Association between lifestyle factors and thyroid function in young euthyroid adults
2024-02-16ElisaMerchanRamirezGuillermoSanchezDelgadobLucasJuradoFasolicFranciscoAcostadManuelMuozTorresJoseLlamasElvirJonatanRuiz
Elisa Merchan-Ramirez*,Guillermo Sanchez-Delgadob,Lucas Jurado-Fasolic,Francisco M Acostad,e,Manuel Muñoz-Torres,Jose M.Llamas-ElvirJonatan R Ruiz*
a Sport and Health University Research Institute (iMUDS),Faculty of Sports Science,University of Granada,Granada 18016,Spain
b Pennington Biomedical Research Center,Baton Rouge 70808,USA
c Faculty of Medicine,University of Granada,Granada 18071,Spain
d Turku PET Centre,University of Turku,Turku 20500,Finland
e Turku PET Centre,Turku University Hospital,Turku 20520,Finland
f Biosanitary Research Institute of Granada (Ibs.Granada),Granada 18012,Spain
g CIBERFES,Instituto de Salud Carlos III,Madrid 28029,Spain
h Endocrinology and Nutrition Service,University Hospital San Cecilio,Granada 18016,Spain
i Nuclear Medicine Service,"Virgen de las Nieves" University Hospital,Granada 18014,Spain
Keywords:Thyroid hormones Dietary intake Sleep Physical activity Euthyroid
ABSTRACT Purpose: The present work examines the associations of dietary habits,sedentarism,physical activity (PA)levels and sleep habits,with thyroid function in young euthyroid adults.Methods:A total of 105 young euthyroid adults participated in this cross-sectional study.Thyroid function was determined in fasting conditions(>6 h).Dietary habits were measured by a food frequency questionnaire and three non-consecutive 24 h recalls,and different dietary intake and patterns were then estimated.The time spent in sedentary,PA levels and sleep habits were objectively measured using a wrist-worn accelerometer.Results:Energy and carbohydrate intake were positively associated with thyroid stimulating hormone (TSH) (β=0.222;R2=0.102;P =0.022 and β=0.425;R2=0.129;P=0.007,respectively) whereas fat intake was negatively associated with TSH (β=−0.428;R2=0.137;P=0.004).Energy intake was also positively associated with free triiodothyronine (β=0.277;R2=0.137;P=0.004).Further,adherence to the Mediterranean diet was negatively related to TSH and free thyroxine (FT4) (β=−0.221;R2=0.113;P=0.020 and β=−0.268;R2=0.071;P=0.007,respectively).Vigorous-intensity and overall PA were negatively associated with FT4 (β=−0.227;R2=0.052;P=0.022 and β=−0.204;R2=0.042;P=0.041,respectively).In contrast,no associations were found between sleep parameters and thyroid function.Conclusions:Lifestyle factors such as dietary intake and PA levels seems to be related to thyroid function even in young euthyroid adults.
1. Introduction
Serum thyroid hormones (THs) concentration is regulated by the hypothalamic-pituitary-thyroid axis to orchestrate processes such as cell differentiation and tissue growth,development,and function[1].The thyrotropin releasing hormone (TRH),which is produced in the hypothalamus,stimulates the pituitary gland,thyroid stimulating hormone (TSH) secretion[1].Subsequently,TSH stimulates the synthesis and secretion of THs (triiodothyronine(T3) and thyroxine (T4)) from the thyroid gland[1].The thyroid gland is the main effector of the hypothalamic regulation of energy expenditure[2,3],and THs are key determinants of basal metabolic rate[1].Importantly,thyroid dysfunction is closely related to metabolic disturbances[3],and even in the absence of thyroid pathology (i.e.,euthyroid individuals),slight differences in THs predict future body weight gain[4].There is increasing evidence suggesting that lifestyle factors including diet,physical activity and sleep influence the THs production,and hence body weight and metabolic homeostasis[1,5].
Nutrient availability is one of the most influential environmental factors modulating the hypothalamic-pituitary-thyroid axis activity[6].During caloric restriction and prolonged starvation,the hormonal milieu is dominated by low levels of insulin and THs[7].This,in turn,reduces whole-body energy expenditure to preserve energy stores by modulating behaviour and energy metabolism[8].On the other hand,an increase in serum free T3 (FT3) might contribute to the increase in energy expenditure observed in response to overnutrition[8-10].Importantly,beyond food availability,different types of diet seem to modulate thyroid hormone concentrations[11,12].For instance,Zupo et al.[13]showed that adherence to the Mediterranean diet was associated to reduced thyroid function in euthyroid individuals.Moreover,micronutrients such as iodine,selenium,zinc,iron,copper,and vitamin A are important regulators of THs synthesis and metabolism[14].Indeed,associations between circulating levels of THs and micronutrient intake have been reported in humans[15,16].However,the heterogeneous results in previous studies prevent drawing firm conclusions[14].Therefore,analysing the association of dietary patterns and habitual nutrient intake with thyroid function is needed to elucidate the role of dietary habits in the thyroid metabolism in euthyroid individuals.
Physical activity is known to play a role in the regulation of many endocrine glands,including the thyroid gland,modulating THs secretion[17].Abnormal THs circulating concentration has a major impact on physical activity tolerance and might result in decreased ability to perform vigorous activities[18].Additionally,physical activity itself could have direct effects on thyroid function because as it produces alterations in the hypothalamic-pituitary-thyroid axis,either due to acute or long-lasting effects[18].Most studies have been conducted in trained subjects and/or patients with thyroid disease[19],thus,it is of great interest to study the relationship between habitual physical activity levels and thyroid function in sedentary and euthyroid subjects.
Furthermore,sleep problems are often related to thyroid problems,since sleep deprivation causes alterations in the hypothalamicpituitary-thyroid axis activity[20].In humans,acute sleep deprivation is associated with an increase of TSH,free T4 (FT4),and FT3 circulating concentration[21,22],whereas sleep seems to acutely inhibit TSH secretion overnight[23].It is also widely known that hyperthyroid subjects are usually short sleepers,and hypothyroid subjects are often long sleepers,which suggests an inhibitory effect of THs on sleep[24].Therefore,thyroid function seems to have an important role in sleep regulation.However,the association between sleep quality and TSH/THs serum levels in euthyroid adults is still controversial and requires further research.
For this reason,the hypothesis of this study was that several lifestyle factors (i.e.habitual nutrient intake,dietary patterns,sedentarism,physical activity levels,and sleep habits) are associated with thyroid function even in young euthyroid adults.
The aim of this study was to analyse the association of several lifestyle factors including habitual nutrient intake,dietary patterns,sedentarism,physical activity levels,and sleep habits,with thyroid function in young euthyroid adults.
2. Materials and methods
2.1 Study design and study subjects
A total of 105 young adults (34 men,71 women,Table 1) were included in this cross-sectional study.All of them were euthyroid adults (TSH,FT4,and FT3 levels within the normal range:0.34−5.60 μUI/mL,0.38−1.50 ng/dL and 2.50−4.94 pg/mL,respectively) and were enrolled in the ACTIBATE study[25]trial(ClinicalTrials.gov ID: NCT02365129).The inclusion criteria of the study were: i) being 18−25 years old,ii) being sedentary (less than 20 min on less than 3 days/week of reported physical activity),iii) not smoking or taking any medication,iv) having had a stable body weight(changes <3 kg in the last 3 months),v) not having acute/chronic diseases,and vi) not being pregnant.The study protocol and written informed consent were performed in accordance with the Declaration of Helsinki (revised in 2013).The study was accepted by the Ethics Committee of Human Research of the University of Granada(No.924) and theServicio Andaluz de Salud(Centro de Granada,CEI-Granada).All measurements were performed in Granada (Spain)during the months of October,November,and December of 2015,and 2016.

Table 1 Characteristics of the study participants.
2.2 Thyroid function
A blood draw was taken intravenously between 8:00 AM and 6:30 PM for determining thyroid function (no associations were found between the time of the day when the samples were collected and the TSH,FT4,FT3 and thyroglobulin (Tg) concentrations),after fasting for >6 h and after avoiding moderate (24 h previous)and vigorous (48 h previous) physical activity.Blood samples were centrifuged and stored at 4 °C until analyses.To determinate the TSH,FT4,FT3,and Tg circulating levels,a Beckman Coulter DXI(33820,33880,A13422,and 33860,respectively) chemiluminescent immunoassay system was used.In addition,the Parametric Thyroid Feedback Quantile-based Index (PTFQI) was calculated-an indicator of resistance to THs-which oscillates between −1 to 1[26].Hence,negative values of PTFQI indicate low TSH values by high inhibition of FT4 (high sensitivity to FT4).In contrast,positive values of this index indicate high TSH by low inhibition of FT4 (low sensitivity to FT4).Consequently,PTFQI determine the set point of the central regulation of THs levels.PTFQI was calculated following adapted Excel spreadsheet formula created by Laclaustra et al.[26].
2.3 Body composition
Fat mass (FM) was determined by dual-energy X-ray absorptiometry scan (Discovery Wi,Hologic,Inc.,Bedford,MA,USA) and data were analysed using the Hologic APEX 4.0.2.(Hologic,Inc.,Bedford,MA,USA) software.Then,FM (%)was calculated.Weight and height were measured by a scale and stadiometer respectively (SECA,model 799,Electronic Column Scale,Hamburg,Germany),and body mass index (BMI) was calculated (kg/m2).
2.4 Dietary assessment
Dietary intake was recorded using three non-consecutive 24 h dietary recalls (2 working days,1 non-working day),in a face-to-face interview performed by qualified and trained research dietitians.In order to improve the accuracy of food quantification,a photograph book of portion sizes was used during the interviews[27].Data were entered independently by two research dieticians in the EvalFINUT®software (https://www.finut.org/evalfinut/),which employs the United States Department of Agriculture (USDA) and Base de Datos Española de Composición de Alimentos (BEDCA) databases,and energy and daily macro/micronutrient intake were estimated.The mean of the two datasets obtained by the two independent researchers was used when the coefficient of variance (CV) was less than 5%.When the CV was greater than 5%,a third dietician analysed the data and the mean for the two datasets with the highest percentage of agreement was used.Dietary energy density was calculated by dividing the energy of foods and beverages (excluding water) by the total weight of foods and beverages consumed (expressed as kcal/g)[28].The 24 h recalls were carried out within a 3-week period and subjects were not informed when they were going to be performed.
In addition to the self-reported energy intake estimated with the 24 h dietary recalls,energy intake was also assessed by anad libitumtest meal.Subjects received a plate of refined wheat pasta with minced pork,fried tomato sauce,and virgin olive oil (45.5% carbohydrates,38.5% fat,16% proteins;3% fiber,dietary energy density=1.54 kcal/g).Four hours and 15 min after consuming a standardized liquid breakfast (energy content equivalent to 50% of the measured resting metabolic rate,T-Diet Energy neutral flavour,Vegenat S.A.,Badajoz,Spain),the subjects were moved into a quiet room without distractions where they found their plate (1 500 g for men and 1 000 g for women) and a glass of water (450 mL).Then,they were left alone and were instructed to eat as much or as little as necessary for feeling pleasantly satiated[29].Food intake was measured as the difference between the plate weight before and after the meal (in grams),and then energy intake was calculated by multiplying the grams of food consumed by their energy content.
A validated food frequency questionnaire (FFQ) of 100-food items was used to record the food frequency consumption[30].The subjects were asked by research dieticians to report their habitual intake frequency of each food item during the last 3 months.To improve measurement accuracy,a lay description of portion size was detailed for each item (e.g.,cups,teaspoons,etc.).Then,each item was converted into standardized portions (weight).
2.5 Dietary patterns
Dietary patterns were determined by analysing the subjects’ data from the 24 h recalls and FFQ.Three Mediterranean dietary patterns were calculated[30-32]: the a priori Mediterranean dietary pattern(MeD-P)[33],the Mediterranean diet score (MeD-S)[31]and the dietary quality index for a Mediterranean diet (MeD-DQI)[32].Of note,a higher score in MeD-P and MeD-S indicates greater adherence to the Mediterranean diet,while a higher score in MeD-DQI indicates poorer Mediterranean diet quality.The Adherence to the Dietary Approaches to Stop Hypertension (DASH) (higher DASH score means better adherence to the DASH guidelines[34]),the diet quality index (DQI)[35](higher DQI score represents a lower diet quality),and the dietary inflammatory index (DII)[36](higher DII score means a more inflammatory diet) were also calculated.
2.6 Sedentary behaviour,physical activity,and sleep habits
Sedentary behaviour,physical activity,and sleep time were objectively assessed for 7 consecutive days by a wrist-worn accelerometer (ActiGraph GT3X+,Pensacola,FL,USA)[25].The participants were instructed to wear the accelerometer on the non-dominant wrist,only removing it when doing water activities.Participants were also instructed to note their daily wake-up time,bedtime,and the time that at which took off and on the accelerometers in a diary.The accelerometers were programmed to archive raw accelerations using an epoch length of 5 s at 100 Hz of sampling frequency[37].Once the record was over,raw accelerations were downloaded and transformed to “.csv” format using ActiLife software,version 6.13.3.(ActiGraph,Pensacola,FL,USA).Afterwards,the GGIR package (version 1.6-0;https://cran.r-project.org/web/packages/GGIR/) in R (version 3.1.2,https://www.cran.r-project.org/) was used to process and analyse the physical activity data as reported elsewhere[38],using the Euclidean norm minus one(ENMO) metrics.The time invested in sedentary behaviour and in different physical activity intensities (light (LPA),moderate(MPA),vigorous (VPA),and moderate-vigorous (MVPA) physical activity) were then calculated by applying age-specific thresholds for ENMO[39,40].The mean ENMO (mG) during waking time was used to obtain a general indicator of physical activity level.
The procedure to process and analyse sleep data was similar to that for the determination of sedentary behaviour and physical activity.A previously proposed algorithm (validated by polysomnography)was used to combine data from the accelerometers and the subjects’diary reports to detect periods of sleep[41].According to this algorithm,sleep is defined as any period of sustained inactivity in which there is only minimal arm angle change (i.e.,<5°) for 5 min during a period recorded as sleep in a subject’s diary.Values for the following sleep-related variables were then determined: (i) night onset (time at which the subject fell asleep);(ii) wake-up time;(iii) sleep time(total time spent in bed excluding sleep latency);(iv) sleep efficiency(percentage of bedtime determined as sleep time),and (v) wake after sleep onset (WASO;total awakens events from sleep onset to the ultimate awakening)[42,43].Moreover,the Pittsburgh sleep quality index (PSQI),a validated self-reported questionnaire,was performed to determinate sleep quality[44].The total score was reversed so that lower values indicated poorer sleep quality.Finally,we classify as good sleepers the participants that obtained an overall PSQI score ≥ −5,and bad sleepers were considered those with overall score of ≤ −6[44].
2.7 Statistical analyses
Data are reported as mean and standard deviation.We conducted simple linear regressions to study the association of TSH,FT4,FT3,and Tg serum concentrations with dietary outcomes,sedentary time,physical activity,and sleep-related variables.Moreover,we also tested these associations using multiple linear regressions adjusting for fat free mass (only for the energy intake analyses),energy intake,sex and BMI.The Sex × TSH/THs/Tg interaction effect on dietary intake,physical activity levels and sleep outcomes was also analysed,and exploratory analyses were performed for men and women separately when significant interaction effects were detected.The Statistical Package for Social Sciences (SPSS,v.22.0,IBM SPSS Statistics,IBM Corporation) was used to perform the analyses.The GraphPad Prism 8 (GraphPad Software,San Diego,CA,USA) was used to build the graphical plots.Statistical significance was set atP<0.05.
3. Results
Descriptive parameters of the participants are presented in the Table 1.Significant gender differences were observed in TSH and FT3 levels,PTFQI,BMI,FM percentage,energy,protein and saturated fatty acid (SFA) intake,adherence to the DASH diet,all physical activity levels and sleep variables such as sleep time and efficiency (P≤ 0.059).
3.1 Association between dietary outcomes and thyroid function
Self-reported energy intake was positively associated with serum TSH (B<0.001;β=0.222;R2=0.102;P=0.022) and FT3 (B<0.001;β=0.277;R2=0.137;P=0.004) levels (Table 2),but no associations were found between energy intake on thead libitummeal test and TSH or THs (Fig.1).On the other hand,carbohydrate intake was positively associated with TSH levels (B=0.005;β=0.425;R2=0.129;P=0.007),whereas fat (B=-0.014;β=−0.428;R2=0.137;P=0.004),SFA (B=−0.036;β=−0.448;R2=0.145;P=0.003) and monounsaturated fatty acid(MUFAs) (B=−0.022;β=−0.326;R2=0.132;P=0.006)intake was negatively associated with TSH levels.Moreover,the associations between energy and fat intake and TSH levels were only found in men (P≤ 0.041),while the associations between fatty acid and cholesterol and TSH levels were only observed in women (P≤ 0.094) (data not shown).These results persisted after adjusting for energy intake,sex and BMI (allP≤ 0.039;Table 2).Further,positive associations were observed between different fatty acids intake and FT3 (SFA (B<0.001;β=0.325;R2=0.097;P=0.032),MUFAs (B<0.001;β=0.328;R2=0.105;P=0.002),polyunsaturated fatty acid (PUFAs) (B<0.001;β=0.337;R2=0.098;P=0.004) and total cholesterol intake(B<0.001;β=0.309;R2=0.096;P=0.004)).However,these results disappeared after adjusting for sex and BMI (allP≥ 0.297,Table 2).PTFQI was also positively associated with SFA(B<0.001;β=0.334;R2=0.046;P=0.032) and MUFAs (B<0.001;β=0.255;R2=0.041;P=0.039),but disappear when adjusted by sex and BMI (allP≥ 0114,Table 2).No associations were found between self-reported energy intake or macronutrient intake and FT4 or Tg(P≥ 0.170;Table 2).We detected a Sex × FT3interaction effect on self-reported energy,energy density,protein and alcohol intake(P≤ 0.029).Associations between self-reported energy and alcohol intake and FT3 were found in women (B<0.001;β=0.280;R2=0.164;P=0.001 andB=−0.038;β=−0.056;R2=0.213;P=0.035) but not in men (P>0.291).No other Sex × TSH/THs/Tg interaction effect on macronutrient intake were observed.

Fig.1 Associations between ad libitum energy intake and thyroid function.Non-standardized B coefficient,R2 and P value from multiple linear regression analyses adjusting for fat free mass.TSH,thyroid-stimulating hormone;FT4,free thyroxine;FT3,free triiodothyronine;Tg,thyroglobulin.

Table 2 Associations between self-reported energy and macronutrients intake with thyroid function.
MED-P was negatively associated with TSH (B=−0.034;β=−0.221;R2=0.113;P=0.020) independently of energy intake,sex and BMI (P≤ 0.037,Table 3) in men,but not in women (data not shown).Furthermore,MED-S was negatively associated with FT4(B=−0.021;β=−0.268;R2=0.071;P=0.007) and the results also persisted after adjusting for energy intake,sex and BMI (P≤ 0.009,Table 3) in men and women.On the other hand,no associations were found between any dietary pattern with FT3 or Tg (P≥ 0.051,Table 3).The MED-P,MED-S,MED-DQI and DASH were also associated with the PTFQI (P≤ 0.034),but the results were attenuated after adjusting for sex and BMI (P≤ 0.217).No Sex × TSH/THs/Tg interaction effect on dietary patterns was detected.
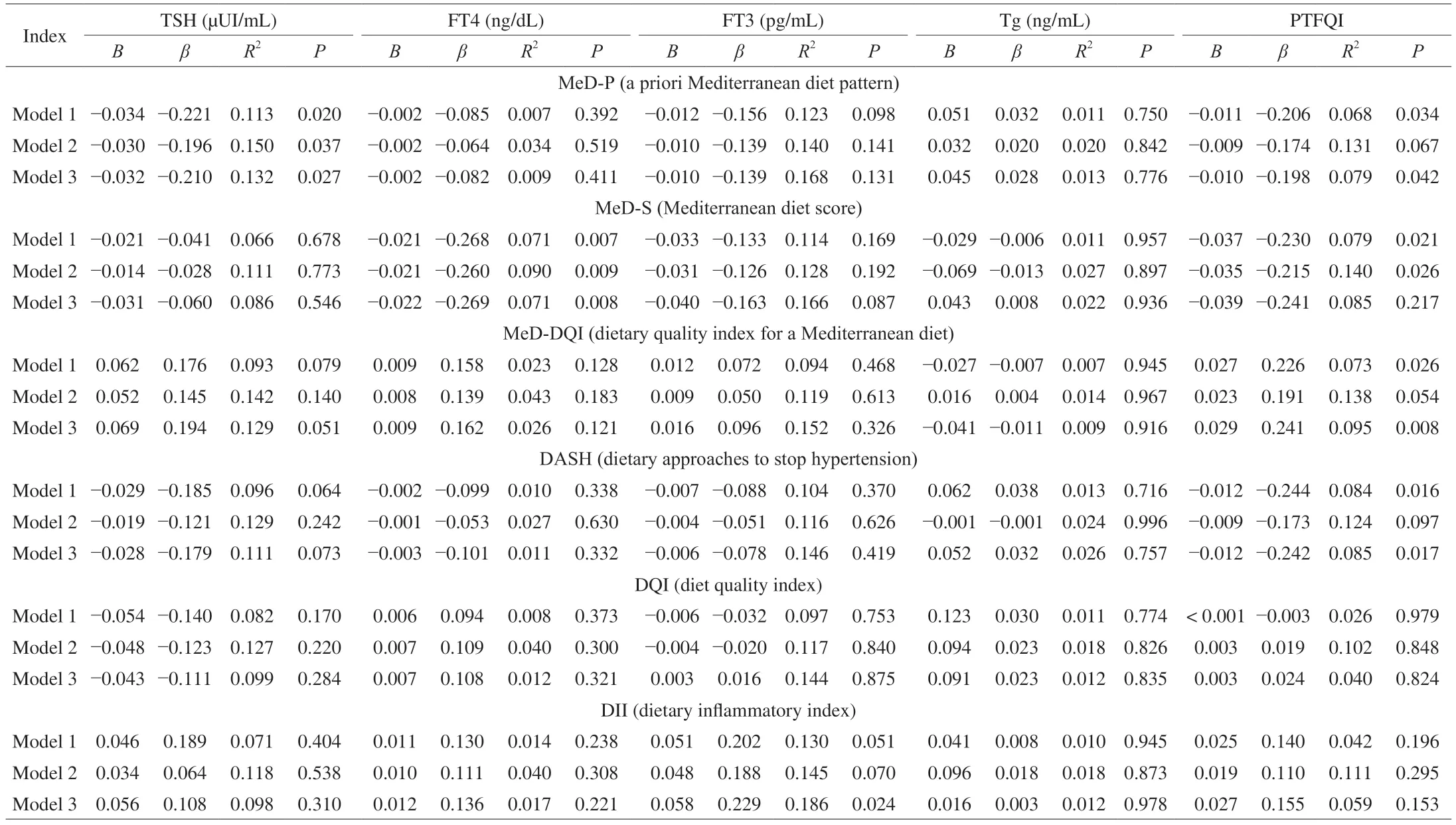
Table 3 Associations between dietary patterns and thyroid function.
Descriptive parameters of micronutrient intake are presented in Table S1.Selenium intake was positively associated with TSH(B=0.008;R2=0.120;P=0.012),whereas folate and vitamin C intake were negatively related to FT3 (B=−0.001;R2=0.149;P=0.014 andB=−0.002;R2=0.137;P=0.032,respectively).The results remained or were attenuated after adjusting for energy intake,sex and BMI (P≤ 0.057) (Table S2).No other associations were found between self-reported micronutrient intake and thyroid function (P≥ 0.060,Table S2).However,Sex × TSH,FT3 and Tg interactions effect was found on tiamine,vitamin B6,vitamin D,niacine and vitamin B5intake.These significant associations were observed in women,not in men,except for niacine that was observed in male but not in female (P<0.032).
Descriptive parameters for the frequency of food groups consumption are presented in Supplementary Table S3.The consumption of vegetables,olive oil,and canned fish were negatively associated with TSH levels,which was positively related to soft drinks consumption (allP≤ 0.048,Table S4).Meat,processed meat,and margarine intake were positively associated with FT4 concentration,while it was negatively associated with eggs and canned fish consumption (allP≤ 0.031).Lastly,negative associations were found between meat,white meat and fish with Tg (allP≤ 0.043).All these associations remained after adjusting for energy intake,sex and BMI (Table S4).No Sex × TSH/THs/Tg interactions effect on food groups consumption was detected.
3.2 Associations between sedentary behaviour and physical activity with thyroid function
Time spent on vigorous and overall physical activity were negatively related to FT4 levels (B=−0.009;β=−0.227;R2=0.052;P=0.022 andB=−0.003;β=−0.204;R2=0.042;P=0.041,respectively),which was attenuated after adjusting for sex and BMI (allP≤ 0.104,Table 4).These associations were found in women (P≤ 0.070) but not in men (P≥ 0.864) (data not shown).Time spent on light,vigorous and overall physical activity were also associated with the PTFQI (P≤ 0.045,Table 4),which were found in women (P≤ 0.089),but not in men (P≥ 0.095) (data not shown).However,the results were attenuated after adjusting for sex and BMI(P≤ 0.302).No associations were found between sedentary time or PA and TSH,FT3,or Tg (allP≥ 0.062,Table 4).No Sex × TSH/THs/Tg interaction effect on PA levels was detected.
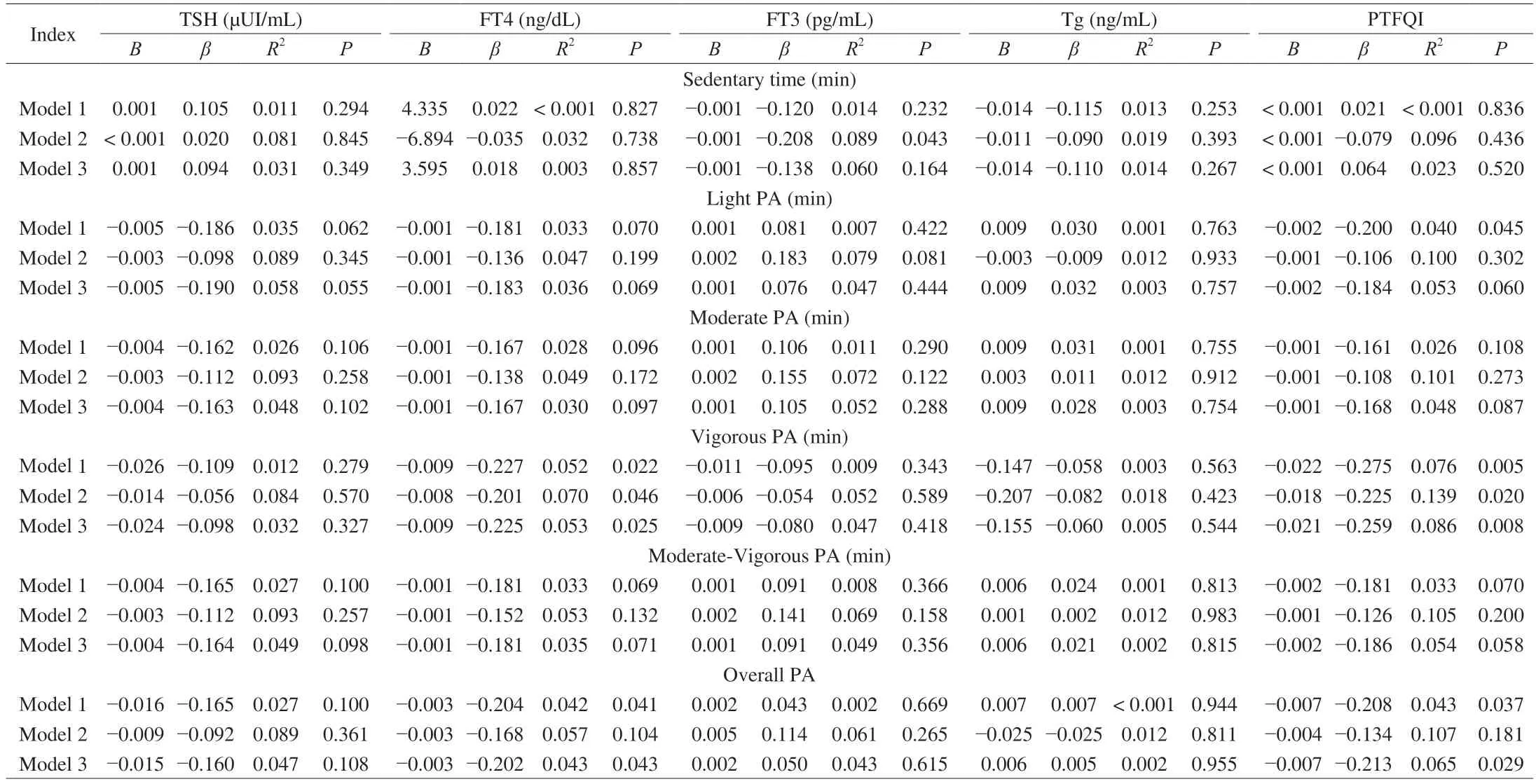
Table 4 Associations between sedentary time and physical activity levels with thyroid function.
3.3 Associations between sleep habits and thyroid function
No associations were found between sleep-related outcomes and TSH,THs,or Tg (allP≥ 0.145,Table 5).Likewise,no Sex × TSH/THs/Tg interaction effects on sleep outcomes were found.
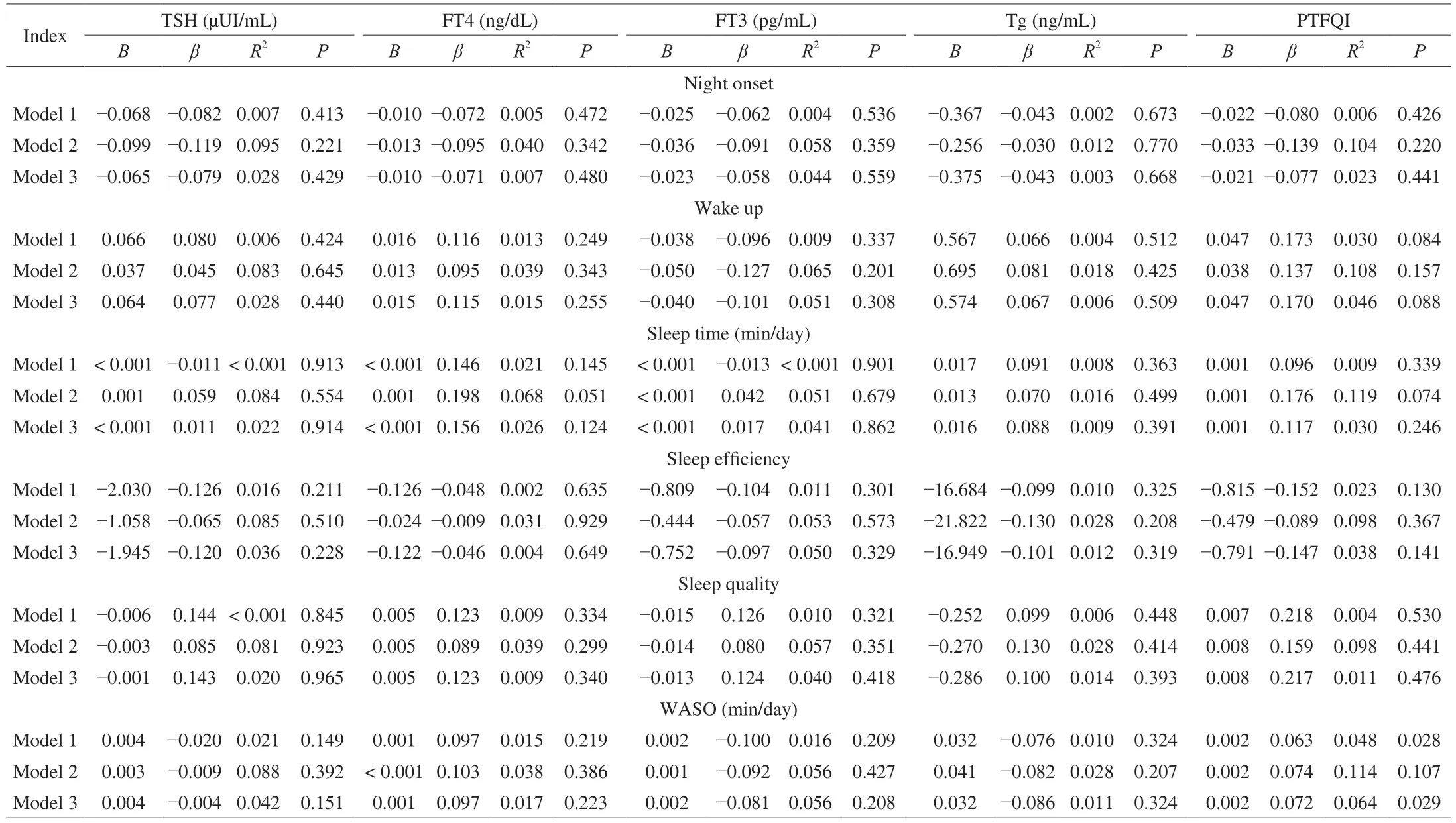
Table 5 Associations between sleep habits and thyroid function.
4. Discussion
The results of this study show that self-reported habitual energy intake is positively associated with TSH and FT3,but energy intake in an ad libitum meal test is not related to TSH,THs or Tg in young euthyroid adults.Additionally,self-reported carbohydrate intake was positively associated with TSH,while fat intake (total fat,SFAs,and MUFAs) was negatively related to TSH levels.Moreover,adherence to the Mediterranean diet was also negatively associated with TSH and FT4 levels.On the other hand,PA levels were negatively associated with FT4 levels.However,no associations were found between sedentary time or sleep habits and thyroid function.These findings suggest that lifestyle factors such as dietary habits and PA levels might modify the thyroid function,even when circulating levels of TSH and THs are within the normal range,in euthyroid young adults.These results are in agreement with data of previous studies observing that lifestyle factors,such as sleep,smoking,diet,exercise and PA,were closely related to thyroid function in euthyroid subjects and with subclinical hypothyroidism[45,46].
Several studies support that decreased THs circulating levels in response to fasting is a relevant adaptive mechanism to preserve the energy during food shortage[47,48].However,the mechanisms involved in the thyroid metabolism regulation during caloric deprivation have not been clarified.In our study,we observed a positive association of self-reported energy intake with TSH and FT3,which concur with others[10].Basolo et al.[10]observed a decrease in serum TSH and FT3 concentrations in response to 36-h fasting.Therefore,the changesproduced in TSH and FT3 levels might indicate an effect of decreased energy intake on both central (TSH response) and peripheral (lower conversion of FT4 to FT3) mechanisms to preserve the energy.Nonetheless,the associations found in our study should be considered with caution,since self-reported energy intake suffers from a very relevant measurement error[49]and we failed to see any association between energy intake,assessed by an ad libitum meal test,and thyroid function.
Variations of diet composition,even in isocaloric conditions,can alter peripheral thyroid hormone metabolism[50,51].Although dietary fat is a crucial component of the human diet[52],excess dietary fat intake has been reported to interfere with the endocrine system[53].We observed a negative association between fat intake (total fat,SFAs,and MUFAs) and TSH levels.A similar trend was observed with FT3 although these results disappeared after adjusting for sex and BMI.This is in accordance,at least in part,with the observations by Otten et al.[51],who documented a decrease of T3 serum levels after highfat diet intervention in humans.The secretion of some gastrointestinal hormones has been documented to be stimulated by dietary fat,amino acids,or glucose[51],which has been speculated to mediate the relation between macronutrient intake and THs metabolism[54].However,no clear relationship between these gastrointestinal hormones and peripheral THs metabolism has yet been established[51].In addition,a high-fat diet rich in saturated and mono-unsaturated fatty acids produce hypothyroxinemia,affecting the lipid profile of the thyroid gland[55].Therefore,dietary fatty acids might influence the fatty acid pool in the thyroid gland,impacting its function.
In addition,studies analyzing the effect of low carbohydrate diets on THs levels have provided heterogeneous results[10,11,50],likely because energy intake was not usually controlled in these studies.A very low carbohydrate diet is known to produce alterations in thyroid hormone metabolism,decreasing the conversion of T4 to T3,and therefore,THs serum levels[56].Contrary,high carbohydrate diets prevent these changes[50].We observed a positive association between carbohydrate intake and TSH levels,which remained after adjusting for energy intake,sex,and BMI.Dietary carbohydrates have been demonstrated to increase the sympathetic nervous system activity[57].Therefore,it is plausible that a high carbohydrate intake could influence the central nervous system activity,stimulating the hypothalamic-pituitary-thyroid axis and increasing the TRH production.Consequently,a rise in TSH levels production might occur,followed by a higher conversion of T4 to T3.
Adherence to the Mediterranean diet has been shown to reduce the risk of suffering cardiometabolic diseases[58].Some reports in the literature have described goitrogenic foods that can affect thyroid function by inhibiting the synthesis of THs[59],such as nuts and vegetables,and specifically cruciferous,or greens,which are consumed abundantly in the Mediterranean diet.In this study,we observed a negative association between adherence to the Mediterranean diet,assessed by two different dietary patterns,and TSH and FT4 levels.We also observed similar associations with some food groups related to Mediterranean dietary pattern such as olive oil and vegetables.These observations are in agreement with the ones reported by Zupo et al.[13],who demonstrated that higher adherence to the Mediterranean diet was independently associated with a reduced thyroid function in euthyroid subjects.
Suddenly my tiredness vanished. I smiled back, and later, when the manager asked me how I d liked my first day, I said, Fine! Those few words of praise had changed everything.
Several micronutrients such as selenium,zinc,iron,copper,vitamin A,vitamin C,and folate,are involved in regulating thyroid function[14].We found a positive association between selenium intake TSH levels,and a negative association between folate and vitamin C intake with FT3 levels.These results are in agreement with the previous evidence that support that several micronutrients are involved in the regulation of THs synthesis and secretion,and protecting the thyroid gland from oxidative stress,interacting with iodine during conversion of the T4 hormone to the metabolically active T3 hormone[14,15].However,our results might seem paradoxical since deficiencies of multiple micronutrients such as iodine,iron,zinc,vitamin A,and folate,seems to exacerbate iodine deficiency and contribute to altered thyroid function.Nevertheless,the paradoxical results and the lack of association between iodine intake and thyroid function,may be due to the limitations of self-reported intake methodology being the urinary iodine concentration the barometer recommended to assess the iodine status[60].
THs have an important effect on numerous systemic actions including muscular and cardiorespiratory function[61].It has been extensively reported that the hypothalamic-pituitary-adrenal axis is stimulated in response to physical activity,and different exercise intensities were also demonstrated to have an important role on thyroid function[18].However,there are few studies exploring the relationship between physical activity levels and thyroid function in euthyroid adults.Tanriverdi et al.[46]demonstrates that women with subclinical hypothyroidism had lower PA levels compared to euthyroid women.We observed a negative association between vigorous and overall PA levels and FT4 concentrations.These results contrast with those reported by Roa Dueñas et al.[61],who found no associations between physical activity and TSH or FT4 levels in adults.More studies are needed to clarify the lack of association between physical activity levels and thyroid function in euthyroid individuals.
TSH and THs secretion can be affected by sleep-wake state[22].In fact,subjects with hyperthyroidism or hypothyroidism suffer numerous sleep disorders,such as insomnia[62].Kim et al.[62]observed an increased risk of subclinical hyperthyroidism (suppression of TSH secretion) in short sleepers,evaluated by a self-reported questionnaire,which contrasts with the lack of association between sleep habits and thyroid function observed in our study.This discrepancy might be explained by the different methodology used to measure the sleep duration,because sleep deprivation was not performed in this study,and due to the participants involved in our study were euthyroid,while in the studies that observed associations between THs and sleep habits,a relatively sleep deprivation was applied to the participants.Moreover,the accelerometers use could be underestimating/overestimating the sleep outcomes,as compared to polysomnography,which is the gold-standard method to properly assess sleep duration and efficiency.
4.1 Limitations
These results need to be interpreted with caution because some limitations are present.First,it is a cross-sectional study and,consequently,no causality can be established.Additionally,only euthyroid young adults are involved in the study,so the results are not extrapolatable to older,younger,or people with thyroid disorders.In addition,blood samples were taken at different times of the day,and due to the circadian variations of THs levels,part of our results could be explained by these variations throughout the day[63].Importantly,other relevant indicators of thyroid function,such as thyroid antibodies (TGAb and TPOAb) were not included in the study.Moreover,despite the largest proportion of the study sample were women,we did not control the menstrual cycle,which might have influenced the results observed.Furthermore,the self-reported dietary intake presents a large measurement error,so the risk of underreporting or misclassification needs to be considered.
4.2 Conclusion
In accordance with the hypothesis proposed,self-reported energy and carbohydrates intake are positively associated with thyroid function,while lipid intake and adherence to the Mediterranean diet are negatively associated with thyroid function,even in young euthyroid adults.Overall and vigorous physical activities are also negatively related to thyroid function,and no relationship seems to exist between sedentarism and sleep habits and thyroid function in young euthyroid adults.
4.3 Clinical implications
The results of the present study suggest that performing intervention studies (e.g.,intermittent fasting,different exercise programs,sleep deprivation/extension,etc.) is important to understand how lifestyle affects to thyroid function in euthyroid adults.Enterprise might bring useful information to the clinical settings since some of these lifestyle factors might be potential strategies for the primary prevention of metabolic diseases.
There are not any competing financial interests in relation to this work.
Acknowledgments
This work is part of a PhD thesis conducted within the framework of the Biomedicine Doctoral Studies Program of the University of Granada,Spain.
This study was funded by the Spanish Ministry of Economy and Competitiveness via the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393),by the Retos de la Sociedad program (DEP2016-79512-R),European Regional Development Funds (ERDF),the Spanish Ministry of Education (FPU13/04365 and FPU19/01609),the Fundación Iberoamericana de Nutrición (FINUT),the Redes Temáticas de Investigación Cooperativa RETIC (Red SAMID RD16/0022),the AstraZeneca HealthCare Foundation,the University of Granada Plan Propio de Investigación 2016-Excellence actions: Unit of Excellence on Exercise and Health (UCEES)-and Plan Propio de Investigación 2018-the Programa Contratos-Puente and Contratos Perfeccionamiento de Doctores,the Junta de Andalucía,Consejería de Conocimiento,Investigación y Universidades (ERDF;ref.SOMM17/6107/UGR),and the Fundación Alfonso Martín Escudero (grant awarded to GSD).
Data availability
The datasets generated and/or analyzed during this study are available upon reasonable request.
Clinical trial registry: NCT02365129 (ClinicalTrials.gov).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250022.
杂志排行
食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing
