The extraction of effective components and an antioxidant activity study of Tulipa edulis
2024-02-16DoudouZhangDongXiaoTingtingYinShuangzhiZhaoOlenaZhurXunXiaoHailunHeLeileiChen
Doudou Zhang,Dong Xiao,Tingting Yin,Shuangzhi Zhao,Olena Zhur,Xun Xiao,Hailun He,*,Leilei Chen,*
a Beijing Advanced Innovation Center for Food Nutrition and Human Health,Beijing Technology and Business University,Beijing 102488,China
b School of Life Sciences,Central South University,Changsha 410013,China
c State Key Laboratory of Coal Resources and Safe Mining,University of Mining and Technology,Xuzhou 221116,China
d Institute of Agro-Food Science and Technology,Shandong Academy of Agricultural Sciences,Jinan 250100,China
Keywords:Tulipa edulis O xidative stress Purification Antioxidant
ABSTRACT Plant extracts from natural sources are an excellent choice for food additives and natural antioxidants. In this study,the active components of Tulipa edulis were extracted and analysed,and their antioxidant capacity was measured.Then,the crude extract mixture was separated and purified using a Sephadex LH-20 gel,and the antioxidant activity of the purified products was determined.Human umbilical vein endothelial human umbilical vein endothelial cells (HUVEC) cells were treated with 35 mmol/L glucose to construct a model of oxidative stress.Then,the cells were treated with the active component to observe whether the products of T.edulis could have a good protective effect on HUVEC cells induced by glucose.Transcriptome analysis was also performed on HUVEC cells after same treatment to explore the possible mechanism of the component F2 protecting HUVEC cells from oxidative stress induced by high glucose.The results showed that component F2 obtained from T.edulis has strong antioxidant activity.Moreover,F2 can play a strong antioxidant protective role in HUVEC cells.Meanwhile,the gene expression of heme oxygenase 1 (HO-1),γ-glutamyl cysteine ligase catalytic subunit (GCLC) and NAD(P)H quinone oxidoreductase-1 (NQO1) in HUVEC cells was up-regulated after treated with F2.This study provides reference value for the further development and application of T.edulis and the d evelopment of functional food.
1. Introduction
Tulipaedulisis a plant of theTulipgenus in Liliaceae,also known as Yingchun grass,that sprouts and flowers in the early spring.T.edulisis a perennial herbaceous plant that is mainly distributed in Shandong,Anhui,Jiangsu and Zhejiang in China[1].Plant extracts contain abundant plant chemicals,with many reports of substances including phenanthrene compounds[2],simple aromatic compounds[3]and their glycosides,sugar and glycoside compounds[4]and flavonoids[5]and so on.In addition,there is a more active component in plant resources: polysaccharides,which are natural non-toxic materials with biological activity that are found in food,biomedicine,cosmetics and other fields and have great application values[6-8].The extracts also contain alkaloids,flavonoids,polyphenols and other bioactive substances in addition to polysaccharides that have a variety of beneficial effects.It has been reported that monomer or complex components in the extract ofT.edulishave significant antihypertensive,lipid-lowering and antitumour effects[6-9].
In the process of life activities,the body will produce free radicals,when the excess of free radicals will cause damage to the tissue,and then lead to the functional impairment of some organs and tissues,leading to a series of diseases.At the same time,the aging process of the human body will also produce a large number of free radicals.Oxidative stress refers to a series of physiological and biochemical stress reactions caused by the imbalance of oxidative and antioxidant homeostasis and excessive accumulation of reactive oxygen species(ROS) under the stimulation of internal and external factors[10,14].Excessive ROS can lead to cell damage,such as mitochondrial DNA mutations and cell membrane structure damage[11,12].At the same time,the production and accumulation of ROS can mediate the inflammatory response,causing cells to secrete a variety of inflammatory factors,stimulate the immune response and induce apoptosis,thus leading to the occurrence of diseases[13,14].Studies have shown that high glucose can induce oxidative stress of vascular endothelial cells,and then cause inflammation[15,16].The damage of vascular endothelial cells is related to many factors,among which oxidative stress is closely related to cardiovascular diseases,atherosclerosis and other diseases[17].In view of the invasion of vascular endothelial cells by external factors such as glucose,the effective use of substances that can inhibit oxidative stress and the inflammatory response is of great significance to maintain body balance,protect body health and prevent related diseases[12,18].
As a kind of plant,T.edulishas very strong application value.However,few studies have been reported on the extraction of the active components and the antioxidant effect ofT.edulisin vitro.The materials used in this study came from the area of Bozhou (Anhui,China),and the local people often used it as food preservation,and the effect was significant.Therefore,we speculated that the extract ofT.edulishas certain anti-corrosion and antioxidant effects and carried out the following studies.This study extracted and analysed the active components ofT.edulis,and its antioxidant capacity was measuredin vitro.Glucose was used to treat human umbilical vein endothelial cells (HUVEC) to construct an oxidative stress injury model and to observe whether the purified product can inhibit glucose-induced oxidative stress damage in HUVEC cells and analyze its potential mechanism by using transcriptomics,to provide a certain research basis and an important theoretical basis for the further development and application ofT.edulisand the development of functional food.
2. Materials and methods
2.1 Main materials and instruments
Part of the stem leaf material ofT.eduliswas derived from Bozhou (Anhui,China).HUVEC were donated by Teacher Xiang Rong of the School of Life Science,Central South University(China),and a Sephadex LH-20 gel column was purchased from GE Healthcare Life Sciences (USA).Bovine serum,DMEM,dual antibody and trypsin were purchased from Biological Industries.Glucose and dimethyl sulfoxide (DMSO) were purchased from Sangon Biotech (Shanghai,China),MTT reagent was purchased from Yanke Biotechnology (Changsha,China).A BCA protein assay kit,a ROS assay kit,a total superoxide dismutase (SOD) activity assay kit,a catalase (CAT) assay kit,a glutathione peroxidase (GSH-PX)assay kit,and a total glutathione (GSH) assay kit were all purchased from Beyotime (Shanghai,China).Following treatment,the total cellular RNA was extracted by Tissue RNA Purification Kit PLUS(EZB-RN001-plus) purchased from EZBioscience (US).The cDNA synthesis using HiScript®II Q RT SuperMix for qPCR (Vazyme,China).The qPCR procedures using a iTaquniversl SYBR Green supermix (Bio-Rad,USA) under the CFX 96 Touch Real-Time PCR system (Bio-Rad,USA).DPPH,AAPH and fluorescein sodium were purchased from Sigma (USA).Trolox and 1,10-phenanthroline(OP) were purchased from Sigma-Aldrich Ltd.(China).Glucose,rutin standard,Al(NO3)3,NaNO2,and NaOH were purchased from Chinese Medicine (Shanghai,China).All biochemical reagents were of analytical purity.A multifunctional enzyme marker (Enspire 2300)was purchased from Perkin Elmer (USA).A vacuum freeze dryer(Scientz-10n) was purchased from Ningbo (China).Fast protein liquid chromatography (FPLC) and gas chromatography mass spectrometry were performed using a chromatograph (7890B-5977A)from Agilent Technologies (Germany).
2.2 Crude extraction of the active ingredients of T.edulis
One to two grams of freeze-dried stem leaf parts ofT.eduliswas weighed,cut into pieces,and placed in a 500 mL beaker,and 100−150 mL of ddH2O was added.The contents were then soaked at room temperature for 2 h,cooked for 1−3 h on an electric stove,cooled,filtered,freeze dried,and then dissolved in sterile ddH2O.The solution was then configured into different mass concentrations of crude extraction mixed solution,which were stored separately in centrifuge tubes in a −20 °C freezer.
2.3 Extraction condition optimization
DPPH radical scavenging activity was used as a reference standard,and time (0.5,1.0,1.5,2.0,2.5 h),temperature (40,55,70,85,100 °C) and ratio of material to liquid (1:50,1:75,1:100,1:125,1:150 g/mL) were selected as the optimization factors.The influence of each factor on the scavenging activity of DPPH radical was measured,and the extraction condition with the highest antioxidant activity was obtained for subsequent experiments.Three parallel experiments were performed in each group.
2.4 Separation and purification of crude extract
The configured coarse extraction mixed solution was subjected to a Sephadex LH-20 column for gel filtration chromatography.The separation conditions were as follows: the mobile phase was ddH2O after ultrafiltration at a flow rate of 0.75 mL/min,as determined by full-wavelength scanning,and the detection wavelengths of the ultraviolet absorption peaks were 220 and 280 nm.
2.5 Determination of the Antioxidant activity of purified products in vitro
The antioxidant activity of the purified products at different peaks was measured,mainly including the scavenging activity of the active products on DPPH and oxygen free radicals.
2.5.1 Detection of DPPH radical scavenging ability
Samples (40 μL) were collected in a 1 mL centrifuge tube,and 200 μL of 0.1 mmol/L DPPH solution was added,mixed well and placed in a 37 °C water bath for 60 min.After the reaction,200 μL of reaction solution was placed into each well of a 96-well plate to determine the absorbance at 517 nm.ddH2O was used as the control(An).Each group had 3 parallel controls,and vitamin C was used as the positive control.The DPPH radical clearance rate was calculated by the following formula:
whereAswas absorbance of sample group at 517 nm.
2.5.2 Detection of oxygen radical scavenging ability (ORAC)
Twenty-microlitre samples were prepared on the plate,100 μL of sodium fluorescein (96 nmol/L,dissolved in PBS buffer with a pH of 7.4 and a concentration of 75 mmol/L) was added,and the plate was preheated for 10 min at 37 °C away from light.Then,30 μL of preheated AAPH solution (320 mmol/L) was added to react at 37 °C for an ELISA.The excitation and emission wavelengths were 485 and 538 nm,respectively,the specific measuring time for every 30 s from the start time,continuous measurement of 2 h,a total of 240 times.Trolox (vitamin E) was used as a reference to calculate the antioxidant equivalent of the sample under test (mmol TE/g),and the evaluation formula was as follows:
where AUCS,AUCCand AUCTrepresent the offline integral areas of the fluorescence attenuation curves of the sample,PBS and Trolox,CTrepresents the molality of Trolox,andWSrepresents the mass concentration of the sample under testing.
2.6 Component analysis of purified products
The total sugar content was determined by phenol-sulfuric acid colorimetry.Under the action of concentrated sulfuric acid,polysaccharides or oligo saccharides were first hydrolysed into monosaccharides and rapidly dehydrated to form glycolaldehyde derivatives with phenol into orange compounds.The colour depth was proportional to the sugar content,and the maximum absorption peak was found at a wavelength of 490 nm,which was determined by the colorimetric method.
The flavonoid content was determined by colorimetry.Flavonoids are a class of natural compounds with benzopyranoid ring structures;flavones are usually bound to sugars to form glycosides in plants,and a few are in the form of free aglycones.The 3-hydroxyl,4-hydroxyl or 5-hydroxyl,4-carbonyl oro-diphenolic hydroxyl of flavonoid compounds can complexate with aluminium salts and react with aluminium ions to form orange-red complexes under alkaline conditions.The final solution obtained has the maximum absorption peak at 500 nm,and the chromogenic reaction is stable within 60 min.
2.7 Analysis of the general composition of the active components using GC-MS and LC-MS
In order to further understand the composition of active components,component F2 was analyzed by GC-MS.The separated component F2 was collected and lyophilized.Then redissolved in chromatographic grade methanol,the concentration is 1 mg/mL.Sample should be centrifuged and filtered with 0.22 μm filter before detection.Chromatographic conditions: DB-5MS quartz capillary column (60 m × 0.25 mm,0.25 μm).The column temperature control process was as follows: GC inlet temperature: 300 °C;Injection amount:1 μL;Injection mode: injection in split mode,with split ratio of 10:1;Flow control mode: linear speed;Column flow rate: 1.0 mL/min.Programmed temperature rise: the initial temperature for sample injection was 40 °C for 10 min,and the temperature was raised to 66 °C at a rate of 3 °C/min,which was maintained for 5 min,and then the temperature was raised to 230 °C at a rate of 20 °C/min,which was maintained for 10 min.Carrier gas: high-purity helium(purity >99.99%).Mass spectrum conditions: electron ionization source detector voltage: 70 eV;Transfer line temperature: 280 °C;Solvent delay time: 0.5 min;Quality scanning mode: full scanning;Full scan rate:m/z50-500.
F2 freeze-dried powder was redissolved in ultrapure water with a concentration of 0.5 mg/mL,and filtered with 0.22 μm filter membrane for LC-MS.Waters UPLC(H-class) and Agilent C18column(2.1 mm × 100 mm,1.8 μm) were used in liquid phase separation,the liquid phase was acetonitrile and water.Chromatographic condition:0−30 min,flow rate was 0.1 mL/min,from 5% to 30% acetonitrile.The mass spectrometry system is the Bruker Impact II Q-TOF MS.Ion source: electron spray ionization (ESI);Dry gas: 8 L/min;Dry temperature: 200 °C;Capillary voltage: 3.5 kV;Funnel 1 RF voltage:300 Vpp;Funnel 2 RF voltage: 300 Vpp;Hexapole RF voltage:60 Vpp;Ion energy: 3 eV;Collision energy: 7 eV;Transfer time: 60 μs;Pre-pulse storage time: 5 μs;Mass range:m/z50−1 500.
2.8 HUVEC cell culture and cell viability assay
HUVEC cells were resuspended using the conventional method,maintained in RPMI 1640 supplemented with 10% fetal bovine serum(FBS) containing 1% penicillin-streptomycin and incubated at 37 °C in a humidified atmosphere with 5% CO2,with one 2−3 day passage.
The cells were plated in a 96-well cell culture plate at a density of 1 × 104cells/well and were allowed to attach for 12 h.Then,replaced the 1640 medium with different final concentrations samples (0,25,50,100 μg/mL),serum-free medium 1640 without glucose and samples was used as blank control,and the cell activity was measured by the MTT assay.The absorbance was measured at the wavelength of 490 nm using a multiscan spectrum.
2.9 Preprotective effect of active ingredients on glucoseinduced oxidative damage in HUVEC cells
Cells were inoculated and cultured in 24-well plates.When the cells grew to appropriate density,washed with PBS for three times,and the model group was added with serum-free medium containing appropriate concentration of glucose,experimental groups were added with 35 mmol/L glucose and different concentrations of samples serum-free medium,and the control group was added with equal volume of serum-free medium.After 24 h,the protection of the active components against oxidative damage in HUVEC cells was investigated by detecting the relative viability and ROS content changes.
2.10 Activity determination of GSH-PX,CAT,SOD in cells
Cells were inoculated into 6-well plates.After protection of HUVEC cells with active components of different mass concentrations and glucose for 24 h.Then,the supernatant was discarded,the cells were washed with PBS 3 times,collected,resuspended in 150 μL of pre-cooled PBS,lysed on ice by ultrasound for 5 min,and centrifuged at 4 °C for 10 min at 12 000 r/min.The supernatant was recovered,and the relative activity levels of GSH-PX,CAT and SOD were detected according to kit instructions.
2.11 RNA sequencing
Totol RNA was extracted from HUVEC cells after the same treated using TRIzol reagent (Invitrogen,USA) and DNase I (TaKaRa,Japan) was used to remove genomic DNA.The RNA quality was checked by Bioanalyzer 2100 (Aligent,USA),and the integrity number (RIN) of all these samples’s RNA were >9.0.
According to the manufacturer’s instructions (Illumina,USA),the sequencing libraries were prepared.Poly-A-containing mRNA was isolated from the total RNA by poly-T oligo-attached magnetic beads,and then fragmented by RNA fragmentation kit.The cDNA was synthesized using random primers through reverse transcription.After the ligation with adaptor,the cDNA were amplified by 15 cycles of PCR,and then 300 bp fragments were isolated using gel electrophoresis.At last,the products were sequenced by an Illumina HiSeq xten/NovaSeq 6000 instrument in Majorbio Co.,Ltd.(China).
2.12 Quantitative real-time PCR (qRT-PCR)
Some gene were selected for quantitative confirmation by qRTPCR analysis with a Bio-Rad CFX96 Touch Real-Time PCR system(Bio-Rad,USA).The totol RNA was extracted from HUVEC cells using Tissue RNA Purification Kit PLUS (EZBioscience,USA).After reversely transcription by HiScript®II Q RT SuperMix for qPCR(Vazyme,China),we obtained the cDNA of the total mRNA.Then,the qPCR reaction used iTaquniversl SYBR Green supermix (Bio-Rad,USA).For each gene,the qPCR reactions were performed in biological quintuplicate.
2.13 Statistical analysis
OriginPro 2018 and SPSS 18.0 statistical software were used for statistical analysis of the data.The data are expressed as mean ±standard error,and attest was used.P<0.05 indicates a statistically significant difference.
3. Results
3.1 Antioxidant activity of crude extracts from T.edulis
Solutions of different mass concentrations were taken,and the scavenging activity of DPPH radicals was measured,with vitamin C (2 mg/mL) used as the positive control.The results (Fig.1A)showed that the DPPH radical scavenging rate gradually increased with increasing sample concentration.When the initial concentration of the sample was 5 mg/mL,the DPPH radical scavenging activity was (79.08 ± 2.39)%,indicating that the mixture solution obtained by crude water extraction had strong DPPH radical scavenging activity and could be used for subsequent studies.
3.2 Optimization of extraction conditions
The DPPH radical scavenging activity was taken as the reference standard,the extraction time,temperature and the ratio of solid to liquid were optimized,and the initial concentration of all samples was 5 mg/mL.The results showed that the optimal antioxidant conditions of the extract were as follows (Fig.1B): extraction time 2 h,temperature 100 °C,and solid-liquid ratio 1:100.These conditions were selected for subsequent experiments.
3.3 Purification and antioxidant activity of different purified products in vitro
3.3.1 Separation and purification
After gel filtration chromatography,there were 5 absorption peaks at 220 nm for the water extract fromT.edulis(Fig.2A),and the components at each peak were collected to obtain a total of 5 separated components,named according to the peak time sequence:F1,F2,F3,F4 and F5.

Fig.2 Purification and antioxidant activity of different purified products and polysaccharide and flavonoid contents of each component.A: Gel filtration chromatography of water extract from Tulipa edulis (LH-20);B: DPPH radical scavenging activity of each component,a: have no significance with vitamin C(P >0.05);b,c,d: have significance with vitamin C (P <0.05);C: Oxygen radical scavenging activity of each component;D: Oxygen radical scavenging activity of the separated component F2 at different concentrations;E: Oxygen radical scavenging activity of the separated component F5 at different concentrations;F: Polysaccharide contents of each component;G: Flavonoid contents of each component.Values are expressed as the mean ± SD of three independent experiments.
3.3.2 DPPH radical scavenging activity
The sample concentration of each component was standardized to 0.33 mg/mL,and the results are shown in Fig.2B.There were some differences among the separated components.In contrast,the DPPH radical scavenging activity of the isolated component F2 was the highest,and the clearance rate reached (72.86 ± 1.64)%,which was significantly higher than those of the other components,thus indicating that F2 was one of the most important antioxidant components.
3.3.3 Oxygen radical scavenging activity
The sample concentration of each separated component was standardized to 0.2 mg/mL,and the results are shown in Fig.2C.The oxygen free radical scavenging activities of the separated components F2 and F5 were both high.When the fluorescence value of the Trolox group dropped to 0,the fluorescence intensities of the F2 and F5 groups were still high,indicating that the separated components F2 and F5 both had strong antioxidant effects.After that,concentration dilution was performed on components F2 and F5 to determine the absorption capacity of oxygen radicals,as shown in Figs.2D and E.When the sample concentration was 0.1 mg/mL,component F2 also had strong antioxidant activity,and the absorption capacity of oxygen radicals of F2 reached (8.74 ± 3.25) mmol TE/g,which was significantly higher than those of the other components,thus indicating that F2 was one of the most important antioxidant components.
3.4 Component analysis of purified products
3.4.1 Polysaccharide content of each isolated component
The polysaccharide contents of each component were different to some extent.The total concentration of each sample was standardized to 2 mg/mL,and the measured polysaccharide concentrations are shown in Fig.2F.The polysaccharide content of each component was between 12% and 45%;in comparison,the polysaccharide content of component F5 was the highest,approximately (42.55 ± 4.08)%,while that of component F2,which had the highest antioxidant activity,was the lowest,only (12.26 ± 3.58)%.
3.4.2 Flavonoid contents of each isolated component
The flavonoid contents of each component were different to some extent.The measured flavonoid contents are roughly as shown in Fig.2G.The flavonoid contents of each separated component were between 0.04% and 3.4%.In contrast,the flavonoid contents of components F1 and F2 were relatively high,at (3.24 ± 0.33)%and (3.35 ± 0.39)%,respectively.Previous research results indicated that the antioxidant activity of the separated component F2 was the highest;therefore,we speculated that flavonoids might be one of the important substances exerting antioxidant effects in F2.
3.5 Preliminary analysis of the rough composition of the active components via LC-MS and GC-MS
The LC-MS and GC-MS method were used to preliminarily identify the compound composition of the active component F2.We obtained the metabolite information for component F2 by comparing the results with a database and then performed a preliminary analysis of the data.By LC-MS component analysis of component F2,we found that component F2 contained epigallocatechin,epigallocatechin dimer and myricetin hexoside.The LC-MS spectra of F2 and the mass spectra of 1.2 and 5.8 min are shown in Fig.3.By L-MS analysis,epigallocatechin,epigallocatechin dimer and myricetin hexoside were detected in component F2.Results are shown in Fig.3B,two peaks with molecular weights ofm/z308.031 1 and 615.061 0 are epigallocatechin and its dimer.In Fig.3C,the substance with a molecular weight ofm/z479.104 1 is myricetin glycoside.They are typical flavonoids with good antioxidant activity[19,20].Flavonoids are common phenolic compounds in plants,which are compounds connected by two benzene rings through heterocyclic pyran rings,so human beings cannot synthesize them themselves.Flavonoids are commonly used antioxidants,but only from exogenous intake[21].The activity of flavonoids depends on its phenolic hydroxyl group,which can provide hydrogen and electrons to hydroxyl radicals and peroxy radicals to stabilize them to scavenge free radicals[22].Studies have shown that the antioxidant activity of catechins can increase with the degree of polymerization[20].Milad et al.have shown that myricetin and myricetin glycosides exert antioxidant effects by scavenging free radicals[19].

Fig.3 LC-MS spectra of component F2.A: Base peak chromatogram of F2 (BPC);B: MS of 1.2 min absorption peak;C: MS of 5.8 min absorption peak.
The GC-MS results revealed that component F2 mainly contained 10 compounds,and the names,retention times and relative contents of the compounds are shown in Table 1.According to their relative contents,the compounds 1,2-15,16-diepoxyhexadecane,9,12-octadecadienoyl chloride,(Z,Z)-,9-octadecenoic acid,methyl ester,(E)-were the main component.9-octadecenoic acid is known as oleic acid,accounts for 20.33% of F2,it also is the main component of olive oil and camellia oil,which has good antioxidant function[23].And 1,2-15,16-diepoxyhexadecane has been shown to have certain antioxidant activity[24].The above results suggesting that these compounds may play major roles in the antioxidant activity of F2.

Table 1 Relative contents of major compounds in F2 as measured by GC-MS.
3.6 Effect of F2 on the oxidative stress of HUVEC cells treated with glucose
The injury of HUVEC cells is closely related to many vascular diseases,among which oxidative stress is an important cause.The protection of HUVEC cells from oxidative stress is important for the prevention and treatment of vascular diseases.The MTT assay was adopted to determine the effects of the purified antioxidant fraction F2 on the viability of HUVEC cells;furthermore,no cytotoxicity was observed,indicating that the purified fraction F2 was safe and could be used in ROS experiments (Fig.4A).The cells were treated with serum-free RPMI 1640 medium,serum-free medium containing 35 mmol/L glucose,serum-free medium containing 35 mmol/L glucose and different concentrations of samples for 24 h,and then the intracellular ROS content in each group was detected using an ROS assay kit.As shown in Fig.4B,the fluorescence intensity of cells treated with 1640 containing 35 mmol/L glucose was significantly higher than that of cells treated with normal 1640,indicating that 35 mmol/L glucose could induce oxidative stress in HUVEC cells.In Fig.4C,The fluorescence intensity of cells treated with purified fraction F2 was significantly lower than that of the glucose-treated model group cells,suggesting that purified fraction F2 could scavenge intracellular ROS and protect HUVEC cells from oxidative damage caused by glucose.The purified fraction F2 showed strong intracellular ROS-scavenging activity at 50 and 100 μg/mL indicating a protective effect of fraction F2 against glucose-induced oxidative damage in HUVEC cells.
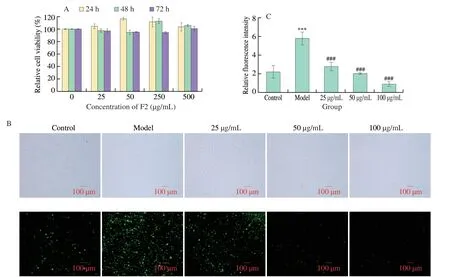
Fig.4 Changes of ROS in HUVEC cells treated with different concentrations of F2.A: Cytotoxicity results;B: Fluorescent images of each group by microscopy;C: Comparison diagram of the relative fluorescence intensity of each group.Compared with Control,*** P <0.001;Compared with Model,### P <0.001.
3.7 Effects of the active component F2 on the activities of associated antioxidant enzymes and GSH in HUVEC cells induced by glucose
After HUVEC cells were treated in the same way,the activity levels of relevant antioxidant enzymes and the change in GSH content in each group were detected.As shown in Fig.5,compared with the normal control group,the GSH-PX activity of cells in the model group after glucose treatment was decreased,with a significant difference,indicating that the glucose-induced oxidative damage model was successfully established.Compared with the model group,the GSH-PX activity of cells in the 100 μg/mL F2+glucose group was increased,and the difference was significant.CAT activity test results showed that compared with the normal control group,CAT activity in the model group was significantly decreased.Compared with the model group,the CAT activity in the 100 μg/mL F2 +glucose group was increased,and the difference was statistically significant.SOD activity detection results showed that compared with the normal control group,the SOD activity in the model group was decreased,and the difference was significant.Compared with the model group,the SOD activity of cells in the protected treatment groups of 50 μg/mL F2+glucose and 100 μg/mL F2+glucose group were increased,and the differences were statistically significant.The experimental results of GSH content showed that compared with the normal control group,the GSH content in the model group after glucose treatment was significantly decreased.Compared with the model group,the cell GSH contents in the 50 μg/mL F2+glucose and 100 μg/mL F2+glucose groups were increased,and the differences were significant.
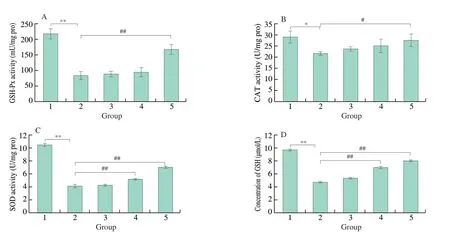
Fig.5 Changes of antioxidant enzyme activity and GSH content in HUVEC cells treated with different concentrations of F2.A: GSH-PX activity in each group;B: CAT activity in each group;C: SOD activity in each group;D: GSH content in each group.1: Control;2: 35 mmol/L glucose (Model);3: 25 μg/mL F2+glucose;4: 50 μg/mL F2+glucose;5: 100 μg/mL F2+glucose.Compared with Control,* P <0.05,** P <0.01;Compared with Model,# P <0.05,## P <0.01.
3.8 Transcriptome analysis of HUVEC cells
Through transcriptome analysis,totally 83 differentially expressed genes were detected withP<0.05 between control and model samples(C vs.M),including 36 up-regulated genes and 47 down-regulated genes,and 403 differentially expressed genes were detected withP<0.05 between control and 100 μg/mL samples (C vs.T),including 114 up-regulated genes and 289 down-regulated genes.Further screening revealed the differentially expressed genes in three groups as shown in Fig.6,in which heme oxygenase 1 (HO-1) and NAD(P)H quinone oxidoreductase-1 (NQO1) were closely related to oxidative stress.Nrf2-ARE-dependent genes includeHO-1,γ-glutamyl cysteine ligase catalytic subunit (GCLC),NQO1,GSH,and many others[27].These genes are closely related to the body’s antioxidant function and can play a powerful ROS scavenging role.At the same time,Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis found that the differential genes were mainly concentrated in diabetes and cardiovascular diseases,in which oxidative stress of vascular endothelial cells was an important cause.Next,the expression of related genes were verified by qPCR.
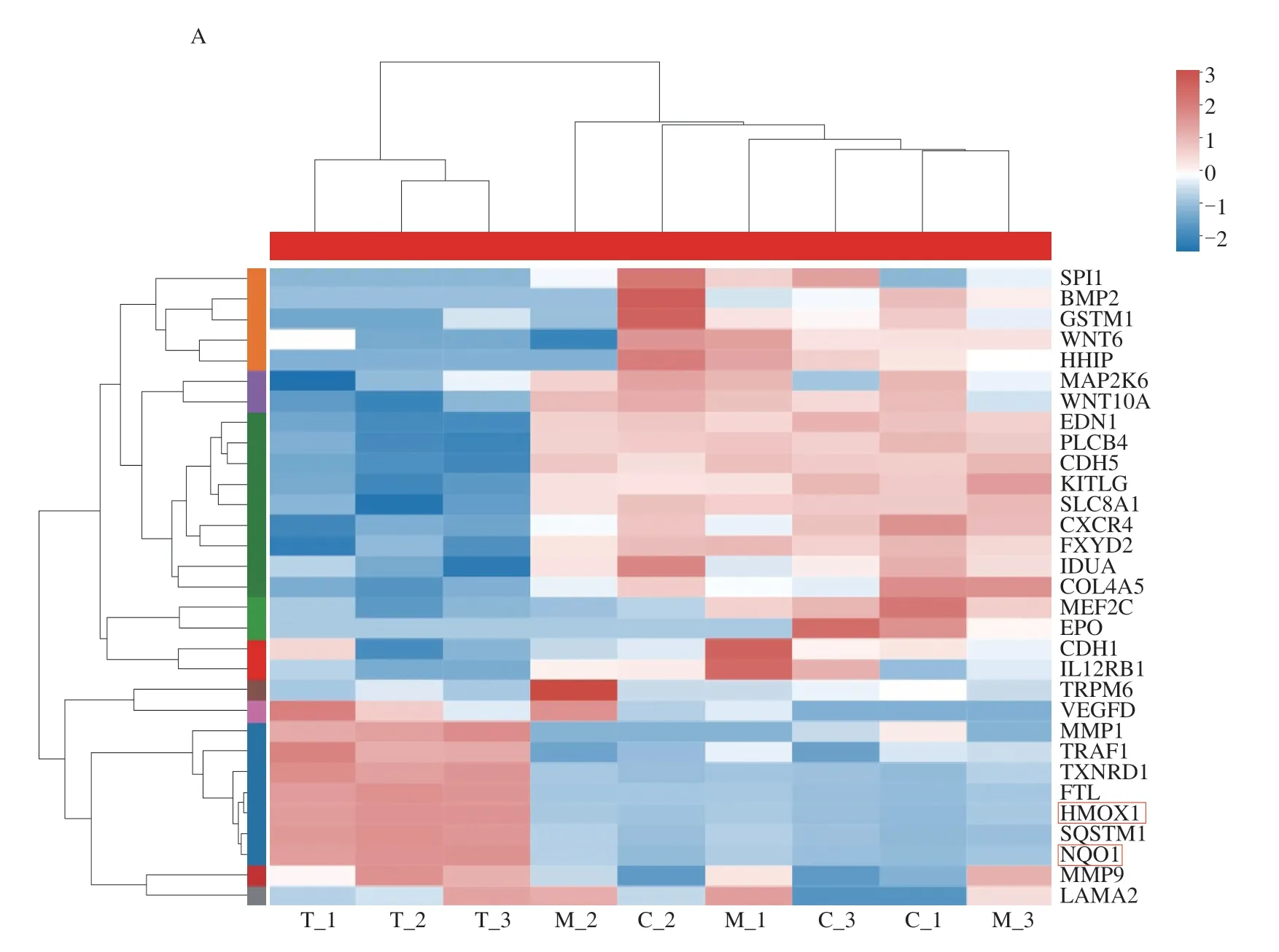
Fig.6 HUVEC cell transcriptome analysis results.A: Differentially expressed genes among Control (C),Model (M) and Test (T) groups;B: KEGG analysis of differentially expressed genes.Two important genes were found in the red box,whose expression levels were subsequently examined.

Fig.6 (Continued)
3.9 qRT-PCR
To further verify and study the antioxidant function ofT.edulis,two differentially expressed genes and its related genes were chosen for qRT-PCR test.After RNA extraction,reverse transcription and qPCR verification,we found that compared with the control group,the expression ofHO-1(HMOX1),NQO1,GCLCin the model group cells were down-regulated,but expression ofNrf2have little change.Meanwhile,compared with the model group,the expression ofHO-1,GCLCandNQO1genes in the 100 μg/mL F2 treated cells were up-regulated,but expression ofNrf2have little change,As we konwn,Nrf2dissociates from Keap1 after activation and is transported to the nucleus,increasing its accumulation in the nucleus and binding with ARE[25],which may also be the reason why its expression level does not change significantly.However,the downstream genesHO-1,GCLCandNQO1that depend on the Nrf2-ARE were significantly up-regulated.This is consistent with the transcriptome results.Therefore,we believe that purified F2 also acts on Nrf2/Keap1/ARE signaling pathway to inhibit oxidative stress induced by high glucose in HUVEC cells,as shown in Fig.7.

Fig.7 Expression levels of differentially expressed genes and related genes and antioxidant mechanism of F2 protective HUVEC cells.A: HO-1;B: NQO1;C: GCLC;D: Nrf2;E: Antioxidant mechanism of F2.Compared with Model,### P <0.001.
4. Discussion
Free radicals are common metabolites in many biochemical reactions,and radicals are usually in a dynamic balance between production and elimination in the body.However,if the radicals accumulate too much or cannot be removed by antioxidants in the body in a timely manner,then cells and tissues may be damaged,which can lead to a series of diseases[26,27].ROS can also cause inflammation[16].Oxidative damage of vascular endothelial cells is closely related to many diseases,such as cardiovascular disease,atherosclerosis and so on[28,29].In fact,the occurrence and development of many diseases in organisms are closely related to the generation and accumulation of radicals[30,31].Protecting the oxidative balance of the body and removing excessive free radicals play an important role in improving the body’s health and immunity,and are of great significance in preventing related diseases.Therefore,it is of great significance to identify antioxidant substances with good effects for the protection of vascular endothelial cell and the prevention of several diseases.
Plant extracts contain abundant natural nontoxic bioactive substances;these active products have strong application value in immune regulation,disease prevention and treatment,oxidation resistance and antiaging[32,33].Therefore,strengthening antioxidant studies of plant extracts can provide more possibilities for the development and application of natural food additives or natural antioxidants.This study mainly discussed the antioxidative aspects of the extract ofT.edulis.Antioxidation is closely related to anti-ageing,and oxidative damage is the main factor of body ageing.The development of antioxidant products can provide more options for the development of the functional food industry and can provide a certain reference basis for disease prevention and treatment.
In this study,we found that the water extract ofT.edulishad good DPPH radical scavenging activity.After the separation and purification of the LH-20 gel,the resulting active component F2 had strong DPPH and oxygen radical scavenging activity.Through a general analysis of the polysaccharide and flavonoid contents of each separated component,we found that the flavonoid content in component F2 was relatively high;thus,we speculated that flavonoids may be one of the important components of F2 to exert antioxidant effects.The components of active component F2 were analyzed by LC-MS and GC-MS,also found epigallocatechin and myricetin glycosides showed strong antioxidant activity[19,20],1,2-15,16-diepoxyhexadecane and 9-octadecadienoic acid had been verified to have certain antioxidant activity[23,24],which may be the reason for the good antioxidant activity of F2.Then,the protective effect of the active component F2 on HUVEC cells was investigated.It was found that after treatment with F2,the activity levels of related antioxidant enzymes and the content of GSH antioxidants in the cells were significantly increased,indicating that the active component F2 had a strong antioxidant protection effect on the cells.Meanwhile,the protective mechanism of F2 on HUVEC cells was also preliminarily studied.However,this study mainly focuses on HUVEC cells and concentrates on their application in the antioxidation industry.Application research in other industries also needs further development and study,may also provide more reference value for clinical research.
5. Conclusion
In this paper,the active components ofT.eduliswere extracted and analyzed,and its antioxidant capacity was determined.Then Sephadex LH-20 gel was used to separate and purify the crude extract,and the antioxidant activity of the purified product was determined.HUVECs were used to establish oxidative stress model to observe the antioxidant effect of F2 at the cellular level,and HUVECs with the same treatment were analyzed by transcriptomic analysis to explore the possible mechanism of F2 component protecting HUVECs from oxidative stress induced by high glucose.The results showed that component F2 had strong antioxidant activity.In addition,F2 showed strong antioxidant protection in HUVECs.Meanwhile,the expressions ofHO-1,GCLCandNQO1genes were up-regulated in HUVEC cells after F2 treatment.This study provides a basis for further application of extracts in food and health care products.
Declaration of competing interest
the authors declare no conflicts of interest.
Acknowledgements
The work was supported by the Open Grant of Beijing Advanced Innovation Center for Food Nutrition and Human Health (20182024),National Natural Science Foundation of China (31370104),The Natural Science Foundation of Hunan Province,China (2021JJ30029),the Taishan Scholar Program of Shandong Province,China(tsqn201909168),“Double Hundred” Program for Foreign Experts of Shandong Province,China (WST2017004),Hunan Province Postgraduate Education Innovation Project and Professional Capacity Enhancement (CX20200297),Project the Fundamental Research Funds for the Central Universities of Central South University(2020zzts424,2020zzts422).
杂志排行
食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
