Protective mechanism of quercetin compounds against acrylamide-induced hepatotoxicity
2024-02-16LinziLiXueyingLeiLinChenMJunLuoXueoLiuXinglinXuGunghongZhouXinhoFeng
Linzi Li,Xueying Lei,Lin Chen*,Y MJun Luo,Xueo LiuXinglin Xu,Gunghong Zhou,Xinho Feng*
a College of Food Science and Engineering,Northwest A&F University,Yangling 712100,China
b College of Animal Science and Technology,Northwest A&F University,Yangling 712100,China
c Synergetic Innovation Center of Food Safety and Nutrition,Key Laboratory of Meat Processing and Quality Control,Ministry of Education,College of Food Science and Technology,Nanjing Agricultural University,Nanjing 210095,China
Keywords:Quercetin compounds Acrylamide Protection mechanism Oxidative stress Antioxidant activity
ABSTRACT Quercetin compounds have antioxidant,anti-inflammatory and anticancer pharmacological functions.Longterm exposure to acrylamide (AA) can cause liver injury and endanger human health.However,whether quercetin compounds can attenuate AA-induced liver injury and the specific mechanism are not clear.Here,we studied the mechanism and structure-activity relationship of quercetin compounds in reducing AA-induced hepatotoxicity in vivo and in vitro.In vivo studies found that quercetin-like compounds protect against AAinduced liver injury by reducing oxidative stress levels,activating the Akt/mTOR signaling pathway to attenuate autophagy,and improving mitochondrial apoptosis and endoplasmic reticulum stress-mediated apoptosis.In vitro studies found that quercetin compounds protected HepG2 cells from AA by attenuating the activation of AA-induced autophagy,lowering reactive oxygen species (ROS) levels by exerting antioxidant effects and thus attenuating oxidative stress,increasing mitochondrial membrane potential (MMP),and improving apoptosis-related proteins,thus attenuating AA-induced apoptosis.Furthermore,the conformational differences between quercetin compounds correlated with their protective capacity against AA-induced hepatotoxicity,with quercetin showing the best protective capacity due to its strongest antioxidant activity.In conclusion,quercetin compounds can protect against AA-induced liver injury through multiple pathways of oxidative stress,autophagy and apoptosis,and their protective capacity correlates with antioxidant activity.
1. Introduction
Quercetin compounds are a class of natural polyphenolic compounds with antioxidant,anti-inflammatory and anticancer pharmacological functions that are widely distributed in common foods,using quercetin as an aglycon to connect different glycosides[1].Previous studies have shown that quercetin can inhibit platelet aggregation and cytotoxicity[2,3]and can exert antioxidant activity to remove free radicals and protect nerve cells from oxidative stressinduced cell damage[4].In addition,quercetin has a protective effect against liver damage caused by the liver and other harmful substances,such as reversing the decrease in glutathione peroxidase(GSH-Px),catalase (CAT) and superoxide dismutase (SOD)activities and the increase in lipid peroxidation products caused by alcoholic liver disease[5],reducing the liver damage and autophagy flux inhibition caused by chronic ethanol consumption through the mTOR-TFEB pathway and reducing the accumulation of abnormal LC3II and p62[6].The mechanism by which quercetin protects against lead acetate-induced liver injury has also been studied.Quercetin inhibited lead-induced apoptosis by changing the ratio of Bax/Bcl-2 and inhibiting the cleavage level of caspase-3[7].Reports have proven that flavonoids with different structures have different effects on cell proliferation and apoptosis[8,9]and anti-inflammatory and antitumor effects and can scavenge reactive oxygen species (ROS)[10,11].In this study,quercetin,quercetin 3-rhamnoside,quercetin-3-β-Dglucoside and quercetin 3-D-galactoside were used for research.The structure of quercetin is shown in Fig.1.Quercetin 3-rhamnoside is based on the structure of quercetin,and C3is linked to rhamnose to form rhamnoside.In quercetin-3-β-D-glucoside,the C3of quercetin is linked to glucose to form glucoside.Quercetin 3-D-galactoside and quercetin-3-β-D-glucoside have the same molecular weight and number of hydroxyl groups.The difference is that quercetin 3-D-galactoside has a hydroxyl group associated with the C3of quercetin that is connected to galactose.Compared with quercetin,these other three quercetin compounds are connected to different glycosides,so there may be differences in chemical properties.However,no data thus far have demonstrated how flavonoids with different structures affect the hepatotoxicity of acrylamide (AA).
AA,a colorless,odorless crystalline substance formed by the hydration of acrylonitrile that is widely used in the production of various industrial compounds,was first detected in starch-rich foods that were fried and baked in 2002[12].AA may be formed by the Maillard reaction;that is,when the processing temperature is higher than 120 °C,the free aspartic acid and sugar in food will react to form AA[13].Numerous experiments have shown that AA has neurotoxicity,hepatotoxicity,reproductive toxicity and potential carcinogenicity[14,15].In recent years,an increasing number of studies on AA have focused on mitochondrial dysfunction and oxidative stress-induced liver toxicity.The liver is an organ of vertebrates with metabolic function that can digest nutrients,synthesize protein and detoxify[16].Therefore,when harmful substances enter the body,they will enter the liver for catabolism.Many studies have found that after entry into the liver,AA,as a harmful substance from food,may cause damage to the structure and function of the liver.Invivotests have demonstrated that AA gavage or feeding causes biochemical levels of hepatic venous dilatation and congestion,abnormal hepatocyte status,inflammatory cell infiltration and increased bile duct wall thickness[17,18].Studies have also found that AA treatment can cause severe cytotoxicity in HepG2 human liver cancer cells,and the results showed that AA significantly increased the expression of cytochrome(CYP),which may lead to mitochondrial dysfunction,apoptosis and liver injury[19,20].In addition,studies have found that AA can affect mitochondrial respiratory function and inhibit the proliferation and differentiation of HepG2 cells through DNA fragmentation and chromatin damage[21].AA accumulation as a result of long-term exposure to fried or baked food increases the risk of poor health in humans.Therefore,it is very important to find effective methods to reduce the toxicity of AA.In recent years,numerous studies have shown that some plant-derived polyphenols and flavonoids can effectively inhibit the toxicity of AA;for example,curcumin,a plant polyphenol,can reduce the content of ROS to lessen the AA-induced cytotoxicity of HepG2 cells[22].
Therefore,the main aim of this study was to determine how quercetin and flavonoids with similar structures,such as quercetin 3-rhamnoside,quercetin 3-β-D-glucoside,and quercetin 3-D-galactoside,affect the hepatotoxicity induced by AA.This study used Kunming (KM) mice and HepG2 human liver cancer cells asinvivoandinvitroresearch models to assess three signaling pathway-related processes (oxidative stress,autophagy and apoptosis) affected by AA and analyze the quercetin class.The effect of compounds on the changes in cell signaling pathways caused by AA was also assessed,and the protective mechanism and structure-activity relationship of quercetin compounds with different structures in AA-induced hepatotoxicity were studied.This study provides a way to solve the hepatotoxicity caused by AA and provides theoretical support for finding more effective quercetin compounds to reduce the harm of AA.
2. Materials and methods
2.1 Experimental animals and treatments
KM mice (male,25−30 g,the experimental animal center of Xi’an Jiaotong University,Xi’an,China),the most widely reared rat in China,were selected as the subject of this experiment in accordance with the guidelines approved by the Animal Care and Use Committee of Northwest A&F University (approved ID: 2015-1-138).Quercetin(98% purity),quercetin 3-rhamnoside (98% purity),quercetin 3-β-D-glucoside (98% purity),and quercetin 3-D-galactoside (98%purity) were purchased from Aladdin (Shanghai,China).Mice were housed (one animal per cage) under a controlled environment of temperature (20−24 °C) and humidity (40%−60%) with a 12 h light/dark cycle and with food and water ad libitum.After two weeks of adaptive feeding,the mice were randomly divided into 8 groups with 10 mice in each group.The groups were as follows: A:Normal saline was given by gavage for 14 days.B: After intragastric administration of normal saline for 7 days,50 mg/(kg·d) AA was injected intraperitoneally for 7 days.C: Quercetin (10 mg/(kg·d))was intragastrically administered for 7 days.On the 8thday,after quercetin (10 mg/(kg·d)) was intragastrically administered,AA 50 mg/(kg·d) was intraperitoneally injected for 7 days.D: Quercetin 3-rhamnoside (10 mg/(kg·d)) was intragastrically administered for 7 days.On the eighth day,after quercetin 3-rhamnoside(10 mg/(kg·d)) was intragastrically administered,AA 50 mg/(kg·d) was intraperitoneally injected for 7 days.E: Quercetin 3-D-galactoside (10 mg/(kg·d)) was intragastrically administered for 7 days.On the eighth day,after quercetin 3-D-galactoside(10 mg/(kg·d)) was intragastrically administered,AA 50 mg/(kg·d) was intraperitoneally injected for 7 days.F: Quercetin(10 mg/(kg·d)) was intragastrically administered for 7 days.G:Quercetin 3-rhamnoside (10 mg/(kg·d)) was intragastrically administered for 7 days.H: Quercetin 3-D-galactoside(10 mg/(kg·d)) was intragastrically administered for 7 days.
Animals were maintained according to the timing layout of the animal test,as shown in Fig.2,for 2 weeks.The mice were weighed and sacrificed by cervical dislocation 24 h after the last drug gavage.Liver tissue was immediately removed from the mice and thoroughly washed with cold normal saline for subsequent use.
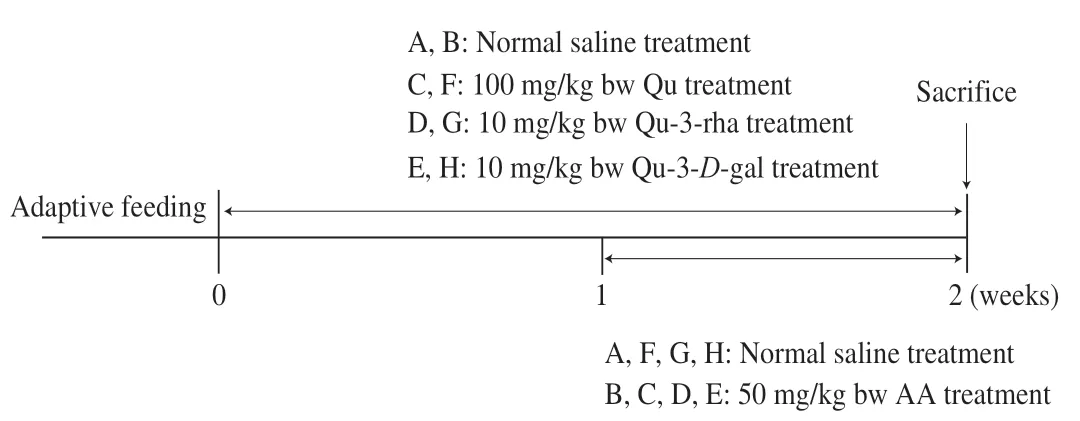
Fig.2 Animal treatment timeline.Qu,quercetin;Qu-3-rha,quercetin 3-rhamnoside;Qu-3-D-gal,quercetin 3-D-galactoside.
2.2 Hematoxylin and eosin (H&E) staining
Liver tissue was fixed in 4% paraformaldehyde for 48 h and then removed for gradient dehydration in ethanol,paraffin embedded and sectioned.Dried section samples were dewaxed and rehydrated in xylene with ethanol,then stained successively with H&E,dehydrated,washed,dried,and finally sealed with neutral glue.
2.3 Immunofluorescence
Tissue paraffin sections were dewaxed in xylene,rehydrated in ethanol,boiled for antigen repair,washed with PBS and incubated with 3% hydrogen peroxide in deionized water for 15 min before blocking endogenous peroxidase.Sections were sealed with 5% goat serum for 30 min,washed with PBS and incubated overnight at 4 °C with the addition of primary antibody (1:100),then incubated with fluorescently labeled secondary antibody for 1 h at room temperature,and finally the coverslip was sealed with DAPI anti-fluorescence attenuation sealing agent,and the slide was photographed with an automatic fluorescence microscope (Lecia,Germany).
2.4 Cell culture
HepG2 cells were purchased from the cell bank of the Typical Culture Preservation Committee of the Chinese Academy of Sciences.They were cultured in minimum essential medium (MEM,HyClone,USA) containing 10% fetal bovine serum (HyClone,USA) and 1%penicillin streptomycin solution (containing penicillin 100 U/mL and streptomycin 100 μg/mL) at 37 °C in an atmosphere of 5% CO2.Quercetin and AA presolubilized in dimethyl sulfoxide (DMSO) were added to the serum-free medium.The test cells were treated in the following manner: quercetin-based compounds were administered as pretreatment for 2 h,AA treatment lasted 24 h,and controls with serumfree medium containing DMSO for the corresponding length of time.
2.5 Cell viability assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.The cells were seeded in 96-well plates at a density of 5 × 104cells/mL.After the cells had adhered to the wall,they were treated with quercetin compounds dissolved in serum-free medium for 2 h and then treated with AA dissolved in serum-free medium for 24 h.After the treatment,MTT was added and incubated for 4 h.Then,the medium was removed,and DMSO (150 μL) was added to dissolve the formazan crystals.The absorbance of the formazan crystals was measured at 490 nm by a Victor X3 multifunctional microplate reader.
2.6 Western blot
Liver tissue proteins were extracted with protein extracts under ice bath conditions,and HepG2 cells were lysed in cell lysis buffer in the presence of protease inhibitor phenylmethanesulfonyl fluoride(PMSF).The protein samples were analyzed by sodium dodecyl sulfate (SDS) gel electrophoresis (BioRad,USA).The PVDF membrane was immersed in methanol for 2 min for activation.Using the semidry transfer method (BioRad,USA) (with an order of transfer filter paper,gel,PVDF film,and transfer filter paper),the transfer time was adjusted according to the molecular weight of the target protein.After the transfer,the membrane was sealed with 5% skim milk for 3 h.TBST (20 mmol/L Tris-HCl,500 mmol/L NaCl,and 0.05% Tween 20) was used to wash the samples 5 times for 8 min each time.A primary antibody corresponding to the target protein was added to the sample,which was then incubated at 4 °C for 12 h.After washing with TBST,the corresponding secondary antibody was selected according to the type of primary antibody,and the sample was placed on a shaker and incubated at room temperature for 2 h.TBST was used to wash the sample again.The PVDF membrane was immersed in enhanced chemiluminescence (ECL) solution (Millipore,Billerica,MA),and the ChemiDox grayscale imaging system and Quantity One software were used to calculate and analyze the gray level of the protein strip.Antibodies against GAPDH,β-actin,mTOR,p-mTOR,AKT,p-AKT,LC3,beclin-1,Atg7,cleaved caspase-3,cleaved caspase-8,Bax,Bcl-2,CHOP,BIP,and p-IRE1 were obtained from Cell Signaling Technology.Antibodies against HO-1 and NQO1 were obtained from Abcam.
2.7 ROS detection
After treatment,ROS in the cells were stained with a ROS detection kit (Beyotime,Shanghai,China) according to the kit instructions.In brief,to a 6-well plate (1 × 106cells/well),1 mL of the fluorescent probe DCFH-DA (diluted with serum-free medium to a final concentration of 10 μmol/L) was added and incubated at 37 °C for 20 min,washed with serum-free medium and observed by fluorescence microscopy (Lecia,Germany).
2.8 MMP detection
After treatment,the cells (2 × 106) were washed with PBS,1 mL serum-free medium and 1 mL JC-1 staining solution were added,mixed thoroughly,and incubated for 20 min.The supernatant was discarded,the remaining cells were washed with JC-1 staining buffer and then 2 mL of serum-free medium was added to observe the samples under a fluorescence microscope (Lecia,Germany).
2.9 SOD and lipid oxidation (malondialdehyde (MDA)) detection
After the cells were processed,a total SOD activity detection kit(employing the WST-8 method) (Beyotime,Shanghai,China) was used to detect the SOD content in the cells.Briefly,cells were washed with precooled PBS at 4 °C.Two hundred microliters of SOD sample preparation were added to a 6-well plate (1 × 106cells/well),properly blown to fully lyse the cells,and centrifuged at 12 000 ×gfor 5 min,and the supernatant was taken as the sample to be tested.The absorbance was measured at 450 nm with a microplate reader (Tecan Austria GmbH,Austria).A lipid oxidation (MDA) detection kit(Beyotime,Shanghai,China) was used to detect the MDA content in the cells.An appropriate amount of standard substance was diluted with distilled water to 1,5,10,50,100,and 200 μmol/L to generate a standard curve according to the kit instructions.The absorbance was measured at 532 nm with a microplate reader(Tecan Austria GmbH,Austria).
2.10 Flow cytometry
After treatment,the apoptotic cells were stained with an annexin V-FITC cell apoptosis detection kit (Beyotime,Shanghai,China).According to the instructions,the cells were collected by digestion with trypsin cell digestion solution and the supernatant was discarded by centrifugation.Then 195 μL of Annexin V-FITC conjugate was added to gently resuspend the cells (1 × 105).Then 5 μL of Annexin V-FITC and 10 μL of propidium iodide staining solution were added successively,gently mixed,and incubated for 20 min at room temperature and protected from light before detection by flow cytometry (PARTEC,Germany).
2.11 Transmission electron microscopy
After treatment,cells were collected by digestion and centrifugation and fixed with 2.5% glutaraldehyde at 4 °C for 24 h.Glutaraldehyde was removed,and the samples were washed with PBS three times and incubated with 1% osmic acid for 4 h at room temperature.Ethanol gradient dehydration was performed.LR White resin was used for embedding and polymerization at 55 °C for 48 h.The sections were stained with 5% uranyl acetate for 20 min and lead citrate for 4 min.The changes in cell internal structure were observed under a transmission electron microscope (FEI,USA).
2.12 Statistical analysis
All data are expressed as the mean ±standard error of mean(SEM).Analysis of variance was performed with GraphPad 6.0,and multiple comparisons were performed with the Duncan’s method.*P<0.05 indicates a significant difference,**P<0.01 indicates a very strong significant difference,and nsP>0.05 indicates no significant difference.
3. Results
3.1 Quercetin compounds have protective effects on AAinduced liver injury in mice
To study what effect AA may have on the livers of mice,H&E staining was used to assess histopathological sections.As shown in Fig.3a,liver sections from the mice in the control group had a normal central venous structure and radial surrounding hepatic cords,and the hepatic cords were separated by hepatic sinusoids.Similarly,treatment with the three quercetin compounds alone did not cause liver disease in the mice (Figs.3f-h),whereas the livers of the AAtreated group of mice exhibited significant pathological changes such as severe venous congestion,infiltration of inflammatory cells and disorganized hepatocyte cords (Fig.3b),indicating that AA has a damaging effect on the livers of mice.As shown in Figs.3c-e,gavage with quercetin,quercetin 3-rhamnoside and quercetin 3-D-galactoside could treat liver injury caused by AA in mice.Venous congestion was improved,and inflammatory cell infiltration was relieved.Only a few inflammatory cells appeared around the veins,and the structures of the hepatocyte cords and hepatic sinusoids were intact.These results indicate that pretreatment with quercetin compounds has a protective effect against liver injury induced by AA.

Fig.3 H&E staining of mouse livers from different treatment groups.(a) Control group (con);(b) acrylamide treatment group (AA);(c) quercetin+acrylamide treatment group (Qu+AA);(d) quercetin 3-rhamnoside+acrylamide treatment group (Qu-3-rha+AA);(e) quercetin 3-D-galactoside+acrylamide treatment group (Qu-3-D-gal+AA);(f) quercetin treatment group (Qu);(g) quercetin 3-rhamnoside treatment group (Qu-3-rha);(h) quercetin 3-D-galactoside treatment group (Qu-3-D-gal).v,central venous structure of the liver;black arrow,inflammatory cells.
3.2 Quercetin compounds can attenuate the oxidative damage in the liver induced by AA
The pathological changes in the mouse and mouse livers induced by AA may be mediated by oxidative stress[23].Therefore,the expression of oxidative stress-related proteins heine oxygenase-1(HO-1) and NAD(P)H:quinone oxidoreductase 1 (NQO1) was examined in the livers of mice from different treatment groups.As shown in Figs.4a-c,the protein expression of HO-1 and NQO1 in the livers of AA-treated mice was significantly increased which could be reversed by oral administration of quercetin,and quercetin alone did not increase the content of HO-1 and NQO1.However,structural differences in quercetin and its glycoside derivatives did not cause significant differences in the protein expression of HO-1 and NQO1.
SOD is an important antioxidant enzyme in biological systems that can eliminate free radicals and maintain cell redox balance.As shown in Fig.4d,quercetin reversed the decrease in SOD induced by AA,and quercetin 3-rhamnoside showed stronger effects on the decrease in SOD induced by AA than quercetin and quercetin 3-D-galactoside.MDA is a lipid peroxide that can reflect the level of lipid peroxidation and thus reflect the oxidative stress of cells.AA increased the MDA content in the liver,while quercetin significantly improved the MDA level (Fig.4e).Moreover,quercitrin and quercetin 3-D-galactoside had stronger effects on the MDA level than quercetin 3-rhamnoside.The results showed that quercetin compounds could reduce AA-induced oxidative stress to varying degrees,which was beneficial for maintaining hepatic redox homeostasis and played a protective role in the liver,among which quercetin 3-rhamnoside had the strongest effect.

Fig.4 Effect of quercetin compounds on various oxidative stress-related indicators affected by AA.(a) Western blot showed that HO-1 and NQO1 protein expression levels in different treatment groups of mice.(b) HO-1 protein gray value analysis.(c) Gray value analysis of NQO1 protein.(d) The content of SOD in different treatment groups of mice.(e) The content of MDA in mice of different treatment groups.Results were presented as mean ± SEM (n=10).*P <0.05;**P <0.01;ns,no statistical significant (P >0.05).The same below.
3.3 Quercetins protect against AA-induced autophagy by affecting the Akt/mTOR pathway
Previous studies have found that AA can not only inhibit autophagy[24]but also enhance autophagy[25],and this apparent contradiction may be related to the intensity of AA treatment.This experiment investigated whether autophagy occurred in the livers of the experimental mice and whether autophagy played a role in the liver injury caused by AA.LC3 is a marker of autophagy.When autophagy is formed,LC3I enzymatically cleaves off a small segment of polypeptide and transforms into LC3II.Compared with the control group,the AA group showed significantly enhanced conversion of LC3I into LC3II (Figs.5a-b),indicating that AA causes autophagy.Similarly,immunofluorescence assay labeling of LC3II showed increased expression of LC3II in the livers of AA-treated mice which was resisted by quercetin compounds (Fig.5g).These results indicate that the ability of quercetin to protect against AA-induced liver injury is related to changes in the autophagy pathway.
Furthermore,to study whether AA has effects upstream of the autophagy pathway,we detected the protein expression of Akt/mTOR signaling pathway factors upstream of the autophagy pathway and the changes in the autophagy pathway after quercetin pretreatment.Western blot results showed that the levels of p-mTOR and p-Akt were remarkably decreased in the AA-treated group compared with the control group,indicating that the pathways upstream of the autophagic pathway were activated and the level of autophagy was significantly increased (Figs.5a,c).Quercetin compounds can inhibit the activation of autophagy by inhibiting mTOR phosphorylation and Akt phosphorylation and reduce AA-induced autophagy (Figs.5a,d).In addition,as shown in Figs.5a,e-f,AA treatment caused a significant increase in the amount of autophagy-related proteins beclin-1 and Atg7 compared with control mice,while quercetin compounds reduced AA-induced autophagy to varying degrees,with quercetin being the most effective in inhibiting autophagy.
3.4 Quercetin compounds can attenuate the apoptosis induced by AA
Autophagy and apoptosis are related to the state of the body.Under normal circumstances,autophagy is the body’s phagocytosis mechanism to eliminate aging-damaged substances,restore the body’s balance,and reduce the occurrence of apoptosis.However,many studies have shown that autophagy and apoptosis have a synergistic effect.Autophagy may promote the occurrence of apoptosis when external stimulation makes autophagy insufficient to maintain balance.Therefore,this experiment further detected indicators related to apoptosis.As shown in Fig.6,AA activated caspase-9 and the downstream apoptotic pathway,resulting in increased expression of the proapoptotic protein Bax and decreased expression of the antiapoptotic protein Bcl-2,which in turn caused cleavage of caspase-3,indicating the onset of apoptosis.Quercetin treatment significantly improved the activation of caspase-3,quercetin 3-rhamnoside did not significantly improve the activation of Bax/Bcl-2 and caspase-9,and quercetin 3-D-galactoside did not significantly improve the activation of caspase-9.Therefore,the effects of quercetins on cellular pathways are complex.In this study,although different quercetin compounds had different inhibitory effects on the activation of caspase-9 induced by AA,they all inhibited the activation of caspase-3 and decreased the apoptosis of liver cells induced by AA.The mechanism of action is worthy of further exploration.

Fig.6 Effects of quercetin on AA-induced apoptosis.(a) Western blot showed that the protein expression levels of Bax,Bcl-2,cleaved-caspase-9,cleavedcaspase-3,BIP,CHOP,p-IRE1 and cleavd-caspase-12 in mice of different treatment groups.(b-h) Bax,Bcl-2,cleaved-caspase-9,cleaved-caspase-3,BIP,CHOP,p-IRE1 and cleavd-caspase-12 protein gray value analysis.
Endoplasmic reticulum stress is closely related to apoptosis,and endoplasmic reticulum stress-related apoptosis is different from mitochondria-mediated apoptosis and involves a unique apoptotic signaling pathway.Some studies have found that ROS may be an upstream signal of endoplasmic reticulum stress,and there is a relationship between ROS and endoplasmic reticulum stress[26].The present pre-experimental study found that AA induced oxidative stress in the livers of mice.Therefore,to determine whether endoplasmic reticulum stress-induced apoptosis is involved in the regulation of AA-induced liver injury,the expression of endoplasmic reticulum stress-related proteins was measured.As shown in Fig.6,BiP,as a key signaling factor of endoplasmic reticulum stress,was significantly increased after AA treatment.AA treatment can also induce IRE1 phosphorylation,activate endoplasmic reticulum stress via the IRE1α-xbp1 pathway,and ultimately activate caspase-12,which can cleave caspase-3 and promote apoptosis.In addition,AA can also induce chop protein expression in the perk pathway to enhance apoptosis.Therefore,AA can induce endoplasmic reticulum stress through activation of multiple pathways.Fig.6 shows that quercetin pretreatment can reduce the expression of proteins related to the endoplasmic reticulum stress pathway and the activation of endoplasmic reticulum stress.In addition,we found that quercetin 3-D-galactoside had no significant effect on the IRE1α-xbp1 pathway,but the three quercetins had significant effects on chop and BiP expression.Similar to mitochondrial apoptosis,the protective mechanisms of quercetin compounds against AA-induced endoplasmic reticulum stress-mediated apoptosis are complex.However,it is worth noting that AA-induced liver injury in mice may be related to apoptosis.Quercetin compounds can improve apoptosis and play a protective role in the liver.
3.5 Effects of AA and quercetin on the viability of HepG2 cells
To detect the effect of AA and quercetin on cell viability,the MTT method was used to detect cell viability.As shown in Fig.7,the cell viability decreased in a dose-dependent manner with increasing AA concentration.When the concentration of AA was more than 1 mmol/L,the cell viability was significantly decreased compared with that of the normal cells;when the concentration of AA reached 16 mmol/L,the cell viability decreased to 52.36% of that of the normal cells.When the cells were treated with 20 μmol/L quercetin compounds,the cell viability did not change significantly.Therefore,the use of 20 μmol/L quercetin compounds in the subsequent treatment process did not cause changes in cell activity.

Fig.7 Effects of different concentrations of AA (a) and 20 μmol/L quercetin compounds on the activity of HepG2 cells (b).Compared with the control,* P <0.05 means significant difference,** P <0.01 means extremely significant,ns,no significant difference (P >0.05) (n=3).Same with Fig.8.
3.6 Quercetin compounds attenuate AA-induced autophagy in HepG2 cells
Autophagy is an important mechanism in cells.In normal cells,autophagy can remove damaged substances and protect cells.When cells are stimulated,autophagy can inhibit apoptosis and protect cells from damage.However,when autophagy is insufficient to respond to external stimulation,it may accelerate cell death[27].To investigate how AA induces autophagy and how quercetin compounds modulate AA-induced autophagy,an autophagy model was developed in this study to determine how AA exerts toxicity in hepatocytes and how quercetin compounds attenuate AA-induced autophagy to protect cells.
In this study,HepG2 cells were treated with different concentrations of AA (1,2,4,8,12,and 16 mmol/L) were set to treat HepG2 cells for 24 h.As shown in Figs.8a-b,when AA concentrations were 1 and 2 mmol/L,the ratio of LC3II/LC3I decreased compared with the control group,indicating that low concentrations of AA inhibited autophagy.In addition,the transformation of LC3 was first increased and then decreased by continuously increasing AA concentration,indicating that 4 and 8 mmol/L AA treatment activates cellular autophagy.Therefore,4 and 8 mmol/L AA were selected as treatment concentrations to establish an AA-induced autophagy model in HepG2 cells.To explore whether quercetin compounds can affect autophagy,this study treated cells with 10 and 20 μmol/L quercetin (ensuring that the concentration of DMSO was within 0.1%) and found that 10 and 20 μmol/L quercetin did not induce activation of LC3,indicating that treatment with this concentration of quercetin did not affect autophagy.In addition,pretreatment with 20 μmol/L quercetin and administration of 4 mmol/L AA significantly reduced the LC3II/LC3I ratio,indicating attenuation of AA-induced autophagy (Figs.8c-d).Based on the above study,an autophagy model of HepG2 cells treated with 4 mM AA and 20 μmol/L quercetin was established for subsequent experiments.
The levels of autophagy pathway-related proteins were detected for different concentrations of AA and quercetin.As shown in Figs.8e-f,pretreatment of cells with the four different quercetin compounds at 20 μmol/L lessened the conversion of LC3 induced by AA;quercetin had the strongest effect,which is consistent with the liver tissue results.Similarly,the results of the immunofluorescence assay for labeling LC3II showed a significant increase in green fluorescence intensity in AA-treated cells,while quercetin pretreatment significantly hindered the activation of LC3 (Fig.8h).In addition,the expression of key signaling factors upstream of the autophagic signaling pathway was detected.AA was found to decrease the phosphorylation of mTOR and enhance autophagy,while the four quercetin compounds improved the phosphorylation level of mTOR and inhibited AA-induced autophagy (Figs.8e,g).

Fig.8 Effects of quercetin compounds on AA-induced autophagy in HepG2 cells.(a and b) The effect of different concentrations of AA and quercetin alone on the expression of LC3 in HepG2 cells.(c and d) LC3 expression levels in HepG2 cells exposed to different concentrations of AA under 10 or 20 μmol/L quercetin pretreatment.(e-g) The expression levels of LC3,p-mtor and mtor in HepG2 cells pretreated with 20 μmol/L quercetin compounds and exposed to AA for 24h.(h) LC3 immunofluorescence in HepG2 cells.
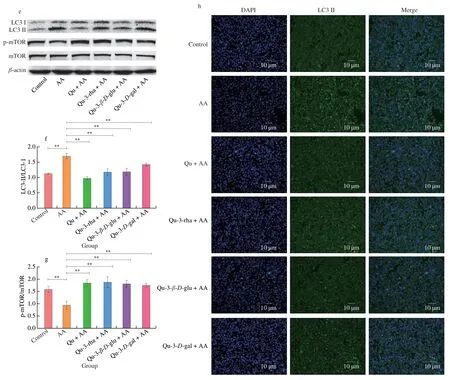
Fig.8 (Continued)
The activation of LC3 causes it to adhere to autophagosomes and serve as an autophagosome structural protein.As observed by transmission electron microscopy,the control group had a normal cell structure with an intact internal structure of the nucleus and organelles and no large number of autophagosomes around the nucleus (Fig.9a).In contrast,double-membrane autophagosome structures were found in the AA-treated group (Fig.9b,arrowheads) and the nucleus was slightly contracted,with marginalized nucleoplasm and some early apoptotic bodies visible,indicating that autophagy was activated in the AA-treated group.Comparatively,the level of autophagy around the nucleus was decreased and the cell structure was relatively intact in the quercetin compound pretreatment group (Figs.9c-f),suggesting that quercetin compound pretreatment could improve AA-induced autophagy.
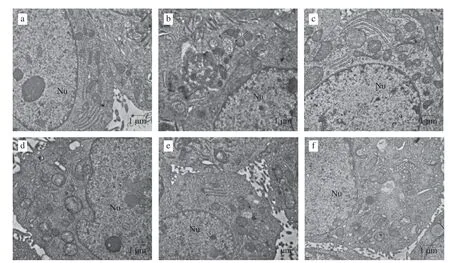
Fig.9 Transmission electron microscopy of HepG2 cells treated with AA and quercetin.Nu: nucleus;the arrow denotes the autophagy with bilayer membrane structure.(a) control;(b) AA;(c) Qu+AA;(d) Qu-3-rha+AA;(e) Qu-3-β-D-glu+AA;(f) Qu-3-D-gal+AA.
3.7 Quercetin can attenuate AA-induced oxidative stress in HepG2 cells
The results of the previous studies in this study revealed that oxidative stress occurs in the mouse liver in the presence of AA,and therefore,the level of oxidative stress was detected in the HepG2 cell autophagy model.ROS are byproducts of aerobic metabolism that regulate physiological processes to adapt to changes in the oxidative environment[28].As shown in Fig.10e,ROS in cells were labeled with a DCFH-DA probe (green fluorescence).Compared with that in the control,the content of ROS increased after AA treatment.Consistent with the results of Cao et al.[22],AA can cause the accumulation of ROS in HepG2 cells,which may cause oxidative damage.When quercetin compounds were used to pretreat cells,the accumulation of ROS induced by AA was alleviated,and this effect may block ROSrelated damage to cells.
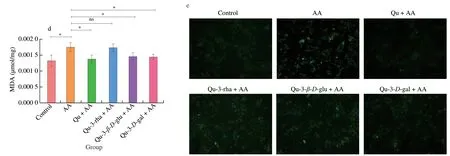
Fig.10 (Continued)
The accumulation of ROS may cause oxidative stress.Detection of oxidative stress-related protein expression revealed that AA treatment upregulated the expression of HO-1 protein and NQO1 protein and increased MDA content,indicating that AA activated oxidative stress in HepG2 cells (Figs.10a-d).All quercetin compounds attenuated the increase in HO-1 protein and NQO1 protein expression,and pretreatment with all three glycosides except quercetin significantly reduced the increase in MDA,indicating that quercetin compounds have a protective effect against AA-induced oxidative stress.Interestingly,quercetin 3-rhamnoside had a better inhibitory effect on AA-induced oxidative stress in the mouse liver than in HepG2 cells,which may be related to the complex structure of organs.The specific mechanism is worthy of further study.
3.8 Quercetin can attenuate AA-induced apoptosis in HepG2 cells
It has been found that an increase in ROS content in cells can lead to a decrease in MMP,change the permeability of the mitochondrial membrane,and cause apoptosis[29].Thus,the JC-1 fluorescent probe detects changes in MMP to assess the level of early apoptosis.When the MMP is high,JC-1 is present as a polymer,producing red fluorescence,and conversely JC-1 is present as a monomer,producing green fluorescence.As shown in Fig.11,AA treatment significantly reduced the aggregation of JC-1 and increased the number of JC-1 monomers in the cells,and these effects reduced MMP,which may lead to apoptosis.In contrast,quercetin compounds significantly inhibited the increase of in JC-1 monomers.Among the 4 compounds,quercetin and quercetin 3-D-galactoside had the most obvious effects in limiting the formation of JC-1 monomers,suggesting that they may have stronger protective effects against AA-induced apoptosis.

Fig.11 JC-1 assessment of the MMP of HepG2 cells.Red fluorescence indicates JC-1 polymer and green fluorescence indicates JC-1 monomer.
To further determine whether quercetin compounds can reduce AA-induced cytotoxicity by affecting cell apoptosis,the expression of apoptosis and endoplasmic reticulum stress-related proteins was detected by Western blot.As shown in Fig.12a,AA treatment significantly increased the activation of caspase-3 and upregulated the expression of the endoplasmic reticulum stress pathway-related proteins chop,BIP and xbp1,while quercetin compound pretreatment significantly improved caspase-3 activation and stunted the upregulation of chop and BIP.Quercetin inhibited xbp1 expression better than other compounds.Therefore,AA can induce apoptosis by affecting endoplasmic reticulum stress,and quercetin pretreatment can reduce apoptosis.
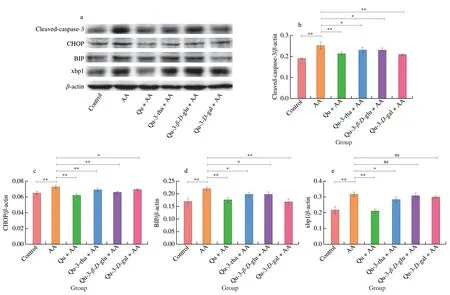
Fig.12 Effects of quercetin compounds on the apoptosis induced by AA in HepG2 cells.(a) Western blot showed that cleaved-caspase-3,CHOP,BIP,xbp1 protein expression levels were in HepG2 cells.(b-e) The gray value analysis of cleaved-caspase-3,CHOP,BIP,xbp1 protein.(f) Flow cytometry showed the apoptosis level of HepG2 cells.
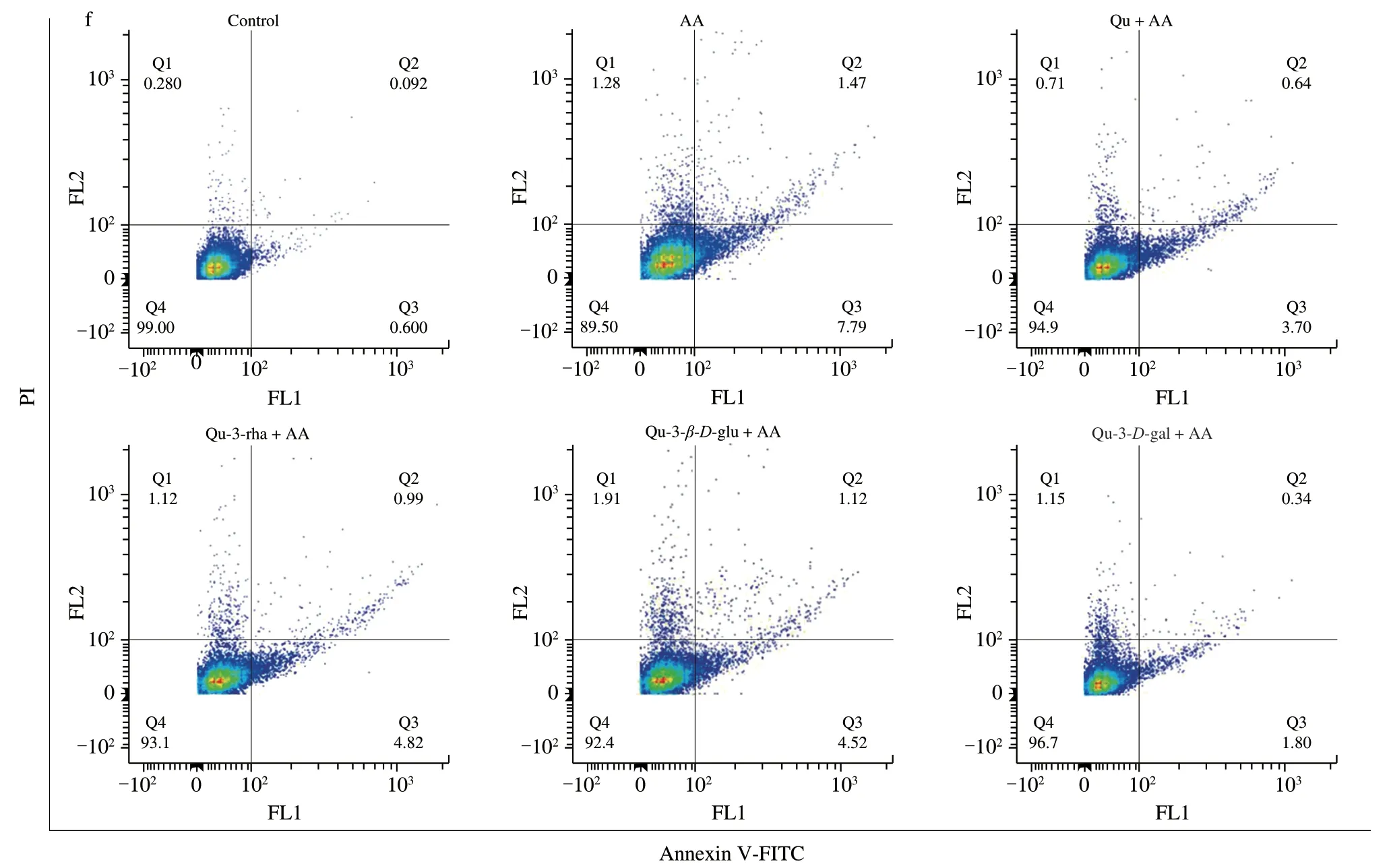
Fig.12 (Continued
In addition,flow cytometry was used to detect the level of apoptosis more accurately to determine whether quercetin compounds can reduce the apoptosis induced by AA.As shown in Fig.12f,compared with the normal cell group,the AA treatment group showed a reduced number of early apoptotic (Q3) and late apoptotic(Q2) cells.Pretreatment with the four quercetin compounds reduced the numbers of early and late apoptotic cells and the quercetin 3-D-galactoside group had the most obvious anti-apoptotic effect,which caused the apoptotic cells to decrease from 9.26% to 2.14%,consistent with the MMP results.Overall,quercetin compounds can play a protective role in AA-induced apoptosis.
4. Discussion
The liver is the main organ of drug metabolism.Toxic substances produced by external or internal metabolism are detoxified in the liver and excreted in bile or urine[30].AA produced by food heating enters the body,causing substantial damage to the liver.Quercetin is a dietary flavonol derived mainly in the form of glycosides[31]and has shown a variety of biological activities,including hypolipidemic,antioxidant and protective effects on liver injury[32].Previous studies have shown that quercetin has a protective effect on oxidative damage and DNA damage in the brain induced by AA[33,34].In addition,quercetin can reverse the changes in SOD content induced by AA in rat serum[35].However,the mechanism by which quercetin compounds protect against the hepatotoxicity of AA has not yet been elucidated.Therefore,in this study,4 different quercetin compounds were used to assess their protective effect against liver injury induced by AAin vivoandinvitro,and the structure-activity relationships of the four quercetin compounds were analyzed.
Invivoexperiments have found that AA treatment leads to pathological changes in liver tissue while upregulating the expression of oxidative stress,autophagy and apoptosis-related proteins.Previous studies have found that AA treatment causes histopathology in mouse liver,and inflammatory cell infiltration,venous and arterial congestion[21],and hepatocyte necrosis were found,in addition to cytoplasmic vacuolation of hepatocytes[36].In the present study,AA treatment was found to induce venous congestion and inflammatory cell infiltration in mouse liver,but no cell necrosis,increased vacuolation and significant abnormalities in the bile ducts or arteries were found in the pathological assessment of the liver sections,which may be related to the treatment time and model.
Oxidative stress is a manifestation of oxidative and antioxidant imbalance,a process that produces excess ROS,increasing the level of lipid peroxidation and causing excessive consumption of antioxidant enzymes[37],which can lead to inflammatory infiltration of neutrophils,production of excessive radicals,and damage to cells and tissues[38].Previous studies have found that AA treatment can significantly upregulate the expression of HO-1 and NQO1,proteins downstream of the oxidative stress pathway[39],decreased SOD activity and reduced MDA content[40,41],causing oxidative damage.In this study,quercetin compounds were able to significantly reduce the AA-induced increase in HO-1 and NQO1 protein expression and improve SOD and MDA levels in mice livers.
Autophagy is a degradation process that ensures cellular stability featuring the transport of damaged organelles or other substances to lysosomes via autophagic vesicles with a double membrane.However,excessive autophagy can increase cell death,called type II programmed cell death.In past studies,AA treatment was found to induce autophagy.Treatment of U2OS cells with 10 nmol/L−10 μmol/L AA for 6 h significantly increased the conversion of LC3I to LC3II and increased the accumulation of p62,which had an effect that was similar to that of the autophagy inhibitor chloroquine (CQ)[24].However,the expression of p62 was decreased and the conversion of LC3 was increased according to Western blot after treatment of PC12 cells for 24 h with 300 μmol/L AA,which proved that AA could cause autophagy[42].In addition to the conversion of LC3,AA can enhance the expression of beclin-1 and activate factors upstream of the autophagy pathway[21].Morin reduces AA-induced testicular toxicity by regulating the Akt/mTOR signaling pathway[43].Therefore,to determine whether autophagy is activated in AAinduced liver injury in mice,we used Western blotting to detect autophagy-related proteins.The results showed that AA activated LC3 and enhanced autophagy.In contrast,quercetin compounds reduced autophagy and reduced the liver injury caused by AA by affecting the AKT/mTOR signaling pathway,decreasing the protein expression of beclin-1 and Atg7 and activating LC3.
Apoptosis is genetically controlled programmed cell death that maintains the homeostasis of cells,and there are 3 main pathways of the death receptor pathway,endoplasmic reticulum stress pathway and mitochondrial pathway[44].Caspases,or cysteine proteases,play a crucial role in the execution of apoptosis,and caspases are activated during apoptosis and undergo a cascade of apoptotic protease reactions.Previous studies have shown that AA treatment can activate the mitochondrial pathway to cause apoptosis[45]and can also affect mitochondrial dysfunction and the release of cytochrome c to increase apoptosis[15].In addition,AA treatment can upregulateCHOPmRNA expression and activate endoplasmic reticulum stress-induced apoptosis[46].In this study,AA could cause apoptosis through the mitochondrial apoptotic pathway,i.e.,increased expression of Bax and decreased expression of Bcl-2 in mitochondria,activation of cleaved caspase-9 to activate the downstream apoptotic pathway,and finally caspase-3 was sheared to cause apoptosis,while quercetin-like compounds significantly improved apoptosis by regulating the expression of Bax and Bcl-2 and inhibiting the activation of caspase-3,the downstream pathway of apoptosis.Previous studies have also found that quercetin can inhibit sodium nitrite-induced apoptosis by changing the expression of Bax and Bcl-2[47].In addition,some studies have found that quercetin can inhibit patulin-induced[48]orα-andβ-zearalenol-induced[49]apoptosis through the endoplasmic reticulum stress pathway.In this study,we found that AA treatment can cause apoptosis through the endoplasmic reticulum stress pathway,i.e.,by increasing the increase of BIP,the signature protein of endoplasmic reticulum stress,and by activating the IRE1α-xbp1 pathway causing increased expression of p-IRE1,cleaved-caspase-12,and thus caspase-3 is sheared to cause apoptosis,and also by activating the expression of CHOP protein of PERK pathway to enhance apoptosis,while quercetin compounds can inhibit apoptosis by suppressing the expression of BIP and CHOP.However,the mechanism of action of quercetin compounds to ameliorate apoptosis is complex,such as the insignificant inhibition of AA-induced activation of caepase-9 and the insignificant inhibition of the endoplasmic reticulum stress IRE1α-xbp1 pathway by quercetin 3-D-galactiside.This may be caused by the difference in bioavailability of different types of glycosides linked to quercetin compounds in mice.In conclusion,invivotests showed that AA can cause liver damage by inducing oxidative stress,autophagy and apoptosis,while pretreatment with quercetin compounds exerted a protective effect on the liver.
Previous studies have found that both quercetin and its glycosides can directly enter cells through transmembrane transport.Therefore,in this study,a model of HepG2 cell autophagy was developed in aninvitroassay to determine how quercetin-like compounds attenuate AA-induced hepatotoxicity at the molecular level.First,the activation of LC3 was used as the assay to determine the concentration of the autophagy model (20 μmol/L quercetin compound and 4 mmol/L AA) to ensure the autophagic activation of HepG2 cells induced by AA treatment and the autophagy inhibitory effect of quercetin.In addition,many studies have shown that AA can induce oxidative damage in HepG2 cells by increasing ROS content[50].In this study,AA treatment was found to increase the level of ROS and induce oxidative stress in HepG2 cells,while the expression of oxidative stress-related proteins HO-1 and NQO1 was increased and MDA content was increased,indicating that AA caused HepG2 cell damage through the oxidative stress pathway.Moreover,increased ROS content in cells leads to a decrease in MMP,causing apoptosis[29].In the present study,AA treatment significantly increased JC-1 monomer in cells thereby downregulating MMP-induced apoptosis and causing apoptosis by upregulating caspase-3 shear,activating the endoplasmic reticulum stress pathway and upregulating the expression of CHOP,BIP,and xbp1 proteins.
Plant polyphenols have a great influence on the content of ROS and the level of oxidative stress factors in cells.Some studies have shown that quercetin has a potential protective effect against the oxidative stress induced by tert-butyl hydroperoxide in HepG2 cells by increasing the SOD,catalase and glutathione catalase contents[51].Other plant polyphenols,such as curcumin[22]and flavonoids with antioxidant functions in blueberry anthocyanin extracts[52]could reduce AA-induced oxidative stress in HepG2 cells and decrease oxidative damage,thus attenuating AA-induced cytotoxicity.In this study,quercetin compounds reduced the level of ROS in cells through antioxidant effects,reduced the occurrence of oxidative stress in cells,and restored the protein expression of HO-1,NQO1 and MDA.In addition,quercetin compounds reduced the number of early apoptotic cells by increasing the MMP and reduced AA-induced apoptosis by increasing the expression of cleaved caspase-3,chop,BIP and xbp1,which are two signaling factors related to granulocyte apoptosis and endoplasmic reticulum stress-mediated apoptosis.Additionally,flow cytometry to detect Annexin V-FITC/PI-stained cells revealed that quercetin compounds reduced AA-induced apoptosis by affecting the number of early and late apoptotic cells.In conclusion,quercetin compounds protect cells by attenuating oxidative stress through antioxidant effects,and by elevating MMP and upregulating endoplasmic reticulum stress-related protein expression to attenuate AA-induced apoptosis.
Autophagy,apoptosis,and oxidative stress do not occur independently and are related in the body.Although the mechanisms of apoptosis and autophagy are different,they share many signaling pathways.The relationship between apoptosis and autophagy is antagonistic,cooperative,and promoting.In many cases,autophagy can maintain cell homeostasis and reduce cell death.Studies have shown that autophagy can reduce endoplasmic reticulum stressinduced apoptosis by leading to protein digestion.Autophagy can also reduce the release of cytochrome c by causing phagocytosis of damaged mitochondria,thereby reducing apoptosis[7].In addition to opposing effects,cooperative effects are also common in the literature.In the prevention and treatment of certain diseases,the use of certain drugs can induce autophagy and apoptosis at the same time.Bromelain induces autophagy and promotes apoptosis of breast cancer cells[53].In addition,as a promoting effect,autophagy does not participate in cell death but provides energy support for apoptosis[54].Oxidative stress is closely related to autophagy and apoptosis.ROS are common factors causing apoptosis and autophagy.ROS can cause mitochondrial damage,reduce the MMP,release cytochrome c,affect the expression of Bax,Bcl-2 and other proteins,cause caspase cascade amplification,and ultimately cause apoptosis[55].However,mitochondrial damage can also affect the activation of adenylateactivated protein kinase (AMPK),which can activate mTOR[56].In addition,ROS can destroy the balance of Ca2+,leading to endoplasmic reticulum stress.Excessive ROS can cause oxidation and modification of proteins in the endoplasmic reticulum;these effects cause the proteins to be retained in the endoplasmic reticulum cavity,resulting in blocked protein synthesis and the depletion of Ca2+in the endoplasmic reticulum,thus causing apoptosis[57].In this study,AA affected autophagy,apoptosis,and oxidative stress pathwaysinvivo,one of which may be affected by ROS.Studies have shown that AA can increase the content of ROS in HepG2 cells and thus cause severe oxidative stress[50].However,excessive ROS may induce autophagy and apoptosis.The different effects of AA on different cellular pathways are still unclear,and the mechanism of action should be further studied.
In addition,this study found that quercetin compounds could reverse AA-induced hepatotoxicity,while the protective effect was differentially influenced by the conformational effect.Compared with other compounds,quercetin had better protective effects against autophagy,apoptosis,and oxidative stress.This finding may be related to the structure of quercetin.As seen in Fig.1,the structural differences in the four quercetin compounds lie in their different C3hydroxyl groups of the C ring.quercetin 3-rhamnoside,quercetin-3-β-D-glucoside and quercetin 3-D-galactoside are linked to rhamnoside,glucoside and galactoside,respectively,where rhamnoside has three hydroxyl groups while glucoside and galactoside have 4 hydroxyl groups.The hydroxyl group at the C3position of the C ring may play an important antioxidative role.Although this point is still controversial,many studies have shown that the antioxidant activity of flavonoids is weakened after the C ring C3hydroxyl group is linked to glycosides[58].Moreover,it was found that the terminal functional groups of antioxidants (primary amino,hydroxyl or aldehyde) may play a role in the reduction of AA[59].Therefore,the better inhibition of AA toxicityinvitroinduced by quercetin may be related to the C3hydroxyl group,while the weaker protective effect of quercetin 3-rhamnoside against AA-induced oxidative stressinvitrocompared with the other two glycoside-substituted quercetins may be related to the number of hydroxyl groups carried by the attached glycosides.
5. Conclusions
In this study,the protective effect of quercetin compounds against AA-induced hepatotoxicity was reflected in the improvement of autophagy,apoptosis and oxidative stress,while quercetin had a better protective effect.This protective effect may be mainly related to the strong antioxidant capacity of quercetin,and the relationship between conformational differences and protective effects is complex and needs further investigation due to differences in their bioavailability.
Conflict of interest
Xuebo Liu and Guanghong Zhou are the editorial board members forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.The authors have no conflicts of interest to disclose.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32 072142;31972099) and Guangxi Science and Technology Base and Talent Special Projects (Guike AD21220004).We thank American Journal Experts (AJE) for the assistance with the English language.
杂志排行
食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing
