类钙钛矿甲酸盐的线性和非线性光学响应
2024-01-20冉丛娇黄智鹏
齐 鲁 冉丛娇 吴 超 黄智鹏 张 弛
(同济大学化学科学与工程学院中澳功能分子材料联合研究中心,上海 200092)
Organic-inorganic hybrid materials are highly attractive in solar cells, photo-detectors, ferroelectrics,optical information processing, and laser frequency conversion due to their excellent performance and versatility[1-5].Nonlinear optical (NLO) crystals can generate coherent laser light, which is widely used in photolithography and semiconductor manufacturing,and plays an important role in solid-state lasers[6-10].Recently, a series of excellent organic-inorganic hybrid NLO crystals, such as (o-C5H4NHOH)2[I7O18(OH)] ⋅3H2O[11], (C(NH2)3)6(PO4)2·3H2O[12], Cs3Cl(HC3N3S3)[13],and (2cepyH)SbBr4[14], have been developed.It is of great importance and interest to search for new functional building blocks as well as the as-constructed new organic-inorganic hybrid NLO crystals.Organic molecules play an important role in modulating the optical properties when designing organic-inorganic hybrid NLO crystals.π-Conjugated organic cations exemplified by (C(NH2)3)+cation[15-16], melamine[17], and 4-hydroxypyridine[18], have been discovered and proved to be efficient NLO-active groups owing to their strong hyperpolarizability and polarizability anisotropy.Recently, Lin et al.adopted melamine and synthesized a potential ultraviolet NLO material (C3H7N6)2Cl2·H2O exhibiting a strong second harmonic generation (SHG)response and large birefringence[17].(C(NH2)2NHNO2)+is an excellent cationic NLO-active group, and the resultant material showed excellent NLO properties[19].Inorganic anions are an important component of NLO materials.Significant efforts have been focused on these inorganic salts containingπ-conjugated planar groups (e.g.BO33-, CO32-, NO3-), such as KBe2BO3F2[20],LiB3O5[21],β-Ba2B2O4[22], ABCO3F (A=K, Rb, B=Mg, Ca,Sr)[23], LiZn(OH)CO3[24], Bi3TeO6OH(NO3)2[25], RE(OH)2NO3(RE=La, Y, and Gd)[26].However, owing to the intricate multiple inter-anion interactions,the combination of two types ofπ-conjugated groups into one structure to create new NLO materials is thus far underexploited[27].
The organic-inorganic hybrid perovskites with general formula ABXn(n=3,4)have attracted extraordinary attention in physics, chemistry, and materials science[28-30].The unique structural feature offers tremendous possibilities for optical property tuning owing to the flexible and replaceable A, B, and X sites[31].We report herein the construction of the NLO-active formate salts (CH(NH2)2)[RE(HCOO)4] (RE=Y, Er) containing theπ-conjugated formamidine cation and formate anion.Both two materials are isostructural with perovskite-like structures.The synthesis, UV-Vis-NIR spectroscopy, SHG response, and birefringence are demonstrated.Density functional theory (DFT) calculation was performed to reveal relationships between linear and nonlinear optical properties and electronic states.
1 Experimental
1.1 Reagents
Yttrium nitrate (Y(NO3)3·6H2O, 99.99%, Xiya Reagent),erbium nitrate(Er(NO3)3·5H2O,99%,Adamas Reagent), formamide (CH3NO, 99%, Adamas Reagent)were commercially available and used as received without further purification.
1.2 Synthesis of (CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4]
A mixture of Y(NO3)3·6H2O (0.192 g, 0.50 mmol)and CH3NO (5 mL) was tightly sealed in a 23 mL autoclave equipped with a Teflon liner.The autoclave was heated at 110 ℃for 72 h and then cooled slowly to room temperature at a rate of 3 ℃·h-1.The product was collected by vacuum filtration, washed with ethanol,and dried in the air.Colorless block crystals of(CH(NH2)2)[Y(HCOO)4] were isolated in a yield of 70%(based on Y) using a microscope.The same process was operated to synthesize compound (CH(NH2)2)[Er(HCOO)4] using Er(NO3)3·5H2O (0.222 g, 0.50 mmol), and CH3NO (5 mL).Pink block crystals were picked out in a yield of 75%(based on Er).
1.3 Single crystal and powder X-ray diffraction
Single-crystal X-ray diffraction data collection of(CH(NH2)2)[RE(HCOO)4] (RE=Y, Er) was carried out on a Bruker D8 VENTURE CMOS X-ray diffractometer using graphite-monochromated MoKαradiation (λ=0.071 073 nm) at room temperature.APEX Ⅱsoftware was applied to collect and reduce data.For (CH(NH2)2)[Y(HCOO)4], in a range of 3.25°<θ<27.12°, a total of 10 270 reflections were collected and 1 150 were independent withRint=0.049 1,of which 1 089 were observed withI>2σ(I).For (CH(NH2)2)[Er(HCOO)4], in a range of 3.255°<θ<27.14°, a total of 9 394 reflections were collected and 1 152 were independent withRint=0.034 3,of which 1 129 were observed withI>2σ(I).Semiempirical absorption corrections based on equivalent reflections were applied for both data sets using the APEX Ⅱprogram.The two structures of (CH(NH2)2)[RE(HCOO)4] (RE=Y, Er) were solved by direct methods and refined onF2by full-matrix least-squares methods using Olex2 software package[32-33].All hydrogen atoms were placed in calculated positions and refined with a riding model.The detailed crystallographic data and structural refinement parameters of the two compounds are summarized in Table S1 (Supporting information).Selected bond distances (nm) and angles(°)are given in Table S2 and S3,while hydrogenbonding interactions are provided in S4 and S5.Powder X-ray diffraction (PXRD) was used to confirm the phase purity of (CH(NH2)2)[RE(HCOO)4] (RE=Y, Er).The PXRD analysis of each sample was carried out on a Bruker D8 X-ray diffractometer equipped with CuKαradiation (λ=0.154 18 nm)in a 2θrange of 5°-80°with a step size of 0.02° at room temperature, and the working voltage and current were 40 kV and 40 mA,respectively.
CCDC:2312145,(CH(NH2)2)[Y(HCOO)4];2312159,(CH(NH2)2)[Er(HCOO)4].
1.4 Energy-dispersive X-ray spectroscopy
Elemental analyses were performed using energydispersive X - ray spectroscopy (EDS) with a field -emission scanning electron microscope(FESEM,Hitachi S-4800, Japan).The EDS analyses on (CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4] confirm the presence of C,H,O,N,and Y/Er,which are evenly dispersed in both samples(Fig.S1).
1.5 Infrared and UV-Vis-NIR diffuse reflectance spectra
The infrared (IR) spectra were recorded on a Nicolet iS10 Fourier transform IR spectrometer (resolution 4 cm-1,spectral range 400-4 000 cm-1).Optical diffuse-reflectance spectra were collected on a Cary 5000 UV-Vis-NIR spectrophotometer over the spectral range 200-800 nm at room temperature and a BaSO4plate was used as a 100% reflectance standard.Reflectance spectra were converted into absorbance spectra using the Kubelka-Munk functionα/S=(1-R)2/(2R), whereαis the absorption coefficient,Sis the scattering coefficient that is practically wavelength-independent when the particle size is larger than 5 µm, andRis the reflectance[34].
1.6 Thermal analysis
A Netzsch STA 409PC thermal analyzer was used to analyze the thermal stabilities of (CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4].The samples were heated from 30 to 800 ℃with a heating rate of 15 ℃·min-1under a nitrogen atmosphere.
1.7 Powder SHG measurements
The SHG intensities of (CH(NH2)2) [Y(HCOO)4]and (CH(NH2)2)[Er(HCOO)4] were measured employing the powder measurement method proposed by Kurtz and Perry[35].A Q-switched Nd∶YAG laser with 1 064 nm radiation was employed for the visible SHG study.Because the SHG efficiency is related to the particle size,the polycrystalline samples of (CH(NH2)2)[Y(HCOO)4]and (CH(NH2)2)[Er(HCOO)4] were ground and sieved into several particle size ranges (0-26, 26-50, 50-74,74-105,105-150,150-200,and 200-280µm).Crystalline KDP with the same particle size ranges was used as references.
1.8 Birefringence measurements
The birefringences of (CH(NH2)2)[Y(HCOO)4] and(CH(NH2)2)[Er(HCOO)4] were assessed with a polarizing microscope (ZEISS Axio Scope.A1) equipped with a Berek compensator.The wavelength of the light source was 546 nm.The positive and negative rotation of compensation affords the relative retardation.
1.9 Theoretical calculations
First-principles calculations on (CH(NH2)2)[Er(HCOO)4] were performed using the CASTEP package[36], a total energy package based on pseudopotential DFT[37].The correlation-exchange terms in the Hamiltonian were described by the functional developed by Perdew, Burke, and Ernzerhof in the generalized gradient approximation form[38-39].Optimized norm-conserving pseudopotentials[40]in the Kleinman-Bylander form were adopted to model the effective interaction between the valence electrons and atom cores, which allows the choice of a relatively small plane-wave basis set without compromising the computational accuracy.A kinetic energy cutoff of 850 eV and dense Monkhorst-Pack[41]k-point meshes spanning less than 1.5×10-5nm3in the Brillouin zone were chosen.
2 Results and discussion
2.1 Synthesis, phase purity, IR, and thermal stability
The mixed organic cationic hybrid formates(CH(NH2)2) [Y(HCOO)4] and (CH(NH2)2) [Er(HCOO)4]was obtained through a mild solvothermal method.Both two crystals were synthesized directly by employing in situ reactions[42].The phase purity of crystalline(CH(NH2)2) [Y(HCOO)4] and (CH(NH2)2) [Er(HCOO)4]were confirmed by PXRD (Fig.S2).The IR spectra of(CH(NH2)2) [Y(HCOO)4] and (CH(NH2)2) [Er(HCOO)4]were similar owing to their isostructural feature.The IR absorption bands and their assignments are shown in Fig.S3, and they are characteristic of N—H vibrations in the formamidine (CH(NH2)2)+cation and formate HCOO-anion.(CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4] also exhibited similar thermal behavior(Fig.S4), and taking (CH(NH2)2)[Y(HCOO)4] as representative.The thermal analysis curve of (CH(NH2)2)[Y(HCOO)4] (Fig.S4a) exhibited three weight - loss steps: in the first stage (220-300 ℃), the weight loss of 28.85% was close to the calculated value of 28.57% by loss of 2CO and 2NH3.The second weight loss of 27.62% was close to the calculated value of 28.25% by loss of 1.5H2,1.5CO,and CO2in a range of 310-410 ℃.The minor weight loss of 6.38% that occurred in a range of 410-800 ℃is due to the loss of CO2(Calcd.6.98%).The residue of (CH(NH2)2) [Y(HCOO)4] and(CH(NH2)2)[Er(HCOO)4] after 800 ℃was confirmed as Y2O3/Er2O3by PXRD(Fig.S5).
2.2 UV-Vis-NIR diffuse reflectance spectra
The study of optical diffuse reflectance spectra showcases that two crystalline materials could be classified among wide optical bandgap semiconductors possessing bandgaps of 5.59 and 5.61 eV for (CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4], respectively.(Fig.1) The bandgaps increased slightly with the increase of atomic number for the lanthanide ions.The UV absorption edge of (CH(NH2)2)[Y(HCOO)4] was 222 nm, and there was no absorption peak ranging from 222 to 800 nm.(CH(NH2)2) [Er(HCOO)4] exhibited sharp absorption bands at 257, 380, 525, and 654 nm.Thef-ford-ftypical transitions of the respective lanthanide(Ⅲ)ions lead to these absorption peaks[43].
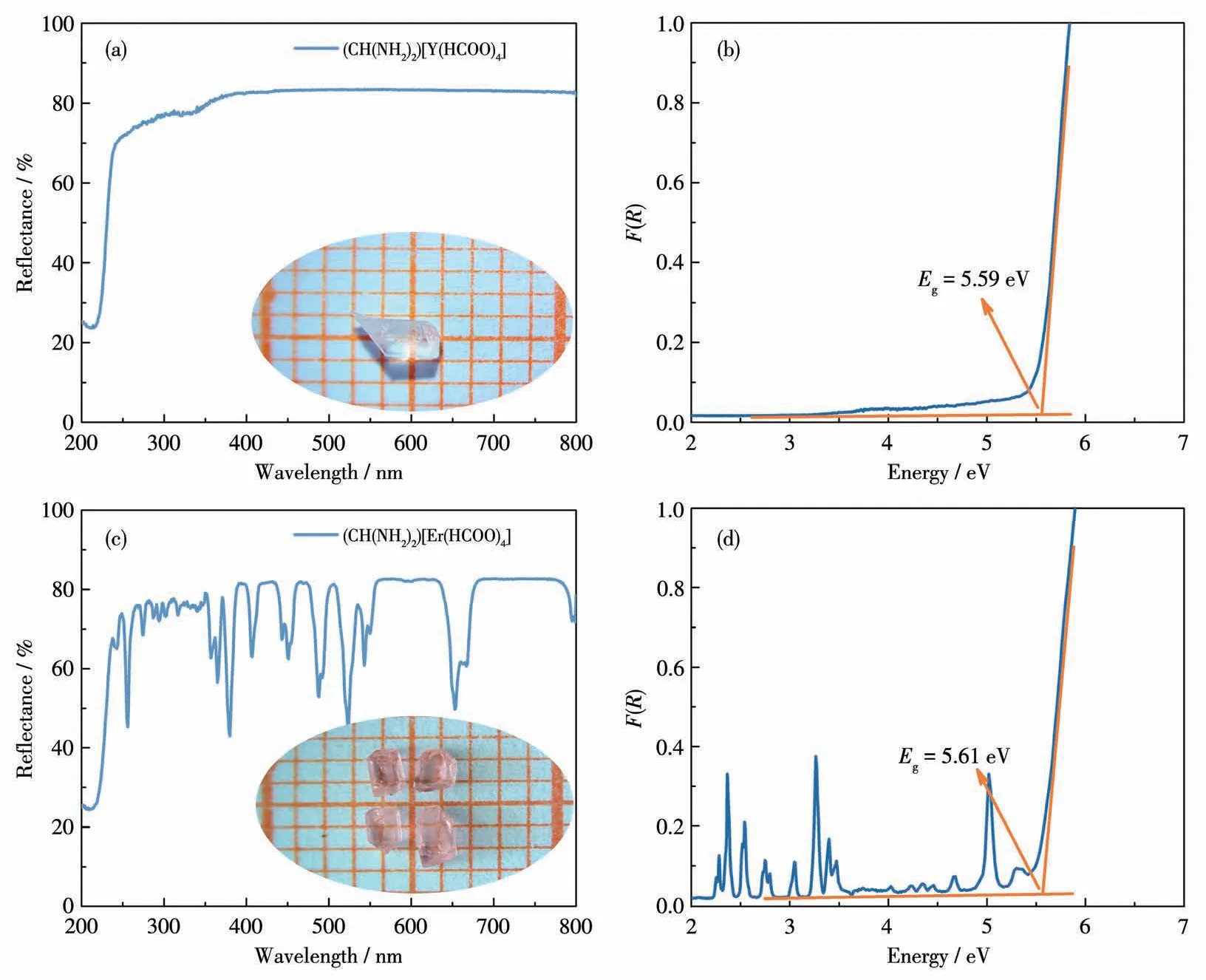
Fig.1 UV-Vis-NIR diffuse reflectance spectra and the corresponding crystal morphologies (Inset)for(CH(NH2)2)[Y(HCOO)4](a)and(CH(NH2)2)[Er(HCOO)4](c);Bandgaps for(CH(NH2)2)[Y(HCOO)4](b)and(CH(NH2)2)[Er(HCOO)4](d)
2.3 Powder SHG response
Based on the chiral structure with a space group ofC2221, the powder SHG response was measured by the Kurtz-Perry method.The SHG intensities increased with the increasing of particle size in a range of 26-280µm (Fig.2a), indicating both (CH(NH2)2)[Y(HCOO)4]and (CH(NH2)2)[Er(HCOO)4] are phase-matchable crystals at the 1 064 nm laser.As shown in Fig.2b,compounds (CH(NH2)2) [Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4] showed the SHG intensity of about 0.32 and 0.37 times that of KDP in a particle size range of 105-150 µm.Because (CH(NH2)2)[RE(HCOO)4] (RE=Y, Er) crystallizes in theC2221space group, both crystals have one non-zero independent SHG coefficient(d14) under the restriction of Kleinman symmetry.The corresponding calculated SHG values of (CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4] were 0.71 and 0.29 pm·V-1, respectively.To quantify the specific SHG contributions from all units, a real-space atomcutting method was employed.As shown in Table S6,the (CH(NH2)2)+units made the dominant contributions to the SHG coefficientd14(58.9% and 48.7%) with the HCOO-anions accounting for 25.0% and 28.2% for(CH(NH2)2) [Y(HCOO)4] and (CH(NH2)2) [Er(HCOO)4],respectively.We conclude that (CH(NH2)2)+and HCOO-groups play a decisive role in the SHG response,and the contributions of Y3+and Er3+to the SHG response should not be ignored.
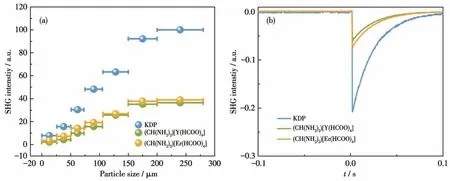
Fig.2 (a)Phase-matching curves of(CH(NH2)2)[Y(HCOO)4],(CH(NH2)2)[Er(HCOO)4],and KDP with 1 064 nm laser radiation;(b)Oscilloscope traces of the SHG signals for polycrystalline(CH(NH2)2)[Y(HCOO)4],(CH(NH2)2)[Er(HCOO)4],and KDP in a particle size range of 105-150µm at λ=1 064 nm
2.4 Birefringence
The birefringence of (CH(NH2)2)[RE(HCOO)4](RE=Y,Er)single crystal was measured on a polarizing microscope (ZEISS Axio A1), which can achieve complete extinction (Fig.3).The optical path difference of(CH(NH2)2) [Y(HCOO)4] and (CH(NH2)2) [Er(HCOO)4]with thicknesses of 158.1 and 167.3 µm were measured to be 2.055 and 2.510µm,respectively.Birefringence occurs and causes the polarized light to decompose into two kinds of polarized light, which are fast polarized light and slow polarized light when polarized light enters an anisotropic single crystal.The generation of optical path difference between the fast polarized light and the slow polarized light is inevitable in this process.The optical path difference ΔRcan be obtained by the Eq.: ΔR=|Ne-No|δ=δΔn, where ΔRdenotes the optical path difference, Δnrepresents the birefringence, andδis the thickness of the crystal.The derived birefringences were 0.013 and 0.015 for(CH(NH2)2) [Y(HCOO)4] and (CH(NH2)2) [Er(HCOO)4],respectively,at the wavelength of 546 nm.
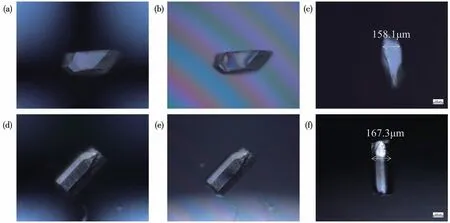
Fig.3 Original crystals(a,d),crystal diagrams to achieve complete extinction(b,e),and thickness diagrams(c,f)of compounds(CH(NH2)2)[Y(HCOO)4](a-c)and(CH(NH2)2)[Er(HCOO)4](d-f)
2.5 Crystal structures and their relationships with optical properties
Both (CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4] are isostructural, and we take (CH(NH2)2)[Y(HCOO)4]asrepresentative.In(CH(NH2)2)[Y(HCOO)4],one Y3+cation, two HCOO-groups, and one formamidinium cation form the asymmetric units.All of the Y atoms in the unit cell are equivalent and located at the origin.The central Y3+is coordinated with the surrounding eight oxygen atoms to compose a YO813-polyhedron with four different Y—O bond lengths.As shown in Fig.4a, the lengths of the Y—O bond are 0.228 6, 0.233 8, 0.239 9, and 0.241 0 nm, respectively.The 1Dchainstructure is composedof YO813-octahedra andπ-conjugated HCOO-planar groups(Fig.4b).The orientation of the C—H bonds in formic acid ligands is diverse, but the alignment in the same direction is consistent when forming 2D and 3D frames.This arrangement can be conducive to the NLO properties of the crystal.Each Y3+ion links to eight neighboring Y3+ions via formate bridges, to form the rhombohedral units′ network, and the unit has the two neighboring grids diagonally crossed by one anti-anti HCOO-.The 2D [Y(HCOO)n]∞layers contain 12-membered rings (MRs) and 16-MRs viewed along the crystallographicc-axis (Fig.4c).The 3D (CH(NH2)2)[Y(HCOO)4]framework possesses perovskite-like structure.The center of the polyhedron is occupied by the Y atom, and (CH(NH2)2+cationsinsituproduced from formamide lie in a cage surrounded by a YO813-polyhedron.(Fig.4d) The (CH(NH2)2)+cations connect the adjacent [Y(HCOO)n]∞layers through N—H…O hydrogen bonds, and the adjacent (CH(NH2)2)+cations are arranged in opposite directions.It is beneficial to break the symmetry of the structure.However, the parallel and consistent orientation of(CH(NH2)2)+in the diagonal rhombohedral cell cavity promotes its SHG response,and the strong covalent bonds of theπ-conjugated cation (CH(NH2)2)+can facilitate a relatively wide UV transparency.The combination ofπ-conjugated formate and formamidine groups can not only enhance the nonlinear optical properties of the compounds but also expand the bandgap.In light of anionic group theory,π-conjugated (CH(NH2)2)+and HCOO-groups are suggested as possibilities to generate the SHG responses while also retaining the short UV absorption edge.
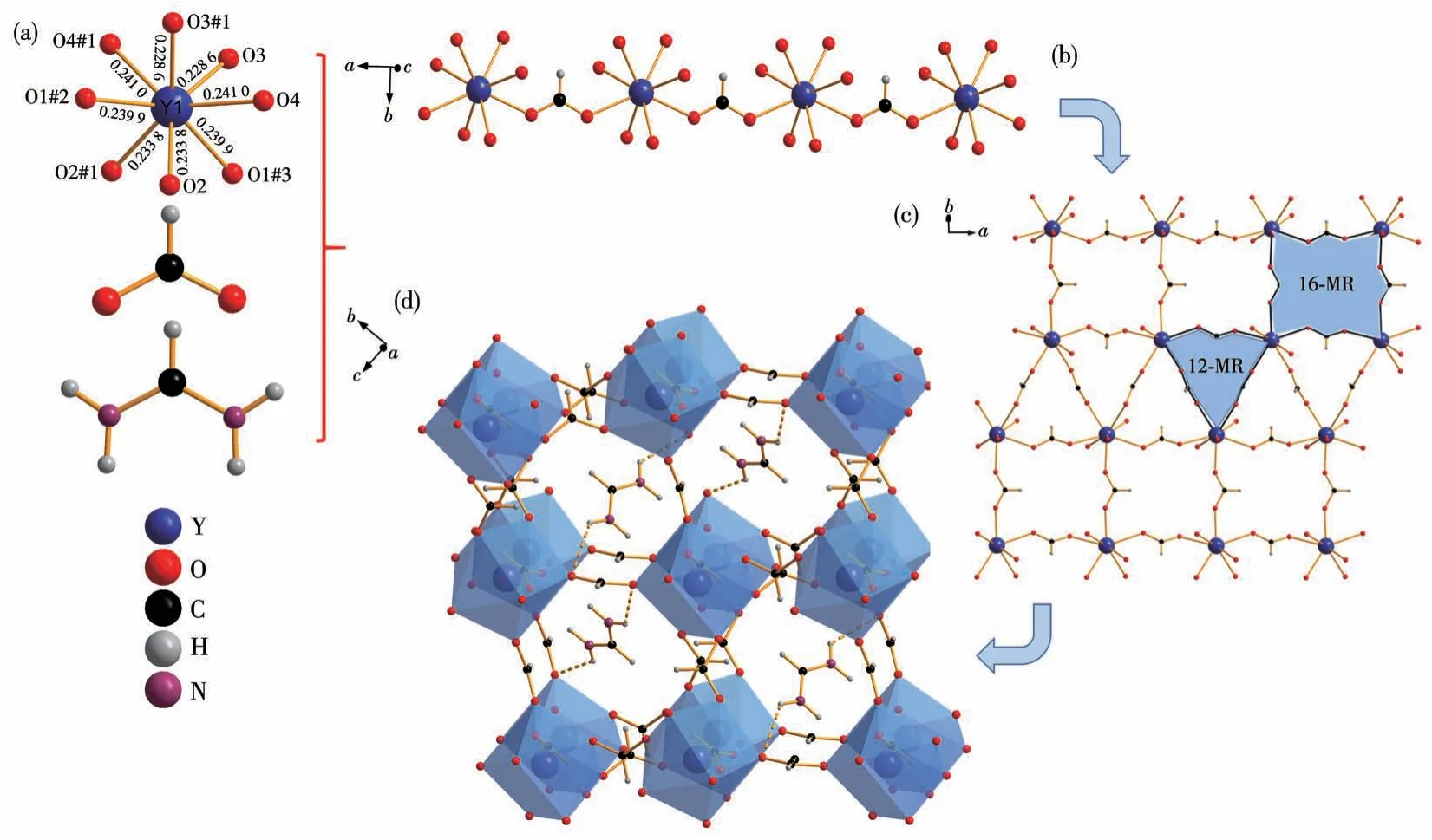
Fig.4 (a)Coordination environment of Y3+in(CH(NH2)2)[Y(HCOO)4],HCOO-ligand group and(CH(NH2)2)+group;(b)1D chain of[Y(HCOO)n]∞;(c)2D[Y(HCOO)n]∞layers containing 12-MRs and 16-MRs viewed from the c-axis direction;(d)3D structure of the cavity-template units in a(CH(NH2)2)[Y(HCOO)4]crystal
2.6 Theoretical calculations
Linear and nonlinear optical properties are closely related to the characteristics of band structure,including bandgap and density of state (DOS).To further comprehend the relationships between electronic structure and optical properties, theoretical calculations using pseudopotential DFT methods were carried out to calculate the bandgap and DOS of (CH(NH2)2)[Er(HCOO)4].As shown in Fig.5a, the calculated band structure of (CH(NH2)2)[Er(HCOO)4] indicates that it possesses a direct bandgap of 4.52 eV with both the conduction band minimum (CBM) and the valence band maximum (VBM) located at the G point.These calculated values were smaller than the experimental value due to the underestimation of the bandgap with the DFT method.Fig.5b shows the total and partial densities of states (TDOS and PDOS) of (CH(NH2)2)[Er(HCOO)4].The VBs are mainly occupied by N2p,C2p, O2pand Er4fstates.The CBs are primarily assigned to C2pand O2pstates, with slight contributions from the Er4dorbital, which indicates that the electron transition is mainly contributed by inside excitation of(CH(NH2)2)+and HCOO-groups, and slight contributions from ErO813-polyhedra.These results indicate that optical performance (bandgap, SHG response, and birefringence) of (CH(NH2)2) [Er(HCOO)4] determined by electronic transitions are mostly governed by the interaction of(CH(NH2)2)+and HCOO-groups.
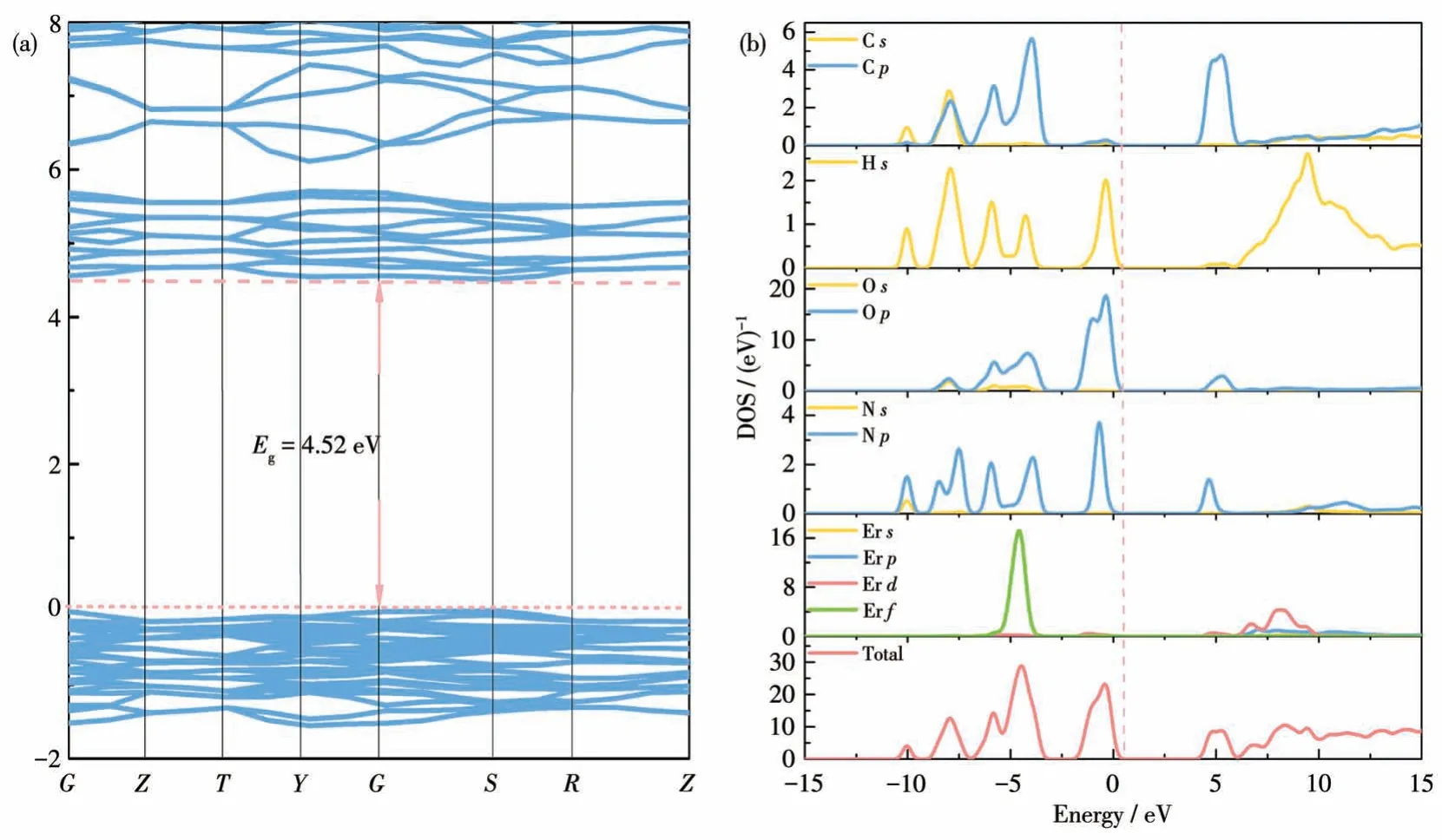
Fig.5 (a)Energy band structures,(b)TDOS and PDOS of compound(CH(NH2)2)[Er(HCOO)4]
3 Conclusions
In summary, two isomeric rare-earth formate nonlinear optical materials (CH(NH2)2)[RE(HCOO)4] (RE=Y, Er) were successfully synthesized through an in situ solvothermal method and comprehensively determined by various types of spectroscopic techniques.They exhibit perovskite-like structures where the negatively charged cavity of the [RE(HCOO)4]-anionic framework is occupied by the(CH(NH2)2)+cations.The powder SHG measurements indicated that (CH(NH2)2)[RE(HCOO)4](RE=Y, Er) possess phase-matchable SHG responses in the visible regions.Linear optical studies show that(CH(NH2)2)[Y(HCOO)4] and (CH(NH2)2)[Er(HCOO)4]exhibited optical bandgaps of 5.59 and 5.61 eV, and birefringences of 0.013 and 0.015, respectively.DFT calculations show that the optical characteristics are mainly attributed to the synergistic effects of twoπconjugated (CH(NH2)2)+and HCOO-groups.This work highlights that the introduction ofπ-conjugated formamidine cations into formate systems may be an effective approach for the development of excellent NLO materials.
Supporting information is available at http://www.wjhxxb.cn
