Sulfated Cyclocarya paliurus polysaccharides exert immunomodulatory potential on macrophages via Toll-like receptor 4 mediated MAPK/NF-κB signaling pathways
2024-02-16YueYuHibinZhuMingyueShenQingYuYiChenShiruMoJinhuXie
Yue Yu,Hibin Zhu,Mingyue Shen,Qing Yu,Yi Chen,Shiru Mo,Jinhu Xie,*
a State Key Laboratory of Food Science and Technology,Nanchang University,Nanchang 330047,China
b International Institute of Food Innovation,Nanchang University,Nanchang 330200,China
Keywords:Cyclocarya paliurus Sulfated polysaccharides Macrophages Immunomodulatory Mechanism
ABSTRACT The biological activity of plant polysaccharides can be enhanced by sulfated modification.In this study,the immunomodulatory effect of sulfated Cyclocarya paliurus polysaccharides (SCP3) on macrophages RAW264.7 and its potential molecular mechanism were investigated.Results showed that SCP3 at 25−100 μg/mL increased viability and improved phagocytosis of RAW264.7 cells.Meanwhile,SCP3 could activate mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathways,which increased the phosphorylation of Erk1/2,JNK,p38 and NF-κB p65,promoting secretion of cytokines tumor necrosis factor α (TNF-α),interleukin 6 (IL-6) and nitric oxide (NO) as well as the production of reactive oxygen species (ROS).In addition,Toll-like receptor 4 (TLR4) receptor inhibitors were able to block the production of NO and TNF-α by SCP3-stimulated macrophages.Based on Western blot analysis and validation using specific inhibitors against MAPK and NF-κB signaling pathways,the results demonstrated that SCP3 induced macrophages activation and enhanced TNF-α and NO production via TLR4-mediated MAPK and NF-κB pathways.In summary,SCP3 has significant immunomodulatory potential. The underlying molecular mechanism was that SCP3 activates macrophages via TLR4 receptors to promote ROS production,which in turn activates the downstream MAPK/NF-κB signaling pathway and then increases the secretion levels of cytokines and NO.
1. Introduction
Immune system is engaged in immune defense,monitoring and regulation of the body,and is closely related to the pathogenesis of various diseases[1].Macrophages are important innate immune cells that are widely distributed in all major organs of the body,capable of phagocytosing pathogens and microorganisms and modulating the body’s immunity with the release of cytokines,chemokines and inflammatory factors[2].During the past decades,a growing number of studies have demonstrated that polysaccharides can modulate the body’s immunity with low toxicity,which are potential candidates for macrophages activation[3,4].There are many pattern recognition receptors on the surface of macrophages,including Toll-like receptor(TLRs),mannose receptor (MR),complement receptor 3 (CR3),and scavenger receptor (SR)[5].Among them,Toll-like receptor 4(TLR4) has been considered as an important membrane receptor for macrophages,which mediates the activation of macrophages by delivering a variety of extracellular signals[6].When polysaccharides bind to TLR4 receptors,signals are transmitted into cells through mitogen-activated protein kinase (MAPK) and nuclear factor kappa B(NF-κB) signal transduction pathways,causing a series of signaling cascades reactions to regulate the body’s immunity[7].Some research has shown that TLR4 was engaged in polysaccharides-induced immune cell activation,such asPolyporusumbellatuspolysaccharides,Platycodongrandiflorumpolysaccharide,SolanumnigrumLinne polysaccharide[8-10].MAPKs are a group of serine/threonine protein kinases in mammalian cells,including three major subunits,Erk1/2,JNK1/2 and p38.MAPKs signaling pathway play an important role in various signaling pathways in immune cells that can influence cellular processes,such as cell proliferation,differentiation and apoptosis[11].NF-κB is an important transcription factor associated with immunomodulatory effects.When cells are not activated,NF-κB is present in the cytoplasm in combination with IκB.Upon activation,NF-κB dissociates from IκB and enters the nucleus to bind to target genes,leading to the gene expression of bioactive substances[12].In recent years,more and more studies have found that polysaccharides exert immunomodulatory activities related to MAPKs and NF-κB signaling pathways,e.g.,astragalus polysaccharide[13],sulfated polysaccharide extracted from the freshwater plantMyriophyllum spicatumL.[14],and blackberry seeds polysaccharide[15].
Cyclocarya paliurus(Batal.) Iljinsk is a well-known medicinal herb and edible plant,widely distributed in the south of China.The leaves ofC.paliuruscontain a high content of bioactive components,such as polysaccharides,flavonoids,proteins[16].Polysaccharides are considered to be an important active substance inC.paliuruswith potential immune enhancing function[17].It has been reported thatC.paliuruspolysaccharide had the immunomodulatory effect in cyclophosphamide-induced immunosuppressed mice[18],as well as to protect against liver inflammation by down-regulating TLR4/MAPK signaling pathways[19].Sulfated modification of polysaccharides could significantly improve structural properties as well as enhance biological activities[20].Sulfated compound of culturedCordyceps militarispolysaccharide (CMPA90-M1)exhibited free-radical-scavenging effects[21].Sulfated derivative ofAgaricus brasiliensispolysaccharide have anti-herpes simplex virus activityin vivowhich can be used as oral agents to reduce the severity of HSV cutaneous and mucosal lesions[22].It is reported thatC.paliuruspolysaccharides (CP) and its sulfated derivative enhance immunoregulatory activity on dendritic cells via TLR2/4-MAPK/NF-κB signaling pathways[23].In our previous research,we found that sulfated CP have higher immunomodulatory activity than unmodified CP,which promoted the proliferation of splenic lymphocytes[24],as well as had better immune functions on cyclophosphamide-induced immunosuppressed mice[25].Based on the potential effect of sulfatedC.paliuruson immune activityin vivo.In this study,the immunomodulatory activity of sulfated CP (SCP3) was evaluated in macrophage (RAW264.7)model.The potential mechanism was then investigated by assessing the effect of SCP3 on the expression of key proteins involved in MAPK and NF-κB signaling pathways in macrophages.
2. Materials and methods
2.1 Materials and reagents
The leaves ofC.paliuruswere purchased from Xiushui County,Jiangxi Province,China.Dulbecco’s Modified Eagle Medium(DMEM) obtained from Solarbio Science &Technology Co.,Ltd.(Beijing,China).Fetal bovine serum (FBS) was purchased from Israel Biological Industries.Cell counting kit (CCK-8) was provides by Dojindo (Kyushu Island,Japan).Lipopolysaccharide (LPS) was obtained from Sigma-Aldrich (Sigma,USA).Primary antibodies of MAPK and NF-κB signaling pathways,including JNK,p-JNK,Erk1/2,p-Erk1/2,p38,p-p38,NF-κB p65,p-NF-κB p65 were all purchased from Cell Signaling Technology (Bever,MA,USA).The related secondary antibodies andβ-actin were bought from ZSGB Biotechnology (Beijing,China).TLR4 inhibitor (TAK242),NF-κB inhibitor (BAY11-7082),p38 inhibitor (SB203580),JNK inhibitor(SP600125),Erk1/2 inhibitor (PD98059) were from Medchemexpress(NJ,USA).All chemicals regents are analytical grade.
CP were extracted from the leaves ofC.paliurusby hot water extraction and ethanol precipitation.The SCP3 were prepared as previously described by our previous report.SCP3 consisted of 29.20% total sugar,8.06% uronic acid and 2.02% protein with average molecular weight of 206 kDa and the degree of substitution(DS) of 0.75[25].
2.2 Cell culture and viability analysis
Macrophages RAW264.7 were purchased from the Chinese Academy of Sciences (Shanghai,China).Macrophages were cultured in DMEM high-glucose medium containing 10% FBS,penicillin(100 U/mL) and streptomycin (100 μg/mL) at 37 °C in humidified atmosphere containing 5% CO2.
The cell viability was assessed using CCK-8 method.The cell density was adjusted to 1.5 × 105cells/mL and seeded in 96-well plates.Then,the medium was replaced with 100 μL polysaccharides(25,50,100 μg/mL) and LPS (1 μg/mL).After incubation for 24 h,10 μL CCK-8 solution was added to each well and the absorbance value was detected at 450 nm in microplate reader.LPS was used for positive control and the cell viability was expressed as percentage of control group.The formula for calculating cell viability as follows:
whereAtestis the absorbance of the sample group,andAcontrolis the absorbance of the control group.
2.3 Phagocytic activity assay
The phagocytic capacity of macrophages was measured by neutral red assay[26].Macrophages were seeded in 96-well plates and stimulated with different concentrations of polysaccharides and LPS at 37 °C under 5% CO2for 24 h.The cells were added with the neutral red dye and incubated for another 2 h.Discard the supernatant and wash the cells with PBS to remove excess dye.Then add cell lysis buffer to lyse the cells.After shaking at room temperature for 10 min,the absorbance was detected at 540 nm.
2.4 Determination of nitric oxide (NO),tumor necrosis factor α(TNF-α) and interleukin 6 (IL-6)
Macrophages (2.5 × 105cells/mL) were seeded into 6-well plates and incubated with polysaccharides at different concentrations as mentioned above.The medium group was used as the control group,and LPS group was used as the positive control group.The NO level was analyzed by using a Griess reagent kit (Beyotime,Biotechnology,China).The TNF-α and IL-6 level in the supernatant were determined by ELISA kits (Boster Biological Technology Co.,Wuhan,China)following its instruction.
2.5 Measurement of intracellular reactive oxygen species(ROS) accumulation
The ROS level was detected by fluorescence microscopy using a cell permeable fluorescent dye 2’,7’-dichlorofluorescein diacetate (DCFH-DA)[27].Cells were treated with polysaccharides(CP and SCP3) and LPS for 24 h,then washed twice with PBS and incubated for 30 min with 10 μmol/L DCFH-DA at 37 °C.After DCFH-DA staining,the cells were washed twice with PBS and the fluorescence intensity was measured with a fluorescence microscope in the excitation wavelength range of 500−530 nm (LEICA DMi8,Germany).
2.6 Western blot analysis
After treatment with polysaccharides (25,50,100 μg/mL) and LPS (1 μg/mL) for 24 h,the cell protein samples were lysed by using Western and IP lysis buffer with 1 mmol/L PMSF in an ice bath for 3 min.Protein samples were separated by 10% SDS-PAGE,and transferred to PVDF membrane.Then,PVDF membranes were blocking with 5% BSA for 1 h by shaking at room temperature,and incubate with the primary antibody (iNOS,Erk1/2,p-Erk1/2,JNK,p-JNK,p38,p-p38,NF-κB p65,p-NF-κB p65,β-actin) overnight at 4 °C.After washing the membrane three times with TBST,and incubate with the secondary antibodies for 45 min.The protein bands were detected with super ECL Plus solution.
2.7 TNF-α and NO levels and Western blot analysis after inhibiting TLR4 or MAPK/NF-κB
Macrophages were cultured in 6-well plates.After cultivated for 24 h,the cells were pretreated with 1 mL of TLR4 receptor inhibitor(10 μmol/L),JNK inhibitor (40 μmol/L),Erk1/2 inhibitor (40 μmol/L),p38 inhibitor (40 μmol/L),and NF-κB inhibitor (10 μmol/L),respectively.After incubation for 30 min,the culture medium was removed and the cells of each well were treated with 100 μg/mL CP and SCP3,1 μg/mL LPS and incubated for another 24 h.The supernatant was collected to determine the content of TNF-α and NO,and the cells were lysed with lysis buffer for Western blot analysis.
2.8 Statistical analysis
All results were presented as the mean ± standard deviations(SD).A one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test was employed to detect the significance differences between groups.Different letters were considered statistically significant (P<0.05).
3. Results and discussion
3.1 Cell viability
The cytotoxicity and proliferation of RAW264.7 cells were measured by CCK-8 assay (Fig.1A).Over a concentration range of 25−100 μg/mL,SCP3 showed no toxicity to RAW264.7 cells.Moreover,SCP3 could promote the proliferation of macrophages compared with the control group (P<0.05).

Fig.1 Viability (A) and phagocytosis (B) of RAW264.7 cells.RAW264.7 cells were treated with SCP3 or CP (25,50,100 μg/mL) and LPS (1 μg/mL) for 24 h.Values are expressed as means ± SD (n =6).Different letters indicated a different significance among all the groups at P <0.05.
3.2 Phagocytic activity
The phagocytic capacity of macrophages was investigated by measuring the amount of neutral red in macrophages.The results showed that SCP3 significantly stimulated the phagocytic activity of RAW264.7 cells in a dose-dependent manner compared to the control and CP group,even better than the LPS-positive control(P<0.05) (Fig.1B).The enhancement of phagocytosis is one of the most significant features of macrophages being activated[28].Thus,it suggests that the enhanced phagocytosis of RAW264.7 cells by SCP3 leads to an increased innate immune response against foreign pathogens.
3.3 Cytokines production
Cytokines are known as biological response modifiers,including TNF and IL,which regulate inflammation,immunity,and defense against external pathogens[29,30].In Figs.2A and B,three concentrations (25,50,100 μg/mL) of CP and SCP3 treatment significantly increased the secretion of TNF-α and IL-6 by RAW264.7 cells compared with control group (P<0.05).It was found that sulfated polysaccharides could regulate the secretion of different types and amounts of cytokines by macrophages to exert immunomodulatory functions,e.g.,sulfated polysaccharide from theMesona chinensisBenth significantly increases the levels of IL-6,IL-1β and TNF-α in macrophages to exert immunomodulatory functions[31],and fucoidans purified fromAgarum cribrosumcould regulate immune activity by inducing IL-10 secretion from macrophages[32].In addition,it has been shown that TNF-α is involved in the early stages of the cytokine cascade and induces NO production[26].In short,the results indicated that SCP3 could stimulate the production of TNF-α and IL-6 to exert immunomodulatory effects.
3.4 NO level and iNOS expression
NO is produced by iNOS catalyzed by arginine which is capable of participating in the killing of microorganisms and tumor cells[33].Compared with control group,NO level increased together with the concentration of polysaccharides.Moreover,SCP3 significantly increased NO secretion of macrophages than CP (P<0.05),with the highest NO secretion of 5.41 μmol/L at a dose of 100 μg/mL(Fig.2C).The DS of the polysaccharide plays an important role in the biological activity of the modified polysaccharide.After sulfation,the DS of SCP3 increased from 0 to 0.75.These results were consistent with those of Wang et al.[34]that the sulfate groups of polysaccharides are key factors affecting the immunomodulatory activities.Treatment with SCP3 in RAW264.7 cells,the protein expression levels of iNOS were significantly upregulated in a dose-dependent manner (Fig.2D).The result was coincident with the above results of NO level in RAW264.7 cells.This suggests that sulfated modification could promote SCP3-induced macrophages expression of iNOS,which modulates the immune response.
3.5 ROS generation
ROS have a key role in macrophage differentiation and activation,which activates MAPK and NF-κB signaling pathways[35,36].ROS production was monitored using DCFH-DA staining.There was a drastic increase for green fluorescence in SCP3 treated cells in a dose-dependent manner compared to control and CP (Fig.3).It is suggested that SCP3 could activate macrophages to produce ROS and participate in the regulation of cellular signaling pathways.Yu et al.[37]found thatGanoderma atrumpolysaccharide was able to induce ROS production by macrophages via TLR4/ROS/PI3K/Akt/MAPKs/NF-κB signaling pathway.

Fig.3 Effect of SCP3 on the intracellular ROS generation in RAW264.7 cells.Fluorescence microscopy images of intracellular ROS (A) and quantitative results (B).
3.6 MAPK and NF-κB signaling pathways activation
The immunomodulatory effects of polysaccharides on the body could be mediated through different intracellular signaling pathways[38].Recent studies have reported that polysaccharides induce immunocyte activation through MAPK and NF-κB signaling pathways[39].To delineate the mechanism of SCP3 modulated the activation of RAW264.7 cells,the expression of JNK,Erk1/2,p38 and NF-κB p65 phosphorylation were analyzed by Western blot (Fig.4).It was found that compared with the control,SCP3 could induce the phosphorylation of three MAPKs and NF-κB phosphorylation in a dose-dependent manner.It suggested MAPKs and NF-κB signaling pathway played a vital role in the activation process of RAW264.7 cells by sulfated polysaccharides SCP3.Similarly,sulfated polysaccharide from freshwaterM.spicatumL.also activated RAW264.7 via NF-κB and MAPK signaling pathways[14].

Fig.4 SCP3 activated the MAPKs and NF-κB pathways in RAW264.7 cells.(A) Western blot analysis.(B-E) Histogram represents quantification of protein expression levels.Values are expressed as means ± SD (n=3),different letters indicated a different significance among all the groups at P <0.05.
3.7 TLR4 participates in SCP3-induced signaling pathways activation
TLRs are considered to be important membrane receptors involved in macrophages activation,among them,TLR4 can mediate polysaccharide-induced macrophages activation[40].However,the role of TLR4 in the response to SCP3 was unclear.To determine whether TLR4 was engaged in macrophages activation by SCP3,we used anti-TLR4 inhibitor (TAK242) to investigate the role of TLR4 in SCP3-induced activation of RAW264.7 cells.SCP3-induced secretion of TNF-α and NO from macrophages was significantly decreased when macrophages were treated with a specific inhibitor of TLR4 receptor(P<0.05) (Fig.5).Meanwhile,the expression of Erk1/2,JNK and p38 phosphorylated proteins was measured to investigate whether inhibition of TLR4 receptor in macrophages could affect the activation of MAPK signaling pathway by SCP3.The results confirmed that SCP3 was unable to promote the phosphorylation of Erk1/2,JNK,and p38 after cells were treated with TAK242,a specific inhibitor of TLR4 receptor.The above results suggest that SCP3 activates MAPK signaling pathway through TLR4 receptor.A previous study showed that sulphated polysaccharides could activate macrophages through TLR2 and TLR4 receptors,such as polysaccharide CPE-II activates RAW264.7 macrophages through TLR4 and TLR2,and promotes IL-6 by regulating NF-κB and MAPKs to promote the production of IL-6 and NO[39].

Fig.5 Effect of TLR4 on SCP3-mediated immunological activities in RAW264.7 cells.(A) Western blot analysis.(B-D) Histogram represents quantification of protein expression levels.(E-F) TLR4 inhibitor treatment inhibits SCP3 induced NO and TNF-α production.Values are expressed as means ± SD (n=3),different letters indicated a different significance among all the groups at P <0.05.
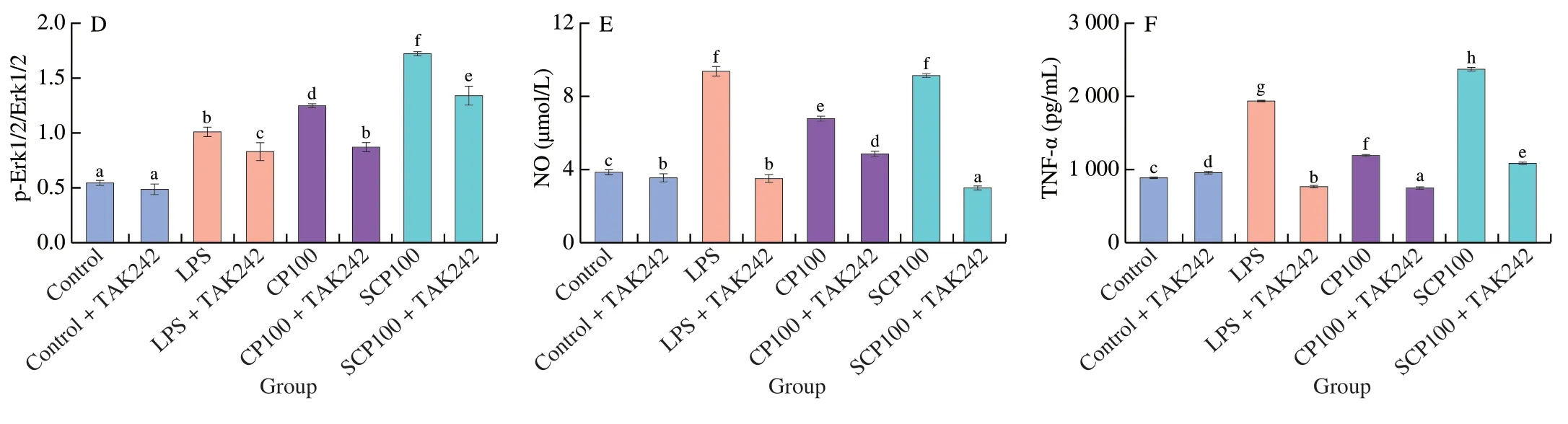
Fig.5(Continued)
3.8 Effects of MAPKs and NF-κB signaling pathway inhibitors on SCP3-induced activation of RAW264.7
With SCP3-induced activation of MAPK and NF-κB signaling pathways confirmed,specific MAPKs and NF-κB pathway inhibitors were used to verify whether MAPK and NF-κB signaling pathways are involved in SCP3-induced macrophages activation.As expected,after pre-treatment of RAW264.7 cells with Erk1/2 inhibitor (PD98059),JNK inhibitor (SP600125) and p38 inhibitor(SB203580) of specific MAPK signaling pathway inhibitors,respectively.It was obvious that phosphorylation of Erk1/2,JNK and p38 was significantly decreased in contrast with SCP3 stimulation of macrophages alone (Fig.6).Moreover,the levels of NO and TNF-α in SCP3-stimulated RAW264.7 were significantly reduced after treatment with the specific pharmacological antagonists of PD98059,SP600125,SB203580 and BAY11-7082,respectively,which inhibit Erk1/2,JNK,p38 and NF-κB p65 (Fig.7).Thus,SCP3 was able to regulate the immune response by regulating the production of RAW264.7 macrophages cytokines and NO through TLR4-mediated MAPK and NF-κB signaling pathways (Fig.8).In addition,Cho et al.[32]reported that sulfated-fucans isolated fromA.cribrosumappeared to active RAW264.7 cells through MAPKs and NF-κB signaling pathways.In addition,the immune response was significantly increased after CP was modified by sulfation,indicating that sulfated modification is an effective method to enhance the immunomodulatory effect of CP.Similarly,sulfated lycium barbarum polysaccharides (sLBPSs)[34]and sulfatedZizyphus jujubacv.Jinchangzao polysaccharides[41]could significantly enhance the immunological activity compared with natural polysaccharides,which indicated that the modified polysaccharide has high potential as candidate immunomodulatory drugs.Ferreira et al.[42]also showed that sulfate groups highly contribute to the immunostimulation by increasing the phosphorylation of Erk1/2,p38,and JNK in MAPK signaling pathways.

Fig.6 Effect of specific MAPKs inhibitors (Erk1/2 inhibitor: PD98059,JNK inhibitor: SP600125 and p38 inhibitor: SB203580) on SCP3-induced phosphorylation of Erk1/2,JNK,p38 MAPK in RAW264.7 cells.Values are expressed as means ± SD (n=3),different letters indicated a different significance among all the groups at P <0.05.

Fig.7 Effect of specific MAPKs and NF-κB inhibitors (Erk1/2 inhibitor: PD98059 (A,B),JNK inhibitor: SP600125 (C,D),p38 inhibitor: SB203580 (E,F) and NF-κB inhibitor: BAY11-7082 (G,H)) on SCP3-induced TNF-α and NO secretion in RAW264.7 cells.Values are expressed as means ± SD (n=3),different letters indicated a different significance among all the groups at P <0.05.

Fig.8 Potential signaling pathways in SCP3-mediated RAW264.7 cells.
4. Conclusion
This research investigated the effect of SCP3 on macrophages RAW264.7 activation and its potential signaling pathways.The results showed that SCP3 has no cytotoxicity,enhanced the phagocytosis of macrophages,and stimulated RAW264.7 cells to produce TNF-α and IL-6,up-regulated the expression of iNOS to promote the synthesis and secretion of NO,which had strong immune activity.Moreover,SCP3 could promote intracellular ROS production,which in turn activates MAPK and NF-κB signaling pathways.Further studies revealed that TLR4 was the main receptor for the interaction between SCP3 and macrophages.SCP3 could activate MAPK and NF-κB signaling pathways through TLR4 receptor.Meanwhile,it was obvious that the phosphorylation of Erk1/2,JNK,and p38 and the content of NO and TNF-α of SCP3 group was decreased after adding the corresponding inhibitors of MAPK and NF-κB signaling pathways.In conclusion,SCP3 was able to induce macrophages activation and regulate immune activity through the TLR4-mediated MAPK/NF-κB signaling pathway,which could better help us understand the mechanism of SCP3 immunomodulatory activity.
Conflict of interest statement
Jianhua Xie is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgements
The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China (82060594),the Natural Science Foundation of Jiangxi Province,China(20202BAB205006).
杂志排行
食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
