Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
2024-02-15JinyueZhouMinTngWnyiLiRuiFngChunlnTngQinwenWng
Jinyue Zhou,Min Tng,Wnyi Li,Rui Fng,Chunln Tng,,c,,Qinwen Wng,
a School of Public Health, Health Science Center, Ningbo University, Ningbo 315211, China
b Department of Neurology, Ningbo Rehabilitation Hospital, Ningbo 3151000, China
c Ningbo Institute of Life and Health Industry, University of Chinese Academy of Sciences, Ningbo 315100, China
d Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province, Ningbo 315100, China
Keywords: Alzheimer’s disease Gut microbiota Brain-gut axis Diet Physical activity
ABSTRACT Alzheimer’s disease is a neurodegenerative disease with complex etiology.Gut microbiota inf luences the gutbrain axis,which may affect pathways related to the pathogenesis of Alzheimer’s disease.Additionally,diet and physical activity are likely to affect the pathology of Alzheimer’s disease as well as the gut microbiota.This demonstrates that it may be possible to prevent or halt the progression of Alzheimer’s disease by regulating the gut microbiota using diet and physical activity strategies.Therefore,the present study reviews the association between these two interventions and gut microbiota in the human body.It also summarizes how these two interventions benef it Alzheimer’s disease.Furthermore,the primary limitations of these two interventions are discussed and promising strategies are proposed,which may be benef icial to further study and develop the intervening measure for the progression of Alzheimer’s disease.
1.Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder with clinical symptoms including cognitive impairment and language deterioration.With the progression of the disease,severe AD patients suffer from malnutrition,reduced mobility,and other complications.The total number of AD patients continues to increase with the increase of the elderly,which remains a major public health problem worldwide.Estimated data shows that 6.2 million (11%) Americans aged 65 and older have Alzheimer’s dementia today[1].The cause of AD is complicated,the main feature of AD in the brain is the presence of amyloid-β (Aβ) plaques accumulation and intracellular tau-containing neurofibrillary tangles (NFTs),accompanied by damaged and reduced neurons,inf lammation,and atrophy[2].To date,there has been no cure for AD or any effective reverse of the disease process.Pharmacologic treatment can only slow the progression[3].The progression of AD includes three phases,preclinical AD,mild cognitive impairment (MCI) due to AD,and dementia due to AD,while this progression of AD is usually unnoticeable in the early stage[4].It is commonly known that interventions are more effective in the early stage.
A healthy lifestyle including regular physical activity,a healthy diet,quitting smoking,blood pressure management,and diabetes management is recommended to reduce the risk of developing AD[5].Notably,diet and physical activity (PA) are suggested to play a part in manipulating the abundance and composition of gut microbiota.The gut microbiota reacts quickly to food intake,although the effect of diet on the core microbial profile is generally temporary[6].Diet inf luences the intestinal barrier and circulating inf lammation through pH changes and metabolites produced by gut microbiota in the intestinal tract.Studies also have suggested that PA could induce alterations in the human gut microbiota and short-chain fatty acids(SCFA) production,reduce inflammatory markers,and modulate gut barrier function,which is related to the duration of exercise and the exercise regime[7-11].
Here,this review summarized the effect of diet and PA on the regulation of AD pathology and gut microbiota.We also looked at how gut microbiota affects AD in relation to diet and exercise.Furthermore,the limitations of current studies and the strategies of diet and PA intervention were proposed for the prevention of the progression of AD.
2.Gut microbiome and AD
The gut microbiota consists of 1013-1014microbial cells.Bacteria dominate the gut microbiota.Among them,Firmicutes and Bacteroidetes dominate the vast majority.Others principally comprise Actinobacteria,Proteobacteria,Verrucomicrobia,and Fusobacteria.95% of the Firmicutes phylum is comprised ofClostridiumgenera.Bacteroidetes consist of predominant genera such asPrevotellaandBacteroides[12-14].The composition of gut microbiota has complex impacts on disease susceptibility.Dysbacteriosis leads to disease,rather than the presence or absence of a single microbe[15].Specific microbiota composition is one of the characteristics of a healthy bond,and microbiota composition is changing during aging.
Thegut-brain axis connects the brain and the gut through extensive interconnected pathways,which affect maintaining homeostasis.Growing findings have shown a certain correlation between the gut microbiome and AD,which is summarized in Table 1.Neurodegenerative diseases seem to have correlations with inflammatory bowel disease (IBD)[16].Nationwide longitudinal research demonstrated the risk of AD is increased in IBD participants,and the risk appeared to increase with IBD chronicity[17].Some studies have demonstrated that the microbiota composition of AD participants is different compared to cognitively healthy people[18-20].Vogt et al.[18]revealed lower microbiota diversity,lower levels of Firmicutes and Actinobacteria,and a higher level of Bacteroidetes in AD patients compared to controls.However,another study reported that MCI subjects and cognitively healthy individuals had similar gut microbiota diversity,which may be due to the difference of disease progress[19].Cattaneo et al.[21]found that the ratio ofEscherichiatoShigellawhich indicated pro-inflammation increased while the abundance of anti-inflammatory bacteria was reduced in cognitively impaired elderly compared to the controls.In a retrospective cohort study,Helicobacter pyloriseropositivity was found to be related to AD mortality[22].In addition,altered gut microbiota composition has also been observed in mouse models of AD[23-24].The change in gut microbiome was correlated with cerebralβ-amyloidosis[25],and higher abundances of Proteobacteria were found in the mouse model of AD.Van Olst et al.[26]discussed studies on microbiota composition in individuals and animal models with AD,which indicated that alteration in specific gut microbiota was inconsistent across studies.But it is pointed out that the effect of gut microbiota on cognitive function is clear,and microbiota balance is quite important rather than specific microbiota.
Modification of gut microbiota composition,such as antibiotic treatment and fecal microbiota transplantation,is a probable option for the prevention or treatment of AD.After transferring a healthy microbiota to the mouse model of AD,the spatial learning and memory behavior of the mouse performed better with alleviated AD pathologies[27-28].But there is still a lack of human studies about fecal microbiota transplantation in AD patients to our knowledge.Treatment with antibiotics on AD patients has led to conflicting results about the effects on cognitive function,probably due to the broad and non-selective effect of antibiotics on certain pathogens[29].Numerous research has reported that probiotic supplementation has a positive effect on cognitive function,inflammation,and oxidative stress in patients with AD[30-31].GV-971 is a new drug that may remodel the gut microbiota,which has been used clinically for mild and moderate AD in China.The contribution of GV-971 to AD pathogenesis is still unclear and under debate.Some studies supported the effect of GV-971 as a therapeutic approach for AD[32-33],but it also has been questioned due to different pharmacological factors and insufficient experimental data.
To the best of our knowledge,the correlation between gut microbiota and AD is obvious,but the specific mechanism of the brain-gut axi is not completely clear,which may mainly involve the immune and nerve system,resulting in aggravation of inflammation in patients with AD (Fig.1).Therefore,the detail underlying mechanisms between gut microbiota and AD were further illustrated.

Table 1 The variation of gut microbiota in AD patients and models.
3.Underling mechanisms between the gut microbiome and AD
Gut microbiota affects the intestinal barrier and inflammation,thus causing AD-related pathological changes.As the most important barrier against the invasion of gut microbiota and toxins,the intestinal epithelium performs the functions of the digestion and immune system.Gut microbiota interacts directly with the intestinal epithelium.Gut microbiota composition affects intestinal barrier dysfunction and mucosal permeability[34].The disrupted intestinal barrier causes a series of inflammatory responses.Depletion of gut bacteria in the host may lead to the alteration of microglia properties[35].Persistent microglia activation (reactive microglia) secretes high concentrations of inflammatory biomarkers,such as interleukin-1β(IL-1β),IL-6,and TNF-α,and produces excessive nitric oxide(NO) and reactive oxygen species (ROS),thus leading to chronic inflammatory response supporting the neurotoxic processes,which cause brain injury and neuronal death[36].Moreover,activated microglia led to neurotoxic reactive astrocytes[37].Evidence suggests that AD correlates with gut permeability biomarkers with inflammatory indicators.The levels of gut permeability biomarkers in subjects with dementia were increased[38].The levels of proinflammatory factors in the brain and CSF in AD patients were higher than those in the control group[39].
The impact of gut microbiota on intestinal health and inflammation is primarily influenced by the product of gut microbiota.Therefore,the effect of different gut microbiota products on intestinal health,inflammation,and AD were summarized as follows (Fig.2).
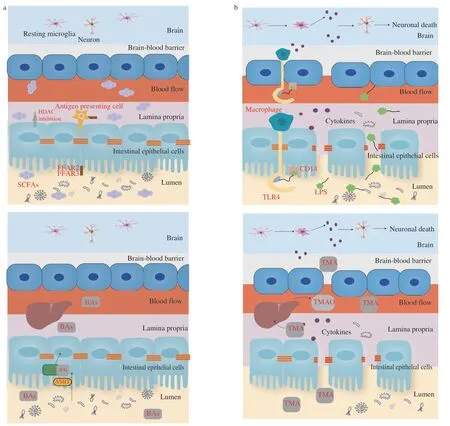
Fig.2 The underlying mechanisms between gut microbiota and Alzheimer’s disease from the perspective of intestinal products including (a) SCFAs,(b) LPS,(c) BAs,(d) TMAO.
3.1 The effect of short-chain fatty acids (SCFAs) on intestinal health and inflammation for AD
SCFAs,namely acetate,valerate,propionate,and butyrate,are metabolized by gut microbiota in the large intestine.As one of the most important energy sources for intestinal epithelial cells(IECs),SCFAs can be absorbed by colonocytes quickly.In the gastrointestinal tract,SCFAs,in particular butyrate,have protective effects on epithelial cells by providing energy for the growth and expansion of IECs[40].With the ability of anti-inflammation and immunomodulation,SCFAs is involved with the maintenance of intestinal health,representing the key point of the relationship between gut microbiota and AD pathology[41].Marizzoni et al.[42]measured the levels of blood SCFAs and cytokines in AD and control participants,levels of acetate and valerate that had an association with most endothelial markers,and butyrate that may have association with the maintenance of the endothelial integrity.Fecal metabolomics studies also have shown the altered concentration of SCFAs in AD patients[43].After entering the systemic circulation,SCFAs exert anti-inflammatory and immunomodulatory effects by activating SCFA receptors,such as free fatty acid (FFA) receptors type 2 and 3 (FFA2 and FFA3 receptors) (Fig.2a)[44].Among SCFA receptors,inhibition of histone deacetylases (HDACs) is considered the most relevant to cognitive function in neurological disease with impacts on chromatin maintenance and neuronal plasticity[45].In mouse models,HDAC3 is involved with the increase of Aβ levels,activation of microglia,and decrease of dendritic spine density[46-47].Furthermore,defective microglia can be restored by reconstitution of diverse gut microbiota or SCFAs supplement.Some studies have agreed with the role of SCFAs (especially butyrate) in the modulation of microglial function[48-49].
3.2 The effect of lipopolysaccharides (LPS) on intestinal health and inflammation for AD
LPS,a structural component of the cell wall of gram-negative bacteria,were also known as endotoxin.A healthy intestinal barrier keeps LPS in the intestinal lumen,but LPS may enter the blood circulation when the intestinal environment is disrupted[50].LPS promotes inflammation based on the formation of the LPS-CD14 complex by binding CD14 on microglia membranes and further associating with Toll-like receptor 4 (TLR4),resulting in the eventual death of neuronal cells (Fig.2b)[51].LPS induces inflammation and disrupts the blood-brain barrier[52].Intracerebroventricular injections of LPS can cause neuroinflammation and cognitive dysfunction[53-54].In the brain of AD patients,LPS can be detected in amyloid plaques and surrounding vessels.Moreover,studies have demonstrated that increased LPS level was in the brain and serum of AD patients[42-43,55].
3.3 The effect of bile acids (BAs) on intestinal health and inflammation for AD
BAs are also one of the important products of intestinal microorganisms in the human body.BAs enter IECs through apical sodium-dependent bile acid transporter (ASBT) and activate the farnesoid X receptor (FXR).BAs can influence epithelial function through the activation of specific cell surfaces of G protein-coupled receptors (GPCRs) and intracellular nuclear receptors (Fig.2c)[56].Alterations in the microbiome lead to alterations in bile acid metabolism.Perrone et al.[57]showed that supplementation of dietary bile acid decreased intestinal apoptosis and improved intestinal integrity in the mice model.Additionally,BAs can lead to perturbations in the plasma membrane that contribute to initiating signaling in epithelial cells[58-59].The serum concentrations of BAs in AD participants are different from the cognitive normal participants,which areassociated with cerebrospinal fluid(CSF) markers for AD[60-61].
3.4 The effect of trimethylamine N-oxide (TMAO) on intestinal health and inflammation for AD
TMAO is a gut microbiota-derived metabolite of dietary choline.Trimethylamine (TMA) is produced in the intestine and then synthesized in the liver as TMAO.TMA can be absorbed quickly through the intestine at low concentrations[62],and disrupt the integrity of the blood-brain barrier (Fig.2d)[63].But there is debate about TMAO’s effect on AD,and the function of TMAO in AD pathology is still unclear.Due to the importance of TMAO as a mediator of inflammatory processes,it has been hypothesized that TMAO concentration relates to neurological diseases.Vogt et al.[64]found increased CSF TMAO levels in participants with MCI and AD.And it was associated with increased CSF biomarkers,including t-tau and neurofilament light chain (NFL).Brunt et al.[65]reported that plasma TMAO level was inversely related to cognitive performance in humans.In the mouse model,TMAO levels of plasma and CSF both increased with age.Another study showed that TMAO had no relevance to the incidence of AD[66].Similarly,Rio et al.[67]indicated that there was little difference in CSF TMAO levels between subjects with AD and other subjects.In addition,the benefits of TMAO for brain health are controversial.Some studies have suggested that TMAO exacerbates the damage of mitochondrial and synaptic[68].Brunt at el.[65]indicated that TMAO supplementation exacerbated cognitive impairment,inflammation,and microglia activation.Conversely,Hoyles et al.[63]showed that TMAO protected the blood-brain barrier to keep the nervous system free from inflammatory infiltrates through tight junction regulator annexin A1.
4.The role of diet
A growing body of literature has demonstrated that healthy diets rich in nutrients and vitamins benefit cognitive function[69-71].Chuang et al.suggested that high intakes of phytonutrient-rich plant foods and protein-rich foodsfor older adults[72].Experimental trials in individuals with AD have shown that cognitive function improved after dietary manipulation[73].Additionally,recent studies have shifted focus from individual nutrients to combinations of specific nutrients for the maintenance of neurocognition,since it is considered that the effect of whole diets is more effective than single nutrient intervention strategies[74].
Diet causes dynamic changes in intestinal flora continuously and alterations in intestinal metabolites.Diet has an effect on SCFAs level,especially dietary fiber[75].Bai et al.[76]analyzed the effect of cereal fiber supplementation on SCFAs production in the human body.The results showed that different durations of intervention resulted in different SCFA increase.Different cereal has different effects on SCFAs production.Additionally,the effect on different people is also different,the effect on obese individuals is more obvious.In addition,dietary intake correlates with human bile acid levels[77].High-fat diets tend to have higher levels of bile acids,whereas diets with higher dietary fiber and lower fat induced lower levels of fecal bile acids[78-79].High fat and high cholesterol diet leads to increased LPS,whereas Mediterranean diet and diet rich in fish cause decreased LPS[75,80-81].High-fat diet and dietary choline intake are associated with higher TMAO levels[82-83].These gut microbiota products have highly relevant to AD,which suggests that AD pathology may be associated with gut microbiota products through gut microbiota.
However,the relationship between specific gut microbiota and corresponding metabolites is quite complicated,the impact of each dietary component and dietary pattern on AD and gut microbiota were further illustrated.Here,the alteration of gut microbiota due to different diet interventions was summarized in Table 2.
4.1 The impact of protein on AD and gut microbiota
Dietary proteins are utilized by gut microbiota,thus contributing to the synthesis of bacterial proteins as substrates and giving rise to metabolites such as SCFAs,BAs,etc.A large-scale investigation totaling more than 500 subjects assessed the relationship between protein intake and the Aβ level in blood and brain.They reported that high dietary protein intake has a potential beneficial impact on the brain Aβ burden in older cognitively unimpaired participants[84].Plant protein and animal protein have different effects on microbiome composition.An animal study indicated that soy protein interventions increased the diversity of gut microbiota and contributed to a relative lower abundance of Bacteroidaceae and Porphyromonadaceae compared to milk protein interventions[85].Moreover,excessive protein supply results in disease pathology through increased inflammation due to the toxicity of protein metabolites[86].Intriguingly,Chen et al.[87]showed that a high-protein diet led to inflammation independent of protein composition,but could alter the microbial composition and reduce the thickness of the intestinal mucus layer.
4.2 The impact of lipids on AD and gut microbiota
It is well known that AD and lipid profile have a close relationship.Studies showed that a high-fat diet impaired cognitive function and promoted brain inflammation in mouse models[88-89].Additionally,a fat diet affects the gut microbiota composition[90].Wan et al.[91]recruited more than 700 subjects and provided threeisocaloric diets with different contents of fat for 6 months.Results showed that the diversity of gut microbiota and SCFAs levels were higher with lower fat intake.Patrone et al.[92]suggested that different fat compositions contribute to differential effects on gut microbiota composition.

Table 2 The role of diet on gut microbiota.
A large cohort study in older adults followed for 12 years reported that lower blood status in polyunsaturated fats and higher saturated fats had a high correlation with a higher incidence of dementia[93].Zhang et al.[94]reported that cognitive performance and hippocampal atrophy in MCI subjects improved after extra DHA intake (2 g/day)for 12 months.However,other studies contradicted the findings of the active role of omega-3 fatty acids (n-3 FA) in alleviating cognitive impairment[95].Interestingly,the efficacy ofn-3 FA supplement on cognitive function relates to APOE genotype,a diet enriched withn-3 FA which is effective to non APOE4 AD carriers was found to be ineffective in APOE4 carriers[96-97].The proportion of butyrate-producing bacteria and other gut microbiota will change withn-3 FA supplement[98-99].
4.3 The impact of dietary fiber (DF) on AD and gut microbiota
DF,described as carbohydrate polymers,stimulates the growth of healthy bacteria in the gastrointestinal tract and produces SCFAs with the fermentation by gut microbiota[100].Studies have revealed that a higher intake of DF contributes to healthy outcomes,which may be due to the immunomodulatory and anti-inflammatory properties of SCFAs[101].An exploratory randomized and placebo-controlled crossover study showed that with specific dietary fiber supplementation in healthy participants,the gut microbiota diversity and inflammatory markers kept similar status,and only cognitive function improved[102].Desai et al.[103]suggested that gut microbiota is influenced by dietary fiber and responded dynamically when dietary fiber intake is changed using a gnotobiotic mouse model.Evidence indicated that the thickness of the colonic mucus layer was related to dietary fiber as fiber deficiency led to the enrichment of mucusdegrading bacteria and promoted degradation.Higher fiber intake tends to be associated with increased abundance ofFaecalibacteriumand decreased abundance ofActinomyces[104].
Furthermore,the fermentation dynamics of DF depend on the chemical structure,the composition of the ileal effluents,and the speed at which digest progresses along the colon[105].Deehan et al.[106]confirmed the importance of the chemical structure of DF on gut microbiota.With three type-IV resistant starches in the general population,different compositions of gut microbiota and different outputs toward SCFAs were shown.
4.4 The impact of vitamin D on AD and gut microbiota
Vitamin D has properties of antioxidant and anti-inflammation,which may be beneficial to cognitive function.A large prospective study with more than 900 cognitively healthy participants was followed for up to 12 years investigated vitamin D level and cognitive function,and found that lower plasma concentrations of 25(OH)D(< 25 nmol/L) had a strong association with increased risk of AD[107].The serum 25(OH)D3 levels were significantly lower in AD participants compared to age-matched cognitively healthy participants[108-109].The reduced concentrations of 25(OH)D may relate to worse cognitive performance in individuals with mild AD[110].A trial reported that 800 IU/day of vitamin D supplement that lasted for one year slowed the decline in cognitive function by reducing some of the Aβ-related biomarkers in AD patients[111].Naderpoor et al.[112]indicated that the abundance of genusBlautiadecreased and the abundance of genusLachnospiraincreased after extra intake of vitamin D.Additionally,the fecal microbiota composition was related to the dose of vitamin D intake[113].Similarly,Luthold et al.[114]found that there was a link between higher vitamin D supplementation and a lower abundance ofHaemophilusandVeillonella.However,the knowledge concerning the effect of vitamin D supplementation on the gut microbiota in individuals with AD is limited.
4.5 The impact of dietary patterns on AD and gut microbiota
Good dietary patterns offer better options for the prevention of AD because of the complexity of the interaction of different components in food.The Ketogenic diet (KD) is characterized by a high proportion of fat and a low proportion of carbohydrate intake and can provide neuroprotective effects[115].Studies have shown that KD could reduce the deposition of Aβ and alleviate the effects of impaired glucose metabolism and inflammation[116-118].
The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet combines the characteristics of the Mediterranean(MD) diet and the Dietary Approaches to Stop Hypertension (DASH)diet.A prospective study investigated the relationship between these three diet patterns and the rate of developing AD.Results suggested that even modest adjustments to the diet correlate with a reduced risk of AD[119],which was consistent with other relevant studies[120-122].Meslier et al.[123]reported that an 8-week MD intervention increased the levels of fiber-degrading bacteria.The specific microbial taxa were related to fecal bile acid degradation and insulin sensitivity.Nagpal et al.[19]observed that a modified Mediterranean-ketogenic diet(MMKD) changed the special gut bacterial taxa in individuals with MCI and suggested that special gut microbiota was associated with CSF biomarkers of AD,such as Aβ42 and tau-p181.A randomized controlled trial in older people for 1-year MD intervention indicated the alteration of gut microbiota related to increased production of SCFAs and a decline in secondary bile acids production[124].
5.The role of physical activity
In recent studies,PA has been shown to have a positive effect on cognitive function in individuals with mild AD,and PA duration of ≥ 150 min/week of PA is recommended[125-126].Aerobic exercise improves VO2peak in subjects with mild AD[127],and cardiorespiratory fitness appears to be associated with cognition function and bilateral hippocampal volume[126].PA promotes the synthesis of myokines and other metabolites contributing to increased expression of brain-derived neurotrophic factor (BDNF),which has been shownto play a role in neuroplasticity[128].Systemically,PA has shown to have a regulatory effect on inflammatory markers,and the different intervention training modes have different effects on neuroinflammatory cytokine[129-131].Endothelial function was also improved after exercise[132-133].Studies reported that PA may regulate Aβ and tau levels in mice model of AD,but consistency is poor inhuman groups[134].However,Lamb et al.recruited more than 400 subjects with dementia and introduced moderate to high intensity aerobic and strengthening exercise training or no PA intervention[135].It turned out that the effect of the training program is not ideal,and might even have adverse effects on cognitive function.

Table 3 The role of physical activity on gut microbiota.
Barton et al.[136]reported that the gut microbiota diversity,levels of SCFAs,and TMAO of athletes are richer and higher than that of ordinary people with different gut microbiota composition,consistent with other studies[137-138].But Magzal et al.[139]indicated that SCFAs level is higher in people with less exercise.Exercise may attenuate LPS-induced memory impairment[140].PA may reduce the expression of LPS-induced neuroinflammatory cytokine in the brain and attenuate astrocyte remodeling[54,141].The alterations of gut microbiota due to PA intervention are summarized in Table 3.However,the literature examining the relationship between PA and gut microbiota in AD patients is sparse.
5.1 The impact of exercise modalities on AD and gut microbiota
Both aerobic and resistance training have beneficial effects on neurocognitive function through different mechanisms.These two modes can increase neurotrophins and reduce some inflammatory cytokines in individuals with MCI[130].Padralli et al.[133]also found aerobic exercise and resistance exercise had a similar effect on endothelial function.Morita et al.[142]indicated that the abundance ofBacteroidesincreased in elderly women after brisk walking for 12 weeks,while there was no obvious change in gut microbiota composition after trunk muscle exercise.Similarly,another human study found an increase in the abundance ofBacteroidesand decreased Firmicutes/Bacteroidetes ratio in both two PA modes (sprint interval training and continuous training)[9].Castro et al.[143]found improved diversity and a decreased abundance ofPseudomonas,SerratiaandComamonasafter resistance training in mouse models.In addition,different effects of resistance and endurance training on plasma bile acids were observed,and both modes of training can change the composition of BAs[144].To sum up,PA can efficiently change the gut microbiota composition and alleviate inflammation with different exercise modalities.
5.2 The impact of exercise intensity on AD and gut microbiota
High-intensity interval training (HIIT) generally has superior aerobic capacity compared to moderate-intensity continuous training(MICT).They have a similar ability to increase mitochondrial content[145].Studies have shown that regardless of HIIT or MICT,the concentration of peripheral BDNF increases after acute exercise.Additionally,the BDND level seems to have nothing to do with the duration of exercise,even a bout of acute training can affect the BDND level[146-147].There was little difference between the effect on improving cognitive function[148].Furthermore,different intensities of PA may induce different effects on gut microbiota composition.Moderate and intense exercise changes the composition of gut microbiota and may increase the bacteria diversity[90,149],but no indepth research has been done on the effect of exercise intensity on gut microbiota.Beale et al.[150]found that light-intensity exercise correlated with some SCFAs,whereas moderate to vigorous exercise correlated with some BAs.
5.3 The impact of exercise duration on AD and gut microbiota
Short-term and long-term exercise can both change microbiota composition[9,11,142,151-152]and reduce inflammatory markers[9,11].Even a single bout of exercise can lead to disturbance of intestinal flora.Tabone et al.[151]reported that gut microbiota composition changed after a controlled acute exercise session.Likewise,Zhao et al.[152]assessed the changes in fecal metabolites and gut microbiota after a half-marathon race.Results showed that gut microbiota responded rapidly after acute exercise.Additionally,the alteration of gut microbiota composition with short-term exercise will be reversed once exercise ceased[7].Sellami et al.[153]showed that compared to short-term activity,long-term exercise results in higher alterations to cytokines.Danese et al.[154]evaluated the acute effect of middledistance running on serum BAs.Results showed the serum BAs were significantly reduced.
6.The combined effect of diet and PA in AD
Diet and PA are connected in some manner,and they have impacts on one another[155].Exercise may affect one’s appetite and food preferences[156].Individuals who performed endurance exercise favored high-fat dietary intakes compared to sedentary individuals[157].Yang et al.[158]reported that exercise reduced predilection for a high-fat diet in mice.The combined effect of diet and PA generally is better than the effect of exercise alone.It has been shown that a ketogenic diet combined with PA has a more potent impact on enhancing fat oxidation and reducing insulin resistance compared to that of a habitual mixed diet combined with PA[159-160].The benefits of exercise may be diminished by inappropriate diet strategies.Zhang et al.[161]found that TMAO supplementation from Western diets eliminated the cardioprotective effects of exercise in mice.
Multidomain lifestyle interventions including diet and exercise are encouraged in AD prevention[162].Multidomain lifestyle intervention is associated with a longer life expectancy compared to zero or one lifestyle intervention in individuals with or without AD[163].Some studies have investigated the combined effects of diet and PA on cognitive health[164],but the mechanism of their interaction on cognitive function remains unclear.
Moreover,the underlying mechanism of the interaction of diet and PA to gut microbiota is uncertain,and corresponding research is limited.The timing of nutrient intake around exercise and which nutrient intake can better improve functional capacity around exercise are to be learned.Murtaza et al.[165]analyzed gut microbial community profiles in athletes with three diet patterns of different contents of carbohydrates before and after an intensified training program.Results showed that compared to that of individuals with a high carbohydrate diet and periodized carbohydrate diet,a greater variation of microbial community profiles was found in individuals with a ketogenic low carbohydrate high-fat diet.And there was an increase in the abundance of lipid metabolism-related bacterial taxa in subjects with a ketogenic low carbohydrate high-fat diet.Yu et al.[166]investigated the impact of PA and butyrate supplementation combined with a high-fat diet on gut microbiota and inflammatory indicators.They hypothesized that exercise may cause an increase in butyrate-producing fecal bacteria.Butyrate-producing bacteria and butyrate have been considered to have beneficial effects on cognitive function[167].Diet combined with PA also brings different effects on intestinal products,which could promote BAs synthesis and reduce the blood total BAs level[168].
7.Discussion
There is a close correlation between AD and gut microbiota.The gut microbiota profile of AD patients is different from that of cognitively normal people.Patients with gastrointestinal disorders are more likely to develop AD compared to the general population.Researchers have hypothesized that it might be possible to affect AD by altering the gut microbiota.Several studies have shown that these strategies such as probiotic supplementation and microbiota transplantation are effective.
As previously mentioned,a suitable ratio of dietary protein but not excessive protein intake,adequate intake of dietary fiber,and lower fat intake are recommended for brain health.Gut microbiota changes dynamically with the alteration of diet,but it is resilient.After digestion in the stomach and small intestines,food is fermented by gut microbiota and produces metabolites such as SCFAs.Additionally,diet alters gut PH value and glucose homeostasis and produces dietary neurotransmitters,thus influencing the gut microbiota.The products of gut microbiota regulate energy metabolism and have anti-inflammatory properties,which ameliorate gastrointestinal inflammation (Fig.3).It is hypothesized that the products can pass BBB and has a role in the CNS.
PA improves VO2peak,cardiorespiratory endurance,endothelial function,as well as the level of BDNF.It affects BBB permeability by reducing oxidative stress and attenuating the inflammatory effect[169-171].Accumulating evidence has indicated that PA increases the abundance of gut microbiota and changes gut microbiota composition.We hypothesize that PA alleviates the inflammation in the CNS due to the anti-inflammatory effects of gut microbiota products.Additionally,BDNF expression affects gut microbiota.In the BDNF gene knock-out mouse,the colonic epithelia barrier was damaged by an invasion of bacteria and a decrease of microvilli[172].On the other hand,gut microbiota affects BDNF levels.Bercik et al.[173]found that the composition of gut microbiota changed and BDNF expression in hippocampal increased in specific pathogenfree (SPF) BALB/c mice with oral antimicrobials administration.The intervention effect of PA on gut microbiota,and then the benefit of AD was summarized in Fig.4.
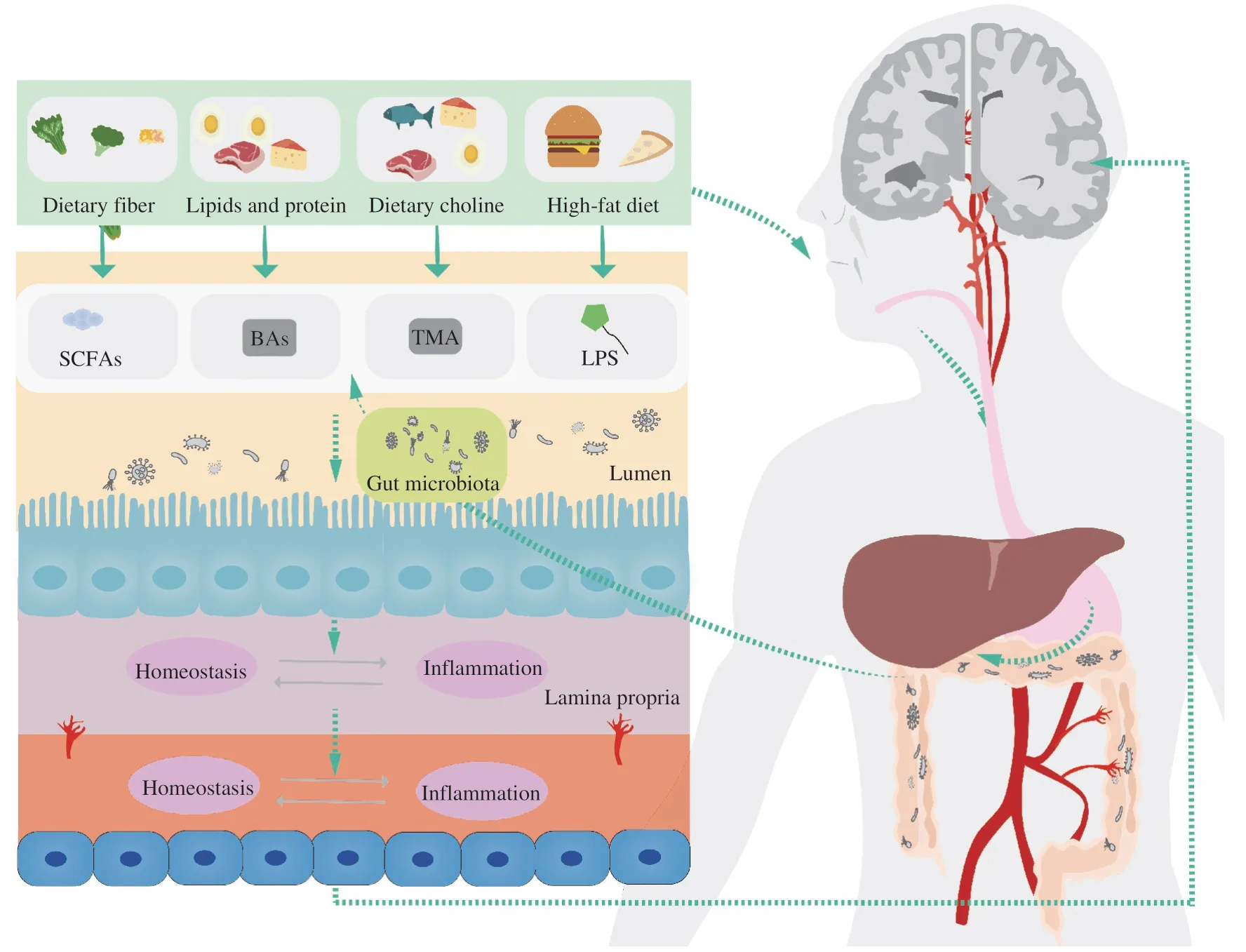
Fig.3 The potential effects of diet on AD through regulation of gut microbiota products.

Fig.4 The potential effects of physical activity on AD through regulation of gut microbiota products.
Many questions remain to be answered on the parameters that make the beneficial effect of AD occur.The duration of interventions is one of the factors for the composition of gut microbiota.David et al.[174]found a significant alteration in gut microbiota composition after a 2-day diet change.It returned to the baseline in 6 days after the end of the dietary intervention.Pagliai et al.[175]reported that a 3-month dietary intervention did not reduce the major change in gut microbiota due to the resilience of gut microbiota.Allen et al.[7]showed gut microbiota composition was significantly altered after 6-week exercise training.It returned to baseline 6 weeks after the end of the exercise intervention.As AD commonly happens among the elderly with difficulties in mastication,swallowing,malnutrition,and other problems,more attention should be paid to daily care and nutritional intake.Moreover,the adherence and adverse events of interventions in old individuals need to be considered.Rigorous exercise is not recommended since exercise-related adverse events such as musculoskeletal injuries are likely to occur in the elderly.A 26-week study investigated the effectiveness and safety of aerobic exercise training,resistance exercise training,and combined aerobic in obese older adults.Results indicated that three modes of excise had excellent PA adherence with a median 96% PA attendance and relatively few adverse events[176].Likewise,Cox et al.[177]reported that a 24-month intervention with 150 min/week of moderate walking,swimming,or biking in the elderly with MCI or subjective memory complaints (SMC) was acceptable and accessible,as the median PA adherence reached 91.67%.
Study related to the role of diet,which is quite complicated,combined with PA on AD through gut microbiota is sparse.Current studies generally lack guidance from experts in various fields for multi-field exploration.As we previously stated,poor nutrition and improper PA may counteract one other’s positive impacts,whereas proper diet and PA may strengthen the benefits of each other,which requires the professional guidance of nutritionists and kinesiologists.Uncovering the underlying mechanisms and guiding the implementation of this strategy to community populations requires the support of clinicians and public health experts.Regarding the underlying mechanisms of diets and PA for the prevention and alleviation of AD through regulating the compositions of gut microbiota,more in-depth research should be considered in future studies.There are many studies on the impact of diet and PA on cognition improvement and alterations of gut microbiota,but there are no in-depth studies on the mechanism of improved cognition properties of diet and PA through gut microbiota.It is suggested to focus on the intricate impact of gut microbiota products on brain health requires in the future.The concentrations of gut microbiota products that can cause or attenuate pathological state are to be learned,and whether there is an inhibitory or promotive relationship between different microbiota products.
To sum up,dietary intervention,as well as exercise programs,should be based on individual body functions.There is a demand for assessment of individual physical conditions and professional guidance.The community needs to increase the availability of community-based services with advanced technologies and support for people living with AD with the help of professional dietitians,fitness experts,and clinicians.
8.Conclusion
Dietary intervention and PA provide a myriad of benefits for the promotion of brain health.They are enticing approaches in the fight against AD since they are relatively easy,benign,and costless.Based on the available evidence,it is conceivable that dietary intervention and PA may change gut microbiota diversity and composition,which contribute to the improvement of cognitive function.However,despite this,the evidence remains controversial.In conclusion,modulation of gut microbiota through diet and PA intervention may be a promising option for AD,but in-depth studies must be carried out to make it more scientific and reasonable.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is financially supported by National Natural Science Foundation of China (32171035),the major fund project of Ningbo Science and Technology Bureau (2019B10034),Opened-end Fund of Key Laboratory (KFJJ-202101,ZPKLP202202),Public Project of Ningbo (202002N3167),Project of Yinzhou(2022AS025) and Ningbo Rehabilitation Hospital (2022KY02),In addition,the work was also sponsored by a K.C.Wong Magna Fund in Ningbo University.
杂志排行
食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18
- Cyanidin-3-glucoside protects the photooxidative damage of retinal pigment epithelium cells by regulating sphingolipid signaling and inhibiting MAPK pathway
