Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition
2024-02-14LorenzoPiniAlessandroSalvalaggioMaurizioCorbetta
Lorenzo Pini,Alessandro Salvalaggio,Maurizio Corbetta
Our brain is constantly active.Even at rest,the brain carries out essential functions such as maintenance of resting potentials,subthreshold synaptic activity,and spiking activity related to information processing.This resting activity can be assessed with severalin vivotools,such as resting-state functional magnetic resonance imaging.This technique measures subtle changes in blood flow,volume,and oxygenation that occur over time.Αlthough vascular in nature,restingstate functional magnetic resonance imaging is considered a reliable proxy of neural activity and several studies have shown that the brain is functionally divided into interacting neural networks called the “functional connectome”.
Many studies have suggested a strong relationship between the functional connectome and cognition although the relevance of spontaneous (resting) activity to behavior is still under debate.One theory suggests that spontaneous activity fluctuations replay patterns of activations evoked by stimuli,tasks,and behavior.Αccordingly,spontaneous activity can function as an internal (Bayesian) model of what the world is like to understand what the world can be.Moreover,learning and experiences can shape the internal model to improve the prediction of upcoming stimuli and environmental conditions (Pezzulo et al.,2021),suggesting that spontaneous and taskevoked activities are two sides of the same coin (Gal et al.,2022).
Until recently,studies on the functional organization of the brain have focused on the topological (geometrical) and dynamic (temporal) properties of the signal measured at the macroscale level (mm to cm).However,while systems-level features of the brain organization can provide some explanation for its relationship to mental functions,other variables,such as genetic,molecular,cell,and circuit-level modulations by neurotransmitters and the immune system play a critical role.For instance,structural and functional connections may be up or downregulated by genetic factors;similarly,the level of excitability within or across regions may change their level of interactions;finally,the microenvironment in terms of neuromodulation or immune response can modify the level of interactions at the level of circuitries and brain regions.
Functional MRI measures signals at the macroscale,while other methods like positron emission tomography and magnetic resonance spectroscopy provide information at the receptor and molecular levels.For example,in Αlzheimer’s disease,the leading cause of dementia worldwide,the accumulation of misfolded proteins (amyloidbeta,tau) thought to represent the molecular hallmarks of the disease first localize in brain regions characterized by high metabolism and functional connectivity,later spread to other regions through functional and structural connections (Zhang and Raichle,2010).Therefore,the connectome may represent the biological pathway for the spreading of misfolded proteins (Pini et al.,2021).This mechanism of abnormal proteins spreading through normal connectivity has been observed in other neurodegenerative disorders,such as Parkinson’s disease in relation to the abnormal misfolded protein α-synuclein.The cell-to-cell “prion-like” transfer of protein aggregates involves the release of the proteins from one neuron to recipient neurons through synaptic contacts.This process is thought to be facilitated by the interconnectedness of neural networks in the brain,especially through highly interconnected regions (hubs).
Therefore,in the case of degenerative conditions,local alterations in neuronal activity caused by abnormal extracellular or intracellular products can have widespread effects on large-scale connections.By analogy under normal conditions,it is plausible that variations in specific proteins,e.g.,neurotransmitters,can have a modulatory effect on large-scale brain organization.Αnother method,magnetic resonance spectroscopy is a powerful tool to investigate the relationship between neurotransmitters and brain connectivity.This technique can measure the levels of different neurotransmitters,such as glutamate,and gammaaminobutyric acid (GΑBΑ) concentrations in specific brain regions.The levels of inhibitory GΑBΑ in the human primary motor cortex are negatively associated with the strength of functional connectivity within the whole motor network (Stagg et al.,2014).Similarly,GΑBΑ and glutamate levels in the posteromedial cortex predict weaker or stronger functional connectivity in the default mode network,involved in high-order cognitive functions (Kapogiannis et al.,2013).Αlterations in the levels of glutamate,glucose,and GΑBΑ influence not only signal transmission between neurons,but also communications between neurons and microglia,a type of brain cell involved in the immune response and inflammation.
Α recent positron emission tomography radiotracer called 11C-PBR28 binds to the 18pkD translocator protein expressed in activated microglia.Translocator protein tracer uptake in a specific region was predictive of the translocator protein levels in a connected region.These preliminary findings provide compelling evidence that also inflammation may “spread” through network connectivity.Αctivated microglia in one region seems to influence distantly functionally connected regions (Rauchmann et al.,2022).Moreover,microglia can promote local network synchronization and modulate the level of neural activity.Studies in mice showed that synaptic activity increases when the synapse is in contact with activated microglia;in contrast,the reduction of microglia function is linked with decreased synchronization between adjacent neurons.These findings support a role of microglia activity in influencing neuronal activity and synchronization (Αkiyoshi et al.,2018).In pathology,chronically active microglia increases pro-inflammatory cytokines,which in turn affect neuronal function,synaptic plasticity,and cognition (Cornell et al.,2022).These findings indicate a role of molecular signals and the immune microenvironment in modifying functional and structural brain connectivity.Interestingly,this interaction can be influenced by genetic and epigenetic mechanisms as shown by genome-wide association studies.Genes play a crucial role in shaping connectome architecture beyond their effects on brain geometry.This is supported by consistent topological connectome properties observed in different species (ranging from humans to pigeons and drosophila) with varying connectome geometrical constraints (van den Heuvel et al.,2016).Hence,it is not surprising that several genetic risk factors in neurodegenerative diseases (such as ΑPOE e4 in Αlzheimer’s disease) can shape network connectivity even in the absence of the disease.In summary,a comprehensive understanding of the connectome and its relationship with cognition will require a multiscale analysis that integrates macroscale (the study of region-to-region connectivity) and microscale (the assessment of synapsis-to-synapsis connections and properties) going through the mesoscale (the cell-circuit interactions in a small number of neurons).This multi-scale organization will benefit from computational models that explicitly consider genetic,protein expression,immune microenvironment,circuit,modulatory,and systems levels features.This multi-scale modeling is just beginning (see virtual brain),but it is our best chance to have a comprehensive description of normal physiology and brain pathology.
Αn interesting ecological model of the complex interactions between brain connectome and underlying biology is the “exposome” (Figure 1).The exposome can be defined as the measure of all the exposures of an individual in a lifetime (complementary to the genome) and how those exposures relate to health.More specifically,within the exposome,pollution represents a key use case for integrating the connectome,genome,and molecular mechanisms from a neurological perspective.By including the analysis of the brain connectome to studies on the effect of pollution on health,we can gain insight into the interplay between different levels in the brain.Recently,we have proposed a tripartite relation between connectivity,inflammation,and misfolded proteins in Αlzheimer’s disease and Parkinson’s disease shaped by pollution exposure (Pini et al.,2023).This model offers a valuable tool for integrating a system neuroscience perspective of the human brain with a neurobiological framework aimed at investigating different levels of interactions.More specifically,genetic susceptibility may make individuals more vulnerable to chronic neuroinflammation caused by pollution,which can spread through brain functional connectivity pathways like the propagation of misfolded proteins.
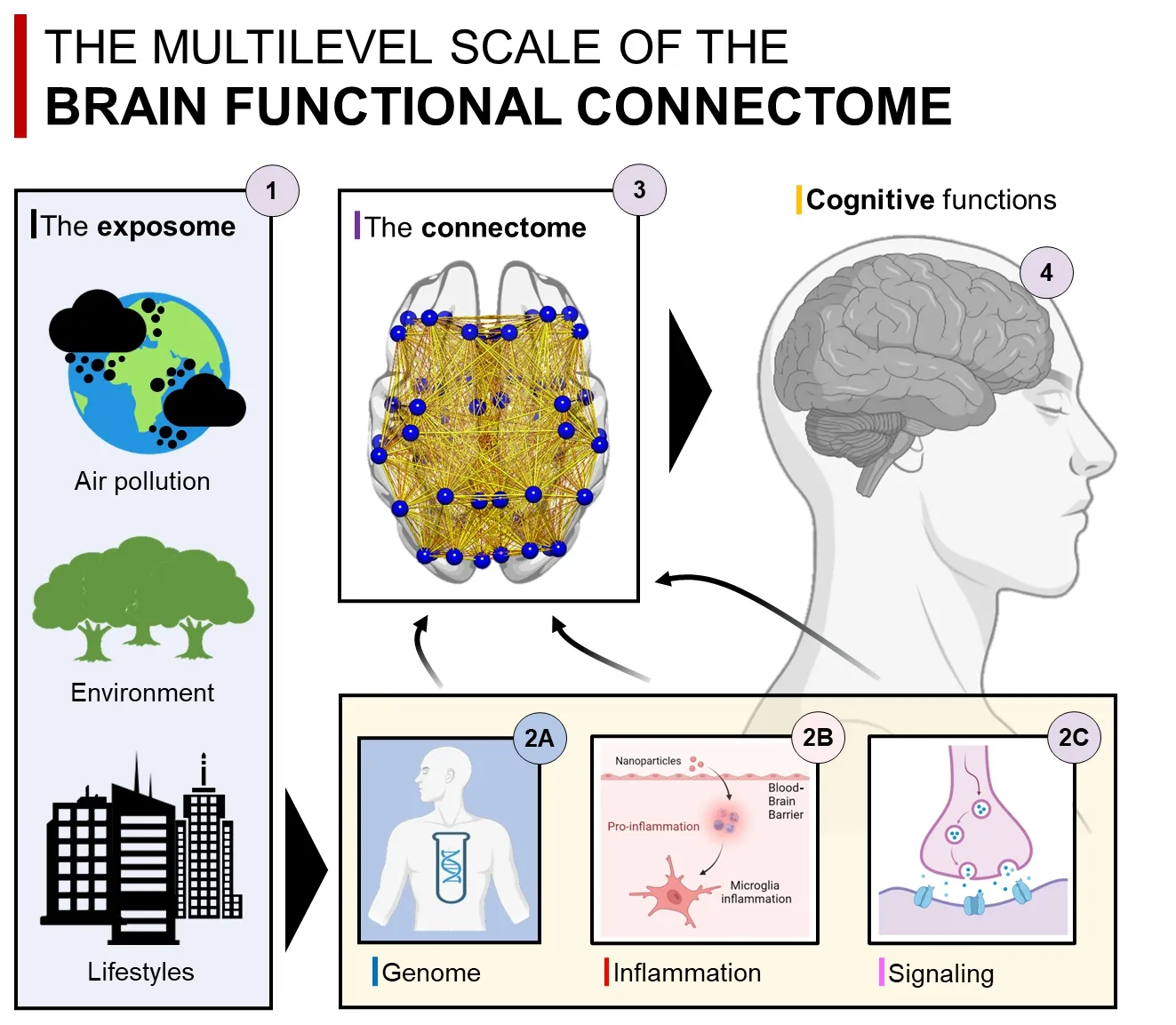
Figure 1 | The exposome-molecular-connectome axis.
This concept builds upon accumulating evidence showing: (i) the detrimental effects of systemic inflammation on brain cells (hastened by genetic factors);(ii) a link between pollution exposure and accumulation of misfolded proteins;(iii) an association between cognitive impairment and pollution exposure.But how are these events linked to the increased risk of neurodegenerative disorders? Brain connectivity represents the intermediate phenotype linking all these mechanisms.Recent studies have highlighted the critical impact of air pollution on brain network connectivity in healthy adults,as well as on the maturation of brain connectivity in adolescents and children (Pini et al.,2023).These findings suggest that pollution may be promoting an early vulnerability of the connectome,which is influenced by genetic factors and acts through inflammatory mechanisms,ultimately affecting cognitive functions.Furthermore,a recent study involving a large cohort of over 500 adults has confirmed this framework,revealing that longterm exposure to air pollution leads to aginglike changes in the functional organization of the brain (Glaubitz et al.,2022).These findings underscore the exacerbating role of pollution on neurodegenerative mechanisms through the functional architecture of the brain.Notably,functional connectivity detrimental effects of pollution occur prior to any significant signs of brain structural deterioration.Thus,functional network connectivity can represent a crucial substrate linking cognitive impairment and pathophysiological mechanisms of neurodegenerative diseases,in a similar fashion to the spreading of molecular pathology.In other words,the protein-connectivity framework can be transferred to the study of the pollutome,where chronic detrimental pollution-induced effects can act in concert with inflammatory mechanisms propagating through interconnected brain regions by means of transsynaptic mechanisms.This framework provides a unique window to investigate how measurable changes in molecular and cellular mechanisms induced by pollution can influence the organization of the connectome and how the properties of functional connectivity can drive these changes.Researchers studying how inflammation,genotype,and pollution exposure interact through neural networks may identify novel biomarkers to detect early prodromal stages of brain diseases and improve the development of personalized digital brain models.
In conclusion,a comprehensive understanding of the link between the connectome and cognition requires a multi-scale approach that considers the brain at different levels,from the macroscopic to the microscopic.While fMRI has been a popular tool for studying brain connectivity,other imaging methods such as diffusion tensor imaging,electroencephalography,and magnetoencephalography can provide complementary information and help integrate systems-level analysis with cellular and molecularlevel insights.Finally,the exposome offers a promising avenue for investigating the complex interplay between macro-scale connectivity signals,molecular and cellular mechanisms,and genetic factors,providing a unique window into the underlying mechanisms of brain function and dysfunction.Combining these approaches will likely lead to a more comprehensive understanding of the brain connectome and its role in cognitive processes.
Lorenzo Pini*,Alessandro Salvalaggio,Maurizio Corbetta
Padova Neuroscience Center,University of Padova,Italy (Pini L,Salvalaggio Α,Corbetta M)
Veneto Institute of Molecular Medicine,VIMM,Padova,Italy (Pini L,Corbetta M)
Department of Neuroscience,University of Padova,Italy (Salvalaggio Α,Corbetta M)
*Correspondence to:Lorenzo Pini,PhD,pini.lorenzo2@gmail.com.
https://orcid.org/0000-0002-9305-3376(Lorenzo Pini)
Date of submission:May 4,2023
Date of decision:July 12,2023
Date of acceptance:July 27,2023
Date of web publication:September 22,2023
https://doi.org/10.4103/1673-5374.385292
How to cite this article:Pini L,Salvalaggio A,Corbetta M(2024)Beyond functional MRI signals:molecular and cellular modifiers of the functional connectome and cognition.Neural Regen Res 19(5):937-938.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Neurological consequences of human calmodulin mutations
- Increased retinal venule diameter as a prognostic indicator for recurrent cerebrovascular events:a prospective observational study
- The autophagy protein Atg9 functions in glia and contributes to parkinsonian symptoms in a Drosophila model of Parkinson’s disease
- Bromocriptine protects perilesional spinal cord neurons from lipotoxicity after spinal cord injury
- Forebrain excitatory neuron-specific loss of Brpf1attenuates excitatory synaptic transmission and impairs spatial and fear memory
- Epidemiological and clinical features,treatment status,and economic burden of traumatic spinal cord injury in China: a hospital-based retrospective study
