phenylacetic acid metabolites (including DHAA) have attracted increasing attention from researchers at the cellular level.
Long-term HFD can cause overweight and metabolic syndromes,which are mainly characterized by abnormal glucose metabolism and dyslipidemia.Elevated fasting blood glucose and insulin resistance levels are two typical clinical symptoms of abnormal glucose metabolism[32].Dyslipidemia mainly causes lipid peroxidation,lipid accumulation,and oxidative stress[5,33].Somein vitrostudies have shown that phenylpropanoic acids (PPAs) can improve glucose and lipid metabolism viaregulating peroxisome proliferator-activated receptors (PPARs),a group of nuclear regulatory factors[34].PPARs are agonists of free fatty acid 4 receptor (FFA4),which can stimulate insulin secretion and control the blood glucose level[35].Previous studies have demonstrated the critical role of PPAs and phenylacetic acids (PAAs) in regulating lipid metabolism,and 4-HPPA and 4-HPAA can effectively limit the formation of foam cells[36,37].The conversion of macrophages into foam cells is mainly related to abnormal lipid metabolism in the cells.
It has been revealed that the oxidative profiles including the ROS,SOD and MDA level,which are key factors maintaining the whole health,are closely associated with the levels of glucose and insulin[38,39].Moreover,oxidative stress can result from lipotoxicity,which is characterized by the accumulation of lipid intermediates[40].Severalin vitroexperiments have demonstrated the ability of PPAs and PAAs to alleviate obesity and balance oxidative stress.Under high glucose conditions,DHAA can prevent the imbalance in redox status in renal tubular cells by suppressing ROS generation and promoting the GSH level as well as the SOD and CAT activity[41].Although further studies are still needed to confirm the role of DHPA and DHAA in alleviating the symptoms and disease process of obesity and metabolic syndrome,the existing evidence suggests that their antioxidant effect may be related to the mitigation of TG accumulation in HepG2 hepatocytes[42].
Taking gut microbiome into consideration,HFD indeed resulted in the disorder of intestinal microbiota,including a decrease in the proportion of beneficialBifidobacteriumand an increase inOscillospiraand Odoribacteraceae associated with disease or obesity[43].Surprisingly,although oral administration of DHAA or DHPA could alleviate the obesity of mice fed with HFD,it had no obvious effect on the intestinal microbiome.Combining with the result that the two compounds were not found in cecal contents,we speculate that DHPA and DHAA may be directly absorbed in the small intestine before arriving in the colon,which is exactly the reason why DHPA and DHAA did not lead to obvious changes in the intestinal microbiome.There has been increasing evidence indicating that the phenolic acid of polyphenol metabolites can be absorbed in the small intestine and liver.Monocarboxylic acid transporter of Caco-2 cell can mediate the transport of phenolic acids,including benzoic acid and 3-HPPA[44].In another study,when the polyphenol metabolites were inoculated with the Caco-2/HepG2 co-culture,approximately 3%–5% of DHPA was observed in the basolateral side.Subsequently,HepG2 cellular metabolism led to obvious increases in DHPA and DHAA by 160%–370%[45].Furthermore,a measurement of the plasma content of DHPA and DHAA also confirmed that they can be absorbed by small intestinal epithelial cells[46].These findings highlight the significant absorption of phenolic acid by the small intestine and liver rather than accumulation in the colon.
In this work,DHPA and DHAA treatment for 16 weeks showed particularly noticeable effects on metabolism,and a total of 54 most significant differential metabolites were identified.Obvious changes in metabolites associated with obesity,such as norepinephrine,butyric,ethyl-4-(acetylthio)-butyrate,ethyl 4-(acetylthio)-butyrate and xi-3-hydroxy-5-phenylpentanoic acidO-β-D-glucopyranoside,were observed in the M group.These differential metabolites are mainly involved in four key metabolic pathways,including pentose and glucuronate interconversion,tyrosine metabolism,pentose phosphate and TCA cycle.Many previous studies have demonstrated that the TCA cycle is disrupted in obese,diabetic or alcoholic liver injury models of mice[10,47-49].Pentose and glucuronate interconversion and pentose phosphate and TCA cycle are interrelated pathways.DHPA and DHAA undergo phase-II metabolism to produce monoglucuronide conjugatesin vivo[29,50,51].Therefore,we speculate that the glucuronidation of DHPA and DHAA directly affect the interconversion of pentose and glucuronate,and then the pentose will enter the TCA cycle.These two metabolic pathways play important roles in regulating obesity,particularly in regulating oxidative stress,glucose metabolism,and lipid metabolism[52-54].The TCA cycle plays a central role in the energy balance and biosynthesis of macromolecules through a series of biochemical reactions[52-55].
Interestingly,the tyrosine metabolic pathway was also altered in mice ingesting DHPA and DHAA,possibly due to the significantly up-regulated levels of sulfated 4-hydroxyphenylpropionic acid or 4-hydroxyphenylacetic acid in the DHPA and DHAA groups compared with other groups.These results indicate that DHPA and DHAA can be converted into 4-hydroxyphenylpropionic acid or 4-hydroxyphenylacetic acidin vivo,which is also consistent with previous studies[13].DHPA and DHAA are also metabolites of phenylalanine and tyrosine[56].Therefore,the intake of DHPA and DHAA may affect the metabolism and absorption of tyrosine.Tyrosine is the precursor of the neurotransmitter dopamine,and tyrosine and its metabolites are important signaling molecules in the brain-gut axis,playing important roles in inflammatory mechanisms,immune responses,and neurobiological functions[57-59].Phenylalanine hydroxylase can be catalyzed and oxidized to tyrosine,which then participates in the synthesis of neurotransmitters and hormones to interfere with glucose and lipid metabolism[60].Another study revealed that there is a negative correlation between the intake of tyrosine and the TC/high-density lipoprotein cholesterol (HDL-c)ratio[61].In addition,there is also a close relationship between tyrosine and other aromatic amino acids (tryptophan and phenylalanine).The aromatic amino acids can affect gastrointestinal motility and intestinal hormone secretion,enhance intestinal epithelial barrier function,and thus play a role in the development of intestinal diseases and metabolic syndrome[62,63].
The limitation of this study lies in the lack of evaluation of the pathways related to energy metabolism at the gene and protein expression levels.Changes in tyrosine metabolism,pentose phosphate,and TCA cycles were observed in metabolomics,suggesting that DHPA and DHAA may alter energy metabolism in mice fed with HFD.PGC1-αis a transcriptional co-activator that regulates the expression of genes related to energy metabolism[64].Activation of PPAR-αinduces fibroblast-to-adipocyte differentiation and promotes the differentiation of white fat to brown fat[65].In addition,PGC1-αalso controls mitochondrial biosynthesis,and jointly regulates the expression of mitochondrial fatty acidβ-oxidase with PPAR-α[65].Moreover,PGC1-αcan significantly increase PPAR-γ,which can coordinately activate the expression of uncoupling protein 1 (UCP1)and promote the non-shivering thermogenesis of brown fat[66].In future studies,we can further monitor the basal metabolic rate of mice and evaluate the expression of thermogenesis-related genes in brown fat (BAT) at the scapula of mice,including theUcp1gene,Pgc1αgene,andPpar-αandPpar-γgenes.
5. Conclusion
In conclusion,DHPA or DHAA treatment can alleviate the weight gain,unbalance of oxidative stress and disorder of glucose and lipid metabolism induced by HFD.Although dietary supplementation of DHPA or DHAA did not reverse the disorder of gut microbial community structure caused by HFD,it led to dramatic changes in metabolism.Metabonomics analysis revealed that tyrosine metabolism and the interconversion of pentose and glucuronate might be important pathways for DHPA and DHAA to alleviate HFDinduced metabolic disorders.
Declaration of interest statement
All authors declare that no conflict of interest exists.
Acknowledgments
This investigation was supported by the project of the National Natural Science Foundation of China (32272331),and the project of Fundamental Research Funds for the Central Universities(2662019PY034).
杂志排行
食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
