Milk fat globule membrane supplementation protects against β-lactoglobulininduced food allergy in mice via upregulation of regulatory T cells and enhancement of intestinal barrier in a microbiota-derived short-chain fatty acids manner
2024-02-16HnGongTingeLiDongLingJingxinGoXiohnLiuXueyingMo
Hn Gong,Tinge Li,Dong Ling,Jingxin Go,Xiohn Liu,Xueying Mo,*
a Key Laboratory of Functional Dairy,Ministry of Education,College of Food Science and Nutritional Engineering;China Agricultural University,Beijing 100083,China
b Henan Engineering Technology Research Center of Food Processing and Circulation Safety Control,College of Food Science and Technology,Henan Agricultural University,Zhengzhou 450002,China
c Applied Nutrition I,China National Center for Food Safety Risk Assessment,Beijing 100022,China
Keywords:Cow’s milk allergy Milk fat globule membrane Gut microbiota Short-chain fatty acid G protein-coupled receptor Regulatory T cell
ABSTRACT Milk fat globule membrane (MFGM),which contains abundant glycoproteins and phospholipids,exerts beneficial effects on intestinal health and immunomodulation.The aim of this study was to evaluate the protective effects and possible underlying mechanisms of MFGM on cow’s milk allergy (CMA) in a β-lactoglobulin (BLG)-induced allergic mice model.MFGM was supplemented to allergic mice induced by BLG at a dose of 400 mg/kg body weight.Results demonstrated that MFGM alleviated food allergy symptoms,decreased serum levels of lipopolysaccharide,pro-inflammatory cytokines,immunoglobulin (Ig) E,IgG1,and Th2 cytokines including interleukin (IL)-4,while increased serum levels of Th1 cytokines including interferon-γ and regulatory T cells (Tregs) cytokines including IL-10 and transforming growth factor-β.MFGM modulated gut microbiota and enhanced intestinal barrier of BLG-allergic mice,as evidenced by decreased relative abundance of Desulfobacterota,Rikenellaceae,Lachnospiraceae,and Desulfovibrionaceae,while increased relative abundance of Bacteroidetes,Lactobacillaceae and Muribaculaceae,and enhanced expressions of tight junction proteins including Occludin,Claudin-1 and zonula occludens-1.Furthermore,MFGM increased fecal short-chain fatty acids (SCFAs) levels,which elevated G protein-coupled receptor(GPR) 43 and GPR109A expressions. The increased expressions of GPR43 and GPR109A induced CD103+dendritic cells accumulation and promoted Tregs differentiation in mesenteric lymph node to a certain extent.In summary,MFGM alleviated CMA in a BLG-induced allergic mice model through enhancing intestinal barrier and promoting Tregs differentiation,which may be correlated with SCFAs-mediated activation of GPRs.These findings suggest that MFGM may be useful as a promising functional ingredient against CMA.
1. Introduction
Cow’s milk allergy (CMA) is an immunologically mediated response to milk proteins including caseins,α-lactalbumin andβ-lactoglobulin (BLG).CMA leads to various adverse anaphylactic reactions such as gastrointestinal disorders,runny nose and wheezing[1].Up to now,no effective strategies and interventions have been developed,and the primary management of CMA involves strictly avoiding relevant allergens.However,it is difficult to avoid allergens completely and it will affect nutrient intake and reduce life quality.Modern processing techniques such as thermal treatment,fermentation and enzymatic hydrolysis are used for reducing the antigenicity of milk proteins,while allergen reactivity cannot be completely eliminated[1].Hence,the development of safe and effective measures is extremely urgent and relies on the understanding of CMA mechanisms.CMA is caused by allergens stimulating naive T cells differentiate into T helper (Th) 2 cells,which further induce B cells to produce immunoglobulin (Ig) E,resulting in the mast cells degranulation and release of histamine,mast cell protease and inflammatory cytokines[2].As major immunosuppressive cells,regulatory T cells (Tregs) express transcription factor forkhead box protein 3 (Foxp3) and inhibit Th2 immune response[3].Dendritic cells(DCs) are critical to the immune responsive directions via determining the way of T cell differentiation[4].CD103+DCs in mesenteric lymph node (MLN) express the retinaldehyde dehydrogenase-2 (RALDH2)(encoded by Aldh1a2) and indoleamine 2,3-dioxygenase (IDO),promoting Tregs differentiation[5,6].Besides,increased intestinal permeability has been identified as risk factor for developing food allergy and maintaining intestinal barrier is beneficial to protect against food allergy by suppressing allergen permeation[7].Therefore,to prevent and alleviate CMA,inhibition of Th2 immune response by promoting Tregs differentiation and suppression of allergen permeation via enhancing intestinal barrier are of great importance.
Gut microbiota has been proven to be involved in the intestinal mucosal barrier and immune response[8].The richness and diversity of the gut microbiota might be reduced or not significantly different in food allergic patients compared to healthy individuals[9-11].Food allergy could be alleviated via regulating gut microbiota metabolitesmediated signaling pathways[5,12,13].G protein-coupled receptors(GPRs) GPR43 and GPR109A are expressed on immune cells and epithelial cells.And they are critical signaling molecules in the immune response,inflammation regulation and intestinal barrier function[14].GPR43 and GPR109A facilitated dietary fiber-induced intestinal homeostasis via regulating inflammasome signaling,thus promoting epithelial barrier integrity[15].GPR109A or GPR43 deficiency caused severe food allergy symptoms,intestinal barrier damage and decreased proportions of CD103+DCs and Tregs in MLN[5,16].Short-chain fatty acids (SCFAs),fermented by commensal gut microbiota,bind GPRs to maintain intestinal barrier and modulate immune responses[17].Butyrate elevated tight junction (TJ) proteins expressions and protected intestinal barrier integrity in a GPR43-dependent manner[18].Human milk-derived SCFAs protected against food allergy through enhancing intestinal barrier and inhibiting Th2 cytokines production[19].Thus,enhancing intestinal barrier and promoting Tregs differentiation through SCFAs-mediated activation of GPRs represent the attractive strategy for the prevention and alleviation of CMA.
Milk fat globule membrane (MFGM),a natural tri-layered membrane surrounding the fat globules in milk,contains bioactive glycoproteins and phospholipids[20].MFGM and related metabolites modulated immune responsesinvitroand played an important role in infant gut health and immunity[21,22].MFGM regulated gut microbiota accompanied by the increased fecal SCFAs levels and the level of GPR43 in the intestinal tract[23].In our previous study,MFGM supplementation increased TJ proteins and alleviated intestinal inflammation in obese mice,thereby enhancing intestinal barrier[24].MFGM restored the intestinal development through increasing TJ proteins expressions,promoting intestinal proliferation and differentiation in infant formula-fed pups[25].Based on these studies,MFGM could exert beneficial effects on immunomodulation and intestinal barrier.However,whether MFGM could alleviate BLGinduced CMA through modulating immune responses and enhancing intestinal barrier remains undefined.
Therefore,the aim of this study was to investigate the effects of MFGM on intestinal barrier and gut microbiota in a BLG-induced allergic mice model.Then,gut microbiota-derived metabolites SCFAs were examined.Additionally,whether the alternations of gut microbiota could modulate immune responses were also explored to further clarify the alleviation of CMA in a BLG-induced allergic mice model after MFGM supplementation.
2. Materials and methods
2.1 Materials
Anti-CD4-AF488,anti-CD25-PE-CY7,anti-CD127-PE,anti-MHC-Ⅱ-PE,anti-CD11c-FITC,anti-CD103-AF647 and anti-mouse CD16/32 (clone 93) antibodies were obtained from BioLegend Inc.(San Diego,CA,USA).Primary antibody against GPR109A was purchased from Novus Biologicals Inc.(Littleton,CO,United States).Primary antibody against GPR43 was purchased from Sigma-Aldrich Inc.(St.Louis,MO,USA).Primary antibodies against zonula occludens (ZO)-1,Claudin-1 and Occludin were purchased from Thermo Fisher Scientific Inc.(Waltham,MA,USA).Primary antibody againstβ-actin was obtained from Bioss Inc.(Beijing,China).Bovine BLG (≥ 90% PAGE),incomplete and complete Freund’s adjuvant were purchased form Sigma-Aldrich Inc.(St.Louis,MO,USA).Lipopolysaccharide (LPS),interleukin-1β(IL-1β),interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)enzyme-linked immunosorbent assay (ELISA) kits were obtained from Enzyme-linked Biotechnology Co.,Ltd.(Shanghai,China).Mouse mast cell protease-1 (mMCP-1) and histamine ELISA kits were obtained from Dogesce Biotechnology Co.,Ltd.(Beijing,China).Transforming growth factor (TGF-β) and interleukin-10(IL-10) ELISA kits were obtained from eBioscience Inc.(San Diego,CA,USA).IFN-γ and IL-4 levels ELISA kits were obtained from Neobioscience Biotechnology Co.,Ltd.(Shenzhen,China).Total IgE and IgG1 ELISA kits were obtained from Immunology Consultants Laboratory Inc.(Newberg,OR,USA).Trizol reagent was obtained from Tiangen Biotech Co.,Ltd.(Beijing,China).RIPA lysis buffer was purchased from Biotechnology Co.,Ltd.(Haimen,Jiangsu,China) and protease and phosphatase inhibitor were purchased from Sigma-Aldrich Inc.(St.Louis,MO,USA).Polyvinylidene fluoride(PVDF) membrane was purchased from Millipore Corp.,(Bedford,MA,USA).
2.2 Preparation of MFGM
MFGM was obtained as previously described with slight modifications[26].Briefly,raw milk was centrifuged and skimmed to obtain cream.Then,cream was washed,churned,and filtered to obtain butter and buttermilk,respectively.Subsequently,butter was melted and centrifuged at 1 800 ×gfor 10 minutes to obtain butter sera.Buttermilk and butter sera were mixed and freeze-dried to obtain MFGM.
2.3 Animal experiments and BLG-induced food allergy model
Four to five-week-old female BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co.,Ltd (Beijing,China).All animals were maintained in a specific-pathogen-free environment ((22 ± 2) °C,12-h light-dark cycle).All animals received food (Research Diets,New Brunswick,NJ,USA) and water ad libitum and were acclimated for 1week before immunizations.
Mice were randomly divided into 3 groups: control group (CON),BLG-allergic group (BLG) and BLG-allergic mice supplemented with MFGM group (BLG+MFGM).The protocol was performed for sensitization and challenge as previously described (Supplement Fig.1)[27].Mice in BLG and BLG+MFGM group were sensitized with 0.2 mL of 2 mg/mL allergen (mixed with complete Freund’s adjuvant) by i.p.injection on day 0.Then incomplete Freud’s adjuvant was used to replace the complete Freund’s adjuvant on days 7,14 and 21.Subsequently,mice were challenged with BLG(50 mg/mouse) by oral gavage on day 28.Mice in BLG+MFGM group were orally administrated with MFGM at 400 mg/kg body weight 4 times per week from day 0 to 28.Loose stools rate and rectal temperature were recorded during 0–60 min after the oral challenge.The method for determining the diarrhea status of mice is as follows:Each mouse in each group was placed in a cage individually and their stools were counted.The proportion of loose stools in the total stools of each mouse were calculated.The proportion of mice in each group was averaged and standard error of mean was calculated[28].Besides,the anaphylactic symptoms of mice were evaluated according to the method as previously described[29].Briefly,grading was based on the following criteria: 0-no symptoms;1-pilar erect,scratching and rubbing around the nose and head;2-decreased activity,puffiness around the eyes and mouth;3-static activity time exceeds 1 min;4-arching from the back,cyanosis around the mouth and the tail;5-convulsion or death.

Fig.1 MFGM supplementation alleviated characterizations of food allergy in a BLG-induced allergic mice model.(A) Body weight on day 28 (at the end of the experiment). (B) Loose stools rate,(C) rectal temperature and (D) anaphylactic symptom score were recorded after oral challenge.(E) Serum total IgE,(F) BLG-specific IgE,(G) IgG1,(H) mMCP-1 and (I) histamine levels were determined.(J) Representative TB staining in jejunum showed mast cells infiltration in intestinal epithelium (K) with corresponding quantification data.Red arrows indicated the mast cells.Magnification: 100×.TB: toluidine blue.Different letters indicate significant difference (P <0.05) (n=6 except n=10 for loose stools rate).MFGM: milk fat globule membrane;BLG: BLG-allergic group;BLG+MFGM:BLG-allergic mice supplemented with MFGM group.
Subsequently,mice were euthanized and blood samples and tissues were collected for analysis.Spleen and thymus indices were calculated using the following formula (1) and (2):
The procedure of the animal experiment was approved by the Animal Ethics Committee at China Agricultural University (the ethical review serial number is AW51101202-4-8).
2.4 Serum biochemical parameters analysis
Serum total IgE,IgG1,mMCP-1,histamine,LPS,IL-1β,IL-6,TNF-α,IFN-γ,IL-4,TGF-β and IL-10 levels were determined by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocols.Serum BLG-specific IgE was measured as previously described.In brief,96-well plates were coated with BLG in coating buffer.Serum samples were diluted prior to being used in the assays.Then,washing,blocking,and incubation were performed,respectively.The absorbance was determined at 450 nm after adding stop solution within 15 min[30,31].
2.5 Intestinal histology evaluations
Hematoxylin and Eosin (H&E) and toluidine blue (TB) staining of jejunum tissues were performed as previously described[12].Jejunum tissues were fixed in 4% paraformaldehyde.After dehydration using graded ethanol,tissues were embedded in paraffin and cut into a thickness.Subsequently,the tissue sections were stained with H&E for observation of intestinal mucosal structure.In addition,the tissue sections were stained with TB to investigate the distribution of mast cells.The section images were observed using an inverted light microscope (Axio Vert.A1,Carl Zeiss Microscopy GmbH,Germany).The number of mast cells was quantified at high power field (100×)and at least 3 images per section were analyzed.
2.6 Fecal SCFAs analysis
The fecal SCFAs levels were determined using the method as previously reported[32].Briefly,feces were mixed with double distilled H2O and 5% (V/V) H2SO4.Then,samples were added with ether and centrifuged at 13 200 ×gfor 20 min.The supernatant was obtained to analyze the SCFAs levels via a gas chromatography-mass spectrometer (GCMS-QP2010 Ultra system,Shimadzu Inc.,Kyoto,Japan) with a Rtx-Wax column (30 m × 0.25 mm,0.25 μm).Helium was used as the carrier gas at 1.0 mL/min.The injection temperature was 240 °C and the ionization temperature was 220 °C.
2.7 Flow cytometry analysis
Single cell suspensions isolated from MLN were stained for flow cytometry analysis as previously reported[33].Briefly,the fresh MLN from mice were minced aseptically by syringe coring and filtered by mesh cell strainer to obtain MLN single cell suspensions.Subsequently,cell suspensions were washed and suspended in sterile PBS,and stained with anti-mouse CD16/32 (clone 93,dilutions ration is 1:50 and final concentration is 0.01 μg/μL),anti-MHC-Ⅱ-PE,anti-CD11c-FITC and CD103-AF647 for CD103+DCs analysis.The gating strategy of CD103+DCs is as follows: Briefly,MHC-Ⅱ+(PE) cells were gated using the 585/15 optical filter (561 nm laser)after excluding doublets and eliminating false signals.Subsequently,CD11c+(FITC) CD103+(AF647) cells were gated using the 515/20(488 nm) optical filter and 660/20 (633 nm laser) optical filter.For Tregs analysis,cells were stained with anti-CD4-AF488,anti-CD25-PE-CY7,and anti-CD127-PE.The gating strategy of Tregs is as follows: Briefly,CD4+(AF488) cells were gated using the 515/20(488 nm) optical filter after excluding doublets.Then,CD25+(PECY7) CD127-/low(PE) cells were gated using the 780/60 (561 nm laser) optical filter and 585/15 (561 nm laser) optical filter.Data were analyzed using BD FACSCalibur instrument (BD Biosciences Inc.,San Jose,CA,USA) and FlowJo 10.0.7 software (Tree Star Inc.Ashland,OR).
2.8 Quantitative real-time PCR
Total RNA was extracted from jejunum using Trizol reagent and cDNA was synthesized using cDNA Synthesis Kit (ABM Inc.,Richmond,BC,Canada).RT-PCR was conducted by a Techne Quantica real-time PCR detection system (Techne,Stone,Staffordshire,UK)[34].The thermal profile is 95 °C for 180 s,followed by 40 cycles of 95 °C for 5 s,60 °C for 30 s,and 72 °C for 30 s.The primers for Aldh1a2,IDO,TGF-β,IL-10,Foxp3 andβ-actin were synthesized by Invitrogen Inc.(Carlsbad,CA,USA).The mRNA levels of the target genes were normalized toβ-actin mRNA levels and the primer sequences are listed in Table 1.
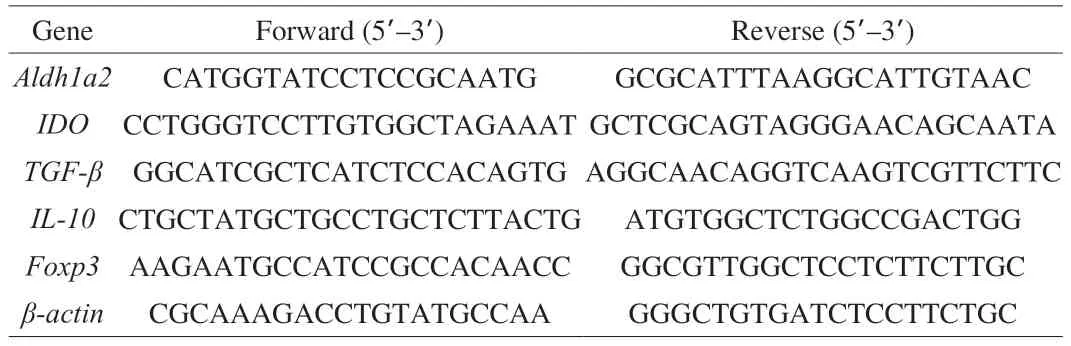
Table 1 Primer sequences used for real-time quantitative PCR.
2.9 Western blot
Jejunal samples were homogenized in RIPA lysis buffer with protease and phosphatase inhibitor to isolate protein.The homogenate was centrifuged at 12 000 ×gfor 15 min to remove insoluble material.Protein concentration was measured by bicinchoninic acid protein assay kit (Thermo Fisher Scientific Inc.,Waltham,MA,USA).Then,the proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes.Subsequently,the PVDF membranes were blocked and incubated with different primary antibodies overnight at 4 °C.After that,the membranes were incubated with secondary antibodies for 1 h at room temperature.The immunoreactive bands were detected with enhanced chemiluminescence reagents and the band intensities were quantified via Image J software (National Institutes of Health,Bethesda,MD,USA).
2.10 Microbiota analysis by 16S rRNA gene sequencing
2.10.1 BacterialgenomicDNAextractionandamplification andIlluminaMiSeqsequencing
Total genome DNA was extracted from mouse feces using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek,Norcross,GA,USA)according to the manufacturer’s instructions.PCR reactions were conducted as previously described.Briefly,the V3 to V4 regions of the bacterial 16S rRNA gene were amplified in accordance with a previous study[25].PCR reactions were conducted in 20 μL mixture including 10 ng DNA template,0.4 μL FastPfu Polymerase,0.8 μL each primer (5 μmol/L),2 μL dNTPs (2.5 mmol/L),and 4 μL 5 × FastPfu Bufferd and reaction system was carried out under the following procedure: 95 °C for 120 s,at 95 °C for 30 s by 27 cycles,55 °C for 30 s and 72 °C for 45 s and finally 72 °C for 10 min.The resulted PCR amplicons were extracted from 2% agarose gels and then purified via AxyPrep DNA Gel Extraction kit (Axygen Biosciences Inc.,Union City,CA,USA) followed by quantified using QuantiFluorTM-ST.Paired-end sequencing was conducted on the Illumina MiSeq platform (Illumina,San Diego,USA) according to the manufacturer’s protocol and the sequencing depth is at least 30 000.
2.10.2 Bioinformaticsanalysis
The bioinformatics analysis was performed according to previously reported[35].Raw fastq files were demultiplexed and quality-filtered by QIIME (version 1.17).Then,tags were assigned to operational taxonomic units using USEARCH (v7.0.1090) at 97%similarity.Subsequently,the 16S rRNA database was used to annotate taxonomic information via Ribosomal Database Project Classifier(version 2.2).α-Diversity including Chao and Shannon index was analyzed with QIIME andβdiversity was analyzed with principal coordinates analysis (PCoA) to further distinguish the between-sample diversity.The correlations between gut microbiota and representative serum biochemical parameters were carried out based on Spearman’s rho non-parametric correlation analysis.Additionally,PICRUSt analysis was performed to predict gene functional profiles of bacterial communities,and to determine compositions of the KEGG pathway(class 3) in the bacterial population.
2.11 Statistical analysis
Data were represented as mean ± standard error of mean (SEM).The statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison test by SPSS software(version 20.0,IBM Inc.,Chicago,IL,USA).Pvalue <0.05 were considered statistically significant.
3. Results
3.1 MFGM supplementation alleviated characterization of food allergy in a BLG-induced allergic mice model
As shown in Fig.1A,BLG group had lower body weight than CON group at the end of the experiment.MFGM supplementation increased body weight as compared with BLG group.Allergy symptoms including loose stools rate,rectal temperature and anaphylactic symptom score were determined.As shown in Fig.1B,loose stools rate was increased in BLG group,while MFGM supplementation reduced the loose stools rate.As shown in Fig.1C,the rectal temperature was reduced rapidly in BLG group compared with that in CON group which exhibited stable rectal temperature.However,MFGM supplementation showed a slower reduction in rectal temperature compared to BLG group.As shown in Fig.1D,the anaphylactic symptom score in BLG group was higher than that in the CON group,while MFGM supplementation decreased the anaphylactic symptom score.Serum total IgE,IgG1,BLG-specific IgE,mMCP-1,histamine levels were increased in BLG group compared to those in CON group,which were reversed by MFGM supplementation (Fig.1E-1I).The mast cells in the jejunum of mice were observed using TB staining.As shown in Figs.1J and 1K,the number of mast cells was increased in BLG group compared to that in CON group,while MFGM supplementation decreased the number of mast cells.
Additionally,spleen index was increased in BLG group,which was reduced by MFGM supplementation.However,there was no significant difference in thymus index among all groups (Supplement Figs.2A and 2B).Inflammatory cytokines including serum LPS,TNF-α,IL-1β,and IL-6 levels were increased in BLG group compared to those in CON group.MFGM supplementation decreased these inflammatory cytokines levels (Supplement Table 1).
3.2 MFGM supplementation regulated the Th1/Th2 responses imbalance in a BLG-induced allergic mice model
As shown in Table 2,BLG group exhibited decreased serum IFN-γ level,whereas showed increased serum IL-4 level compared with CON group.However,MFGM supplementation ameliorated these abnormalities.Serum levels of IFN-γ and IL-4 were determined by ELISA kits.Different letters indicate significant difference (P<0.05) (n=6).BLG: BLG-allergic group;BLG+MFGM: BLGallergic mice supplemented with MFGM group;IFN-γ: interferon-γ;IL-4: interleukin-4.
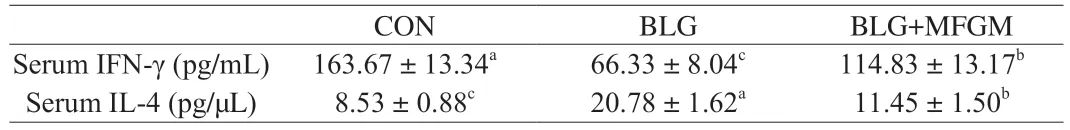
Table 2 Effect of MFGM on Th1/Th2 responses imbalance in a BLG-induced allergic mice model.
3.3 MFGM supplementation induced CD103+ DCs accumulation and promoted Tregs differentiation in MLN in a BLG-induced allergic mice model
As shown in Fig.2A and 2B,the proportion of MHC-Ⅱ+CD11c+CD103+in MLN was increased in BLG+MFGM group compared to that in CON and BLG groups.However,there was no significant difference in proportion of CD103+DC in MLN between CON and BLG groups.As shown in Fig.2C,the mRNA levels of Aldh1a2 and IDO were increased in jejunum in BLG+MFGM group compared to those in CON and BLG groups.Nevertheless,no significant differences were observed regarding the mRNA levels of Aldh1a2 and IDO in jejunum between CON and BLG groups.
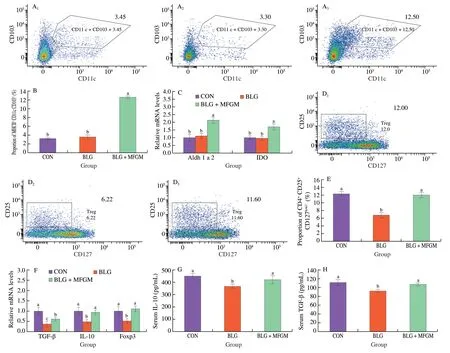
Fig.2 MFGM supplementation induced CD103+ DCs accumulation and promoted Tregs differentiation in MLN in a BLG-induced allergic mice model.(A)Representative flow cytometry plots of CD103+ DCs and (B) Percentages of CD103+ DCs in MLN.(C) The mRNA expressions of Aldh1a2 and IDO in jejunum.(D) Representative flow cytometry plots of Tregs and (E) Percentages of Tregs in MLN.(F) The mRNA levels of TGF-β,IL-10 and Foxp3 in jejunum.(G) Serum IL-10 and (H) TGF-β levels were determined.Different letters indicate significant difference (P <0.05) (n =6).MFGM: milk fat globule membrane;BLG: BLG-allergic group;BLG+MFGM: BLG-allergic mice supplemented with MFGM group;DCs: dendritic cells;Tregs: regulatory T cells;MLN: mesenteric lymph nodes.Aldh1a2: aldehyde dehydrogenase 1 family member A2;IDO: indoleamine 2,3-dioxygenase;TGF-β: transforming growth factor-β;IL-10:interleukin-10;Foxp3: forkhead box protein 3.
Then,effects of MFGM on Tregs differentiation were explored in MLN in a BLG-induced allergic mice model.The proportion of CD4+CD25+CD127low/−in MLN was decreased in BLG group compared to that in CON group.However,the proportion of CD4+CD25+CD127low/−in MLN was increased in BLG+MFGM group compared to BLG group (Figs.2D and 2E).BLG group showed lower mRNA levels of Tregs-associated cytokines (TGF-β and IL-10) and transcription factor (Foxp3) in jejunum compared to CON group,while MFGM supplementation increased these mRNA levels in jejunum compared to BLG group (Fig.2F).Serum Tregs cytokines including IL-10 and TGF-β levels were also determined.BLG group had a lower IL-10 and TGF-β levels compared to CON group,while MFGM supplementation reversed the trend (Figs.2G and 2H).
3.4 MFGM supplementation promoted intestinal epithelium integrity and enhanced intestinal barrier in a BLG-induced allergic mice model
As shown in Fig.3A,signs of severe mucosal damage occurred in the BLG group.The villous shape was irregular,shortened and atrophic.Besides,mucosal epithelial cells were necrotic and exfoliated.However,mucosal damage was alleviated in jejunum of BLG+MFGM group compared to that of BLG group.As shown in Figs.3B and 3C,BLG group showed a decrease in the protein expression levels of Occludin,Claudin-1 and ZO-1 in jejunum compared to CON group.However,MFGM supplementation increased these protein expression levels in jejunum compared to BLG group.
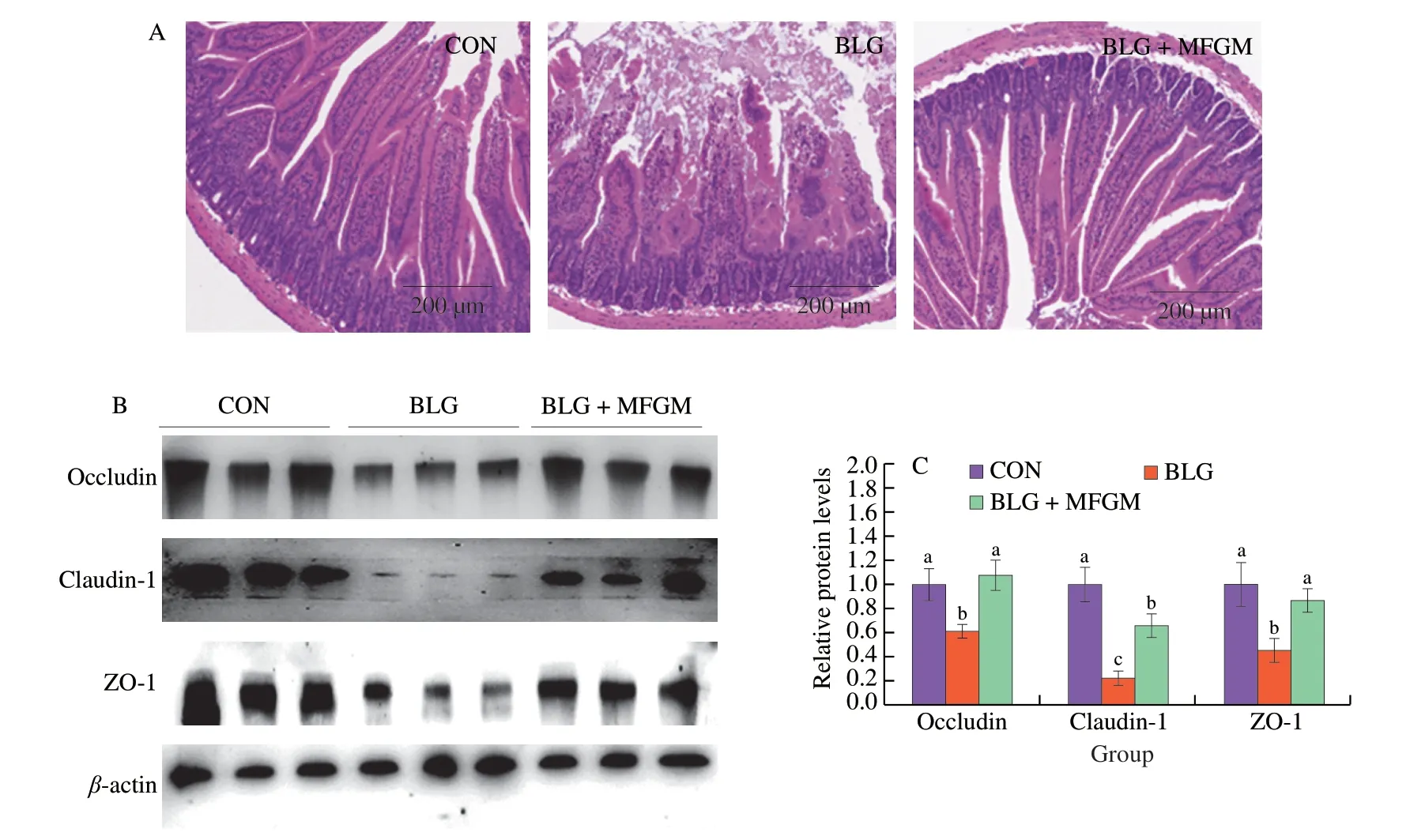
Fig.3 MFGM supplementation promoted intestinal epithelium integrity and enhanced intestinal barrier in a BLG-induced allergic mice model.(A)Representative H&E staining in jejunum.Magnification: 100×.(B and C) The protein levels of Occludin,Claudin-1 and ZO-1 in jejunum with corresponding quantification data.Different letters indicate significant difference (P <0.05) (n=6).MFGM: milk fat globule membrane;BLG: BLG-allergic group;BLG+MFGM:BLG-allergic mice supplemented with MFGM group;H&E: hematoxylin-eosin;TB: toluidine blue;ZO-1,zonula occludens-1.
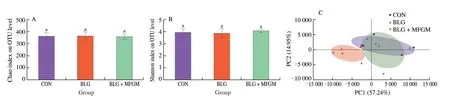
Fig.4 MFGM supplementation regulated the overall structural alterations and the compositions of gut microbiota in a BLG-induced allergic mice model.(A) Chao index at the OTU level as the estimators of the community richness of gut microbiota.(B) Shannon index at the OTU level as the estimator of the community diversity of gut microbiota. (C and D) Principal co-ordinates analysis with each point represented the gut microbiota of one mouse.(E and F) Phylumlevel and (G and H) family-level taxonomic distributions of the microbial communities in feces.Different letters indicate significant difference (P <0.05) (n=5–6).MFGM: milk fat globule membrane;BLG: BLG-allergic group;BLG+MFGM: BLG-allergic mice supplemented with MFGM group.
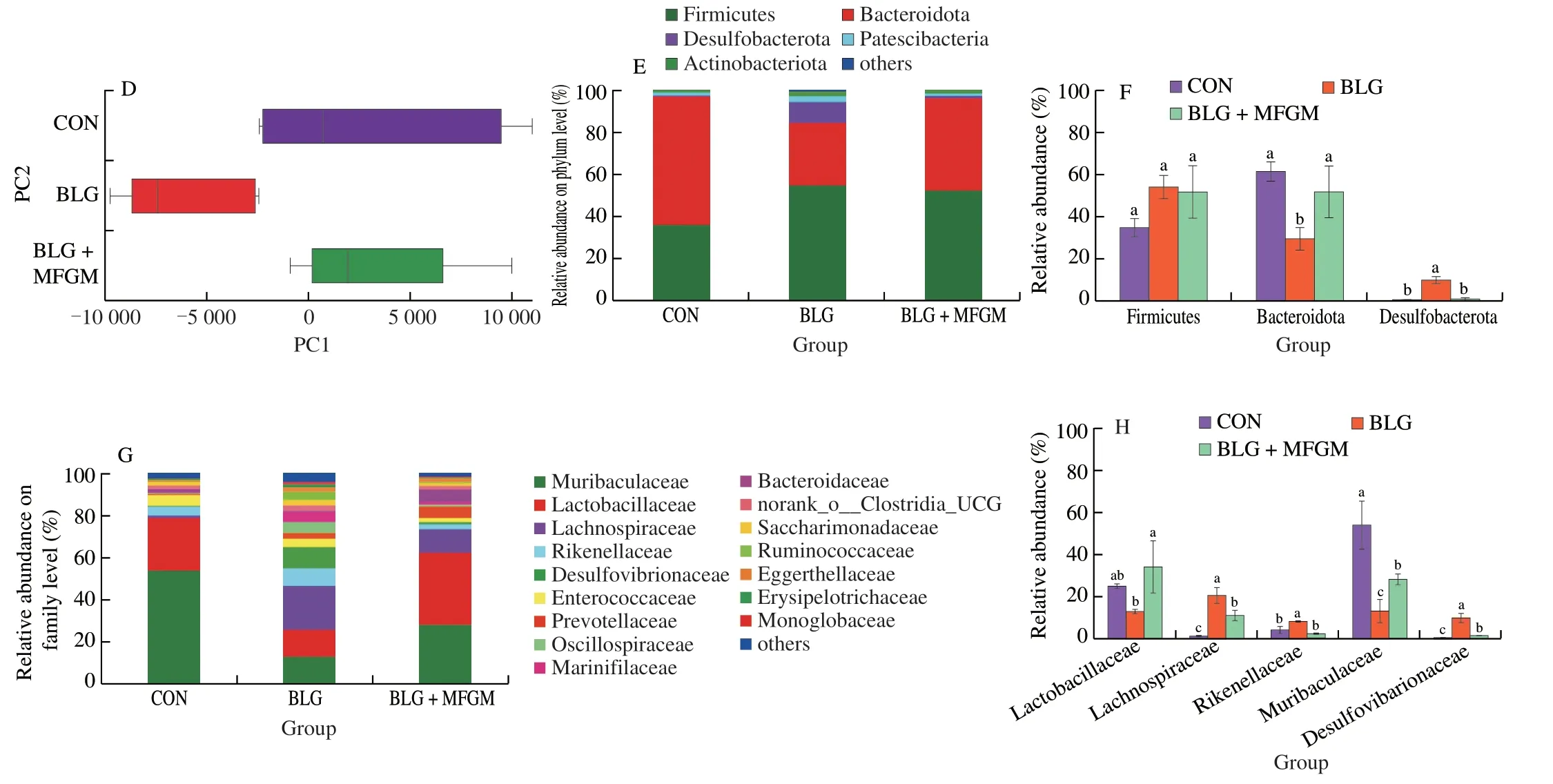
Fig.4 (Continued)
3.5 MFGM supplementation regulated the overall structural alterations and the composition of gut microbiota in a BLGinduced allergic mice model
Community richness and diversity measured by Chao and Shannon index respectively showed no differences among three groups (Figs.4A and 4B).PCoA showed that the bacterial communities among all groups separated significantly with the PC1 percent variation explained=57.24%,and the PC2 percent variation was explained=14.95% (Figs.4C and 4D).The cluster formed in BLG+MFGM group was similar to that of CON group.
At phylum level,compared to CON group,BLG significantly increased the relative abundance ofDesulfobacterota,while decreased the relative abundance ofBacteroidetes,which were reversed by MFGM supplementation (Figs.4E and 4F).At family level,BLG group had a higher relative abundance of Desulfovibrionaceae,Rikenellaceae and Lachnospiraceae,but had a lower relative abundance of Lactobacillaceae and Muribaculaceae than CON group.MFGM supplementation decreased the relative abundance of Desulfovibrionaceae,Rikenellaceae and Lachnospiraceae,while increased the relative abundance of Lactobacillaceae and Muribaculaceae (Figs.4G and 4H).
3.6 Correlation between gut microbiota and representative inflammatory cytokines and immune cytokines
Correlations between gut microbiota (30 major family) and representative inflammatory cytokines and immune cytokines were calculated based on the Spearman’s rho non-parametric correlation analysis.As shown in Fig.5,Muribaculaceae,Tannerellaceae were positively correlated to serum TGF-β and IL-10.Lachnospiraceae,Desulfovibrionaceae and Oscillospiraceae were positively correlated with serum LPS,IL-1β and IgE.Rikenellaceae was positively correlated to serum mMCP-1 and Helicobacteraceae was positively correlated to serum mMCP-1 and LPS.In contrast,Lachnospiraceae,Desulfovibrionaceae and Oscillospiraceae were negatively correlated to serum TGF-β and IL-10.Muribaculaceae and Tannerellaceae were negatively correlated to serum LPS,IL-1β and IgE,and Lactobacillaceae was negatively correlated to serum IL-1β and IgE.
3.7 Functional prediction of gut microbiota
PICRUSt analysis was further performed to predict gene functional profiles of bacterial communities and determine compositions of the KEGG pathway (class 3) in the bacterial population.The gene abundance in pathway of epithelial cell signaling inHelicobacter pyloriinfection was decreased in BLG+MFGM group.However,the gene abundances in pathways of GPRs,glycosphingolipid biosynthesis-ganglio series,retinoic acid inducible gene I (RIG-I),and carbohydrate metabolism were increased in BLG+MFGM group compared to BLG group (Fig.6).
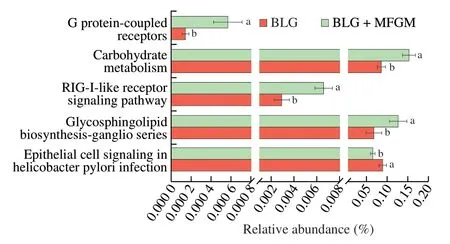
Fig.6 Comparison of gut microbiota function prediction.KEGG pathway (level 3) was compared by PICRUSt-predicted between BLG and BLG+MFGM groups.Different letters indicate significant difference (P <0.05)(n=5–6).MFGM: milk fat globule membrane;BLG: BLG-allergic group;BLG+MFGM: BLG-allergic mice supplemented with MFGM group.RIG-I:retinoic acid inducible gene I.

Fig.7 MFGM supplementation elevated the short chain fatty acid levels in feces and protein expressions of GPR43 and GPR109A in in a BLG-induced allergic mice model.The concentrations of acetic acid (A),propionic acid (B),butyric acid (C),isobutyric acid (D),valeric acid (E),isovaleric acid (F) in feces.(G)The protein expression levels of GPR43 and GPR109A in jejunum and (H) quantification data.Different letters indicate significant difference (P <0.05) (n=6).MFGM: milk fat globule membrane;BLG: BLG-allergic group;BLG+MFGM: BLG-allergic mice supplemented with MFGM group.GPR43: G protein-coupled receptor 43;GPR109A: G protein-coupled receptor 109A.
3.8 MFGM supplementation elevated the short chain fatty acid levels in feces and protein expressions of GPR43 and GPR109A in a BLG-induced allergic mice model
As shown in Figs.7A-F,the fecal propionic acid,butyric acid,isobutyric acid,valeric acid and isovaleric acid levels were decreased in BLG group as compared with CON group.However,MFGM supplementation increased these SCFA levels in feces compared to BLG group.There was no significant difference in acetic acid level among all groups.
As shown in Figs.7G and 7H,the protein expression levels of GPR43 and GPR109A in jejunum were decreased in BLG group compared to CON group.However,MFGM supplementation increased protein expression levels of GPR43 and GPR109A in jejunum compared to BLG group.
4. Discussion
CMA is a serious public health problem all over the word involving damaged intestinal barrier function and immunological mechanisms.In the present study,we demonstrated that MFGM alleviated food allergy symptoms and abnormalities of serum inflammatory cytokines and immune cytokines.It also enhanced intestinal barrier,induced Tregs differentiation and modulated gut microbiota accompanied by increased fecal SCFAs levels and GPRs protein expressions,thus alleviating CMA (Fig.8).

Fig.8 Possible underlying mechanism for the protective effect of MFGM on cow’s milk allergy in a BLG-induced allergic mice model.
Firmicutes and Bacteroidota are the two most dominant phyla in microbial community of human and in rodents[36].Bacteroidota abundance was both reduced in gut microbiota of BLG-allergic patients and mice[29,37].Polar lipids-enriched MFGM supplementation increased the abundance of Bacteroidota in high-fat diet (HFD)-induced obese dams[38].Desulfobacterota,associated with intestinal inflammation and serum LPS level,was increased in colitis mice[39].Depolymerized sulfated galactans alleviated food allergy through decreasing the relative abundance of Desulfovibrionaceae and maintaining intestinal barrier function[40].Lactobacillaceae,which was reduced in BLG-allergic mice,alleviated food allergy via inducing Tregs differentiation and immune tolerance[27,41,42].MFGM supplementation in infant formula elevated the abundance of Lactobacillus belonging to Lactobacillaceae in rat pups[25].Muribaculaceae,also known as S24-7,prevented intestinal inflammation and played an important role in the innate immunity system[43].A reduced abundance of Muribaculaceae was found in BLG-allergic mice compared with normal mice[29].Lachnospiraceae has been involved in the development of inflammation response and metabolic syndrome[44].Enrichment of Lachnospiraceae was observed in BLG-allergic mice and infants with CMA[27,45].In our previous study,MFGM supplementation decreased the abundance of Lachnospiraceae and attenuated metabolic endotoxemia in HFD-fed mice[24].Food allergic mice were demonstrated to have a specific microbial signature characterized by higher abundance of Rikenellaceae[46].Consistently,in our study,the abundance of Bacteroidota,Lactobacillaceae and Muribaculaceae were decreased,while that of Desulfobacterota,Desulfovibrionaceae,Lachnospiraceae and Rikenellaceae were increased in BLG-allergic mice,but MGFM supplementation reversed these changes.Besides,the consumption of milk polar lipids could modulate gut microbiota[47].After sphingomyelin digestion and absorption,the rest of sphingomyelin and its hydrolytic products can reach the colon to interact with the gut microbiota[48].Xanthine oxidase and mucins,which are major components of MFGM protein,can protect host from pathogenic bacteria[49,50].Therefore,the polar lipids and protein components of MFGM may play a key role in the gut microbiota modulation.Levels of SCFAs were significantly reduced in gut microbiota of allergic infants and rodents compared to those in normal individuals[29,51].MFGM promoted the SCFAs productionin vitroandin vivo.MFGM glycoproteins could be fermented to produce SCFAs and MFGM supplementation during late gestation increased SCFAs in feces of sows[23,52].In the present study,MFGM supplementation increased the abundance of Muribaculaceae and Lactobacillaceae,which are SCFA-producing bacteria[53,54].MFGM supplementation increased fecal SCFAs levels in BLG-allergic mice.Taken together,MFGM may exert beneficial effects on the gut microbiota and microbiota-derived SCFAs,thereby alleviating BLG-induced allergy.
Due to the key role of gut microbiota and SCFAs in intestinal barrier,immune responses,immune cell differentiation[8],the effects of MFGM on the predicted functions of gut microbiota were investigated.The gene abundances in pathway of epithelial cell signaling inH.pyloriinfection,GPRs,RIG-I signaling pathway,and carbohydrate metabolism were notably different between the BLG and BLG+MFGM groups.H.pyloridestroy TJs and cause intestinal barrier impairment[55].Mucins inhibited theH.pylorifrom binding to epithelial cells by steric hindrance and acting as a releasable decoy[50,56].RIG-I is an intracellular pattern recognition receptor involved in the immune and inflammatory response.Toll-like receptor 4 (TLR4) signaling induced the expression of RIG-I to regulate inflammatory reactions[57].Maternal MFGM supplementation increased the expression of TLR4 in intestine of piglets[23].Thus,MFGM might regulate the RIG-1 signaling by increasing the expression of TLR4.Besides,RIG-1 was down-regulated in intestinal epithelium of patients with intestinal inflammation and RIG-I−/−mice were susceptible to colitis[58,59].Therefore,increased gene abundance in the RIG-I signaling is correlated with amelioration of intestinal inflammation after MFGM intervention.Retinoic acid is involved in Tregs differentiation.Gut microbiota metabolizes carbohydrates to produce SCFAs.Therefore,shifts of functional gene profiles responding to alterations of bacterial community induced by MFGM may be related to intestinal barrier,GPRs signaling,SCFAs production and Tregs differentiation.Activation of GPRs by SCFAs had a vital function in the immune response and protected against food allergy.Dietary fiber and microbiotaderived SCFAs induced oral tolerance and alleviated food allergy,which was dependent on activation of GPR43 and GPR109A[5].Lactobacillus acidophilussuppressed BLG-induced allergic inflammation via upregulating SCFAs and GPR43 levels in the intestine[60].Mice lacking GPR43 or GPR109A showed fewer CD103+DCs and Tregs in MLN,resulting in the aggravation of Th2 immune response[5,16].Probiotics alleviated food allergy by DCs-dependent induction of Tregs differentiation and inhibition of Th2 immune response[3,41].Besides,milk fat globule-epidermal growth factor 8,a major component of MFGM,promoted DCs maturation and induced Tregs differentiation[61].Consistent with these results,MFGM supplementation increased the fecal SCFAs levels accompanied by the increased expressions of GPR43 and GPR109A.Activation of GPRs induced intestinal CD103+DCs accumulation and promoted Tregs differentiation to a certain extent,which inhibited Th2 immune response as evidenced by decreased serum IL-4 level in BLG-allergic mice.
The occurrence of food allergy is related to the destruction of intestinal barrier[13].Upon exposed to the allergen,the IgE bind to high-affinity receptor FcεRI on mast cells and cross-link with the allergens.Such allergen provocation triggers degranulation of mast cell and release of inflammatory mediators,which induced allergic inflammation response and disrupted intestinal barrier integrity.Destruction of intestinal barrier can in turn accelerate the development of food allergy[62-64].TJ proteins such as ZO-1,Claudins and Occludin connect adjacent intestinal epithelial cells and integrate paracellular pathways.Increased expressions of TJ proteins could enhance intestinal barrier,resulting in the alleviation of food allergy[65].In the current study,MFGM decreased the serum inflammatory cytokines levels and alleviated the infiltration of mast cells,while elevated TJ proteins expressions in BLG-allergic mice.These results suggested that MFGM could alleviate CMA via enhancing intestinal barrier function.SCFAs exert a protective effect on intestinal barrier through modulating GPRs.Butyrate and sodium butyrate promoted anti-inflammatory properties and elevated TJs expressions in a GPR109A-dependent manner[66,67].The enhancement of intestinal barrier was accompanied by the increased GPR43 expression,SCFAproducing bacteria and fecal propionate levels in mice[68].Consistent with these reports,MFGM could alleviate CMA via enhancing intestinal barrier and suppressing intestinal inflammation,which may be attributed to the activation of GPRs by SCFAs.
5. Conclusion
In conclusion,MFGM supplementation alleviated food allergy symptoms in a BLG-induced allergic mice model.MFGM modulated gut microbiota accompanied by the increased SCFAs levels,thereby elevating the protein expressions of GPR43 and GPR109A.The increased expressions of GPRs enhanced intestinal barrier,induced CD103+DCs accumulation and promoted Tregs differentiation in MLN to a certain extent,thus alleviating CMA.These findings suggested that the modulation of gut microbiota-derived SCFAs may contribute to the alleviation of CMA after MFGM supplementation.
Conflict of interests
Xueying Mao is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No.2019YFC1605000),National Natural Science Foundation of China (Grant No.31871806),and the Beijing Livestock Industry Innovation Team (BAIC05-2023).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250010.
杂志排行
食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
