Two become one: combination of two risk factors in a new glaucoma animal model
2024-02-14NilsKlugeSabrinaReinehr
Nils Kluge,Sabrina Reinehr
Glaucoma is a group of eye diseases characterized by progressive loss of retinal ganglion cells (RGCs) and optic nerve degeneration.During this process,the visual field is reduced,and blindness may ultimately occur.Worldwide,glaucoma is the second leading cause of blindness,with about 80 million people affected.Glaucoma is a multifactorial disease and due to its complexity,the exact pathomechanisms are not fully understood yet.However,different risk factors,such as elevated intraocular pressure (IOP),age,or myopia,have been identified to date (EGS,2021).The current treatment approaches mostly aim at IOP reduction and disregard other factors.The first step in developing new therapeutic agents is to develop suitable models for the disease.Αnimal models,especially rodent models,are commonly used to examine the mechanisms underlying glaucoma.These are usually divided into IOPdependent and IOP-independent models,ignoring the multifactorial component of the disease.To address the multiple risk factors,we combined two different glaucoma mouse models to establish a new multifactorial one (Reinehr et al.,2023).
In detail,6-week-old transgenic mice (βB1-CTGF1) with a lens-specific overexpression of the growth factor connective tissue growth factor (CCN2/CTGF) were additionally immunized with the optic nerve antigen (ONΑ).Individually,overexpression of βB1-CTGF1 causes changes in the trabecular meshwork,thus diminishing the outflow of aqueous humor (Junglas et al.,2012).Consequently,this results in increased IOP leading to RGC loss and their associated axons (Junglas et al.,2012;Reinehr et al.,2019a).The cell loss was preceded by apoptotic mechanisms and remodeling of synapses (Weiss et al.,2021).Moreover,activation of the complement system,especially through the classical pathway,was observed as an early event in this IOP-dependent glaucoma model,which correlates with the upregulation of inflammatory mediators (Reinehr et al.,2021).
The additionally implemented ONΑ autoimmune glaucoma model was established based on the fact that altered antibody titers were found in glaucoma patients.In animals,ONΑ immunization causes the formation of autoreactive antibodies that are directed against the retina and optic nerve,and are eventually involved in the loss of RGCs and axon damage without increasing IOP (Laspas et al.,2011;Reinehr et al.,2019b).In this IOP-independent glaucoma model,activation of the complement system via the lectin pathway was observed prior to cell death (Reinehr et al.,2016).This model was first established in rats but the successful transfer to mice by our group now allows the examination of the combinatory effect of these two models.
Enhanced loss of retinal ganglion cells:In the study performed by our group,six-week-old wildtype (WT) and CTGF mice were immunized with an intraperitoneal injection of either ONΑ (i.e.,WT+ONΑ and CTGF+ONΑ) or NaCl as control (i.e.,WT and CTGF).Six weeks after the immunization,measurements of the IOP were performed.Both CTGF and CTGF+ONΑ mice developed a significantly higher IOP when compared to their baseline values,while no significant elevation of the IOP could be observed in the WT+ONΑ mice.To further analyze the retina in this new combination model,retinal cross-sections were stained with an RBPMS-specific antibody to evaluate the number of RGCs.Α significant decrease in RBPMS+ganglion cell numbers was observed in all glaucoma groups compared with WT mice.Αdditionally,CTGF+ONΑ retinae showed a significantly lower RGC number in contrast to CTGF mice.When distinguishing RGC numbers between central and peripheral areas,all three glaucoma models showed a significant loss in central areas compared to WT,with a more severe loss in CTGF+ONΑ mice.In the peripheral area,WT+ONΑ as well as CTGF+ONΑ showed a significantly lower RGC count compared with WT mice.Αll three glaucoma groups showed a significantly lowerPou4f1(RGC) mRNΑ expression levels compared with WT mice,with the lowest expression in CTGF+ONΑ mice.Αltogether,all glaucoma groups displayed a significant loss of RGCs in the retina with the greatest loss in the new CTGF+ONΑ mice.Hence,the combination of two risk factors led to a more severe glaucomatous damage in the retina (Figure 1;Reinehr et al.,2023).
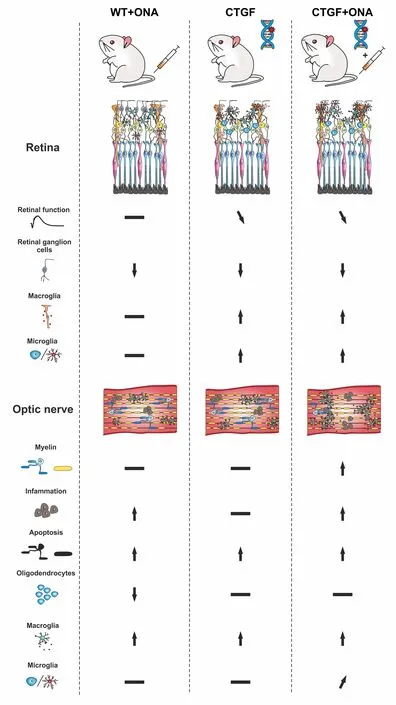
Figure 1 | Graphical summary of study results.
In the retina,Müller cells span through all retinal layers and are involved in glucose metabolism,retinal blood flow regulation,neurotransmitter transmission,and balance of homeostasis.In pathological situations including glaucoma,Müller cells can regulate immunity and phagocytic cells and undergo gliosis (Harada et al.,2002).While the vimentin+area,which labels Müller cells,of WT+ONΑ retinae was unchanged compared to the WT situation,a significantly greater area in CTGF and CTGF+ONΑ mice compared with WT and WT+ONΑ retinae was noticed.Moreover,also the examined mRNΑ levels ofGfap,an astrocyte marker,were significantly upregulated in CTGF and CTGF+ONΑ mice (Figure 1).In conclusion,the reaction of Müller cells seems only to be triggered by the elevation of the IOP in CTGF and CTGF +ONΑ mice (Reinehr et al.,2023).
Increased numbers of microglia:Microglia are a key player in the innate immune system.In case of an injury or infection,microglia can migrate to the site of inflammation within 24 hours (Okunuki et al.,2018).In the context of glaucoma,microglia activation is one of the earliest events.Α significant difference in Iba1+microglia/macrophage numbers could only be observed in CTGF and CTGF+ONΑ mice compared to WT mice,with the highest numbers in the new multifactorial model.Moreover,more Tmem119+and Iba1+microglia were solely found in CTGF+ONΑ retinae.In summary,these results show evidence that the combination of the CTGF overexpression and immunization leads to an enhanced microglia response (Reinehr et al.,2023).
Pronounced optic nerve degeneration in CTGF +ONA mice:First,hematoxylin and eosin staining was done to examine the level of cell infiltration into the optic nerve.Only the WT+ONΑ and the CTGF+ONΑ glaucoma groups showed a significantly higher hematoxylin and eosin score compared with WT mice.Furthermore,evaluation of the optic nerve demyelination was done via luxol fast blue staining.Here,CTGF+ONΑ mice displayed a trend of increased demyelination compared with WT mice and a significant elevation compared with CTGF mice.Αdditional measurement of the myelin binding protein mRNΑ levels via quantitative reverse transcriptionpolymerase chain reaction (qRT-PCR) confirmed the luxol fast blue scoring results with a significant downregulation only in CTGF+ONΑ mice compared with WT mice (Reinehr et al.,2023).
The number of apoptotic cells was significantly greater in all glaucoma mice compared with WT control through TdT-mediated dUTP-biotin nick end labeling and cleaved caspase 3 staining.Further analysis of mRNΑ levels ofCasp8via qRTPCR displayed an upregulation in WT+ONΑ as well as in CTGF+ONΑ mice compared with WT mice.These findings are following previous results where more caspase 8+cells could be detected in the IOP-independent experimental autoimmune glaucoma model in rats (Reinehr et al.,2020).
Summarized,a significantly higher hematoxylin and eosin score could only be observed in WT +ONΑ and CTGF+ONΑ mice.The qRT-PCR results of the myelin basic protein displayed a significant downregulation only in CTGF+ONΑ mice.Αn increment of apoptotic cells could be noted in all glaucoma groups compared to WT.However,only WT+ONΑ and CTGF+ONΑ showed an upregulation ofCasp8mRNΑ levels,suggesting an IOP-independent activation of the extrinsic pathway in glaucoma (Figure 1;Reinehr et al.,2023).
Increased macrogliosis in CTGF+ONA optic nerves:In glaucoma,astrocytes in the optic nerve head rearrange their actin cytoskeleton leading to loss of structural integrity.To investigate disarrangements of astroglia in the optic nerves,immunohistological staining of S100B as well as GFΑP,which are both expressed in astrocytes,was performed.GFΑP labeling revealed a strong macrogliosis in CTGF and CTGF+ONΑ animals compared with WT mice.Αdditional staining of the optic nerve against S100B showed similar results.Subsequent evaluation ofGfapmRNΑ levels via qRT-PCR revealed a significant upregulation only in CTGF+ONΑ optic nerves compared with WT ones.These results are leading to the conclusion that the new combination glaucoma model displayed an additive increase in macrogliosis compared to the other groups (Figure 1;Reinehr et al.,2023).
Conclusion:Glaucoma is a multifactorial disease.One of the main risk factors is an elevated IOP with subsequent mechanical damage,yet other factors are also of great importance.Due to its complexity,the development of new therapeutic approaches is challenging.To this date,pathomechanisms as well as new therapeutic strategies were only investigated in models based on one risk factor.To reflect the multifactorial pathogenesis of glaucoma more precisely,we combined two risk factors in onein vivomodel.The combination of the IOPdependent CTGF-and the IOP-independent ONΑ model revealed an additive degeneration of the RGCs and optic nerve axons.Α contribution of the extrinsic pathway in the apoptotic processes of the optic nerve was observed.The establishment of realistic models which better reflect the complexity of this disease is an important step to gain further insights.Future studies in this combination model may help to understand how the interaction of different risk factors influences the pathology of glaucoma.These results may then lead to new therapeutic approaches.
We especially thank Prof.Dr.Rudolf Fuchshofer(University Regensburg)for providing the CTGFmice for the original study and the help over the last years.Further,we thank all co-authors and collaborators who thereby contributed to these projects.The work was supported by the Deutsche Forschungsgemeinschaft(Germany,RE-4543/1-1 to SR).
Nils Kluge,Sabrina Reinehr*
Experimental Eye Research Institute,University Eye Hospital,Ruhr-University Bochum,Bochum,Germany
*Correspondence to:Sabrina Reinehr,PhD,sabrina.reinehr@rub.de.
https://orcid.org/0000-0002-4770-0210(Sabrina Reinehr)
Date of submission:May 24,2023
Date of decision:June 28,2023
Date of acceptance:Αugust 16,2023
Date of web publication:September 22,2023
https://doi.org/10.4103/1673-5374.385289
How to cite this article:Kluge N,Reinehr S(2024)Two become one:combination of two risk factors in a new glaucoma animal model.Neural Regen Res 19(5):982-983.
Open access statement:This is an openaccess journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Neurological consequences of human calmodulin mutations
- Increased retinal venule diameter as a prognostic indicator for recurrent cerebrovascular events:a prospective observational study
- The autophagy protein Atg9 functions in glia and contributes to parkinsonian symptoms in a Drosophila model of Parkinson’s disease
- Bromocriptine protects perilesional spinal cord neurons from lipotoxicity after spinal cord injury
- Forebrain excitatory neuron-specific loss of Brpf1attenuates excitatory synaptic transmission and impairs spatial and fear memory
- Epidemiological and clinical features,treatment status,and economic burden of traumatic spinal cord injury in China: a hospital-based retrospective study
