The competition between Bidens pilosa and Setaria viridis alters soil microbial composition and soil ecological function
2024-01-17QiaoLiJianyingGuoHanZhangMengxinZhao
Qiao Li ,Jianying Guo ,Han Zhang, ,Mengxin Zhao#
1 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,China
2 College of Life Science,Yangtze University,Jingzhou 434025,China
Abstract Bidens pilosa is recognized as one of the major invasive plants in China.Its invasion has been associated with significant losses in agriculture,forestry,husbandry,and biodiversity.Soil ecosystems play an important role in alien plant invasion.Microorganisms within the soil act as intermediaries between plants and soil ecological functions,playing a role in regulating soil enzyme activities and nutrient dynamics.Understanding the interactions between invasive plants,soil microorganisms,and soil ecological processes is vital for managing and mitigating the impacts of invasive species on the environment.In this study,we conducted a systematic analysis focusing on B. pilosa and Setaria viridis,a common native companion plant in the invaded area.To simulate the invasion process of B.pilosa,we constructed homogeneous plots consisting of B.pilosa and S.viridis grown separately as monocultures,as well as in mixtures.The rhizosphere and bulk soils were collected from the alien plant B.pilosa and the native plant S.viridis.In order to focus on the soil ecological functional mechanisms that contribute to the successful invasion of B.pilosa,we analyzed the effects of B.pilosa on the composition of soil microbial communities and soil ecological functions.The results showed that the biomass of B.pilosa increased by 27.51% and that of S.viridis was significantly reduced by 66.56%.The organic matter contents in the bulk and rhizosphere soils of B.pilosa were approximately 1.30 times those in the native plant soils.The TN and NO3- contents in the rhizosphere soil of B.pilosa were 1.30 to 2.71 times those in the native plant soils.The activities of acid phosphatase,alkaline phosphatase,and urease in the rhizosphere soil of B.pilosa were 1.98-2.25 times higher than in the native plant soils.Using high-throughput sequencing of the 16S rRNA gene,we found that B.pilosa altered the composition of the soil microbial community.Specifically,many genera in Actinobacteria and Proteobacteria were enriched in B.pilosa soils.Further correlation analyses verified that these genera had significantly positive relationships with soil nutrients and enzyme activities.Plant biomass,soil pH,and the contents of organic matter,TN,NO3-,TP,AP,TK,and AK were the main factors affecting soil microbial communities.This study showed that the invasion of B.pilosa led to significant alterations in the composition of the soil microbial communities.These changes were closely linked to modifications in plant traits as well as soil physical and chemical properties.Some microbial species related to C,N and P cycling were enriched in the soil invaded by B.pilosa.These findings provide additional support for the hypothesis of soil-microbe feedback in the successful invasion of alien plants.They also offer insights into the ecological mechanism by which soil microbes contribute to the successful invasion of B.pilosa.Overall,our research contributes to a better understanding of the complex interactions between invasive plants,soil microbial communities,and ecosystem dynamics.
Keywords: plant invasion,Bidens pilosa,soil microbial composition,soil properties,soil enzyme activities
1.Introduction
With the development of the world economy and trade,as well as the increasing frequency of human activities,invasions by alien species have become increasingly prominent and garnered much attention (Wanetal.2002).Invasions by alien species pose a challenge to the economic development of countries worldwide.In China,invasive plants account for more than half of all invasive species,with the Compositae family being the most dominant (Weberetal.2008).The successful invasion of exotic plants leads to a series of severe consequences,including reduced biodiversity,economic losses,and threats to human health (Wanetal.2003).Studying the mechanisms underlying alien plant invasion is a core focus in invasion ecology research,as it can contribute to better prediction,management and protection of the ecological environment (Roiloaetal.2020).Soil serves as the foundation for the growth of terrestrial plants,and soil ecosystems play a crucial role in the invasion of alien plant species.Understanding the role of soil ecosystems in alien plant invasion is critical for the effective control and mitigation of the negative impacts of invasive plants on the environment.
Soil ecological function refers to the set of processes and interactions occurring in the soil,including the physical,chemical,and biological components,all of which contribute to maintaining the health and productivity of terrestrial ecosystems (Nikitinetal.2022).Soil enzymes act as catalysts,accelerating chemical reactions in the soil and affecting soil nutrient cycling,biogeochemical processes,and overall soil ecological functions.They play a crucial role in the decomposition of organic matter and the release of nutrients,making them available for plant uptake (Kompała-Bąbaetal.2021).The production of most soil enzymes is closely related to the metabolic activity of soil microorganisms (Datt and Singh 2019).For instance,sucrase and protease(involved in the decomposition of organic matter) as well as phosphatase and urease (responsible for mineralizing nutrients),are enzymes secreted by soil microorganisms(Tabatabai and Dick 2002).Sucrase catalyzes the hydrolysis of sucrose into glucose and fructose,and plays a significant role in the carbon and nutrient cycling within the soil ecosystem (Guanetal.1986).Soil phosphatases break down phosphate esters and anhydrides,releasing inorganic phosphate and making it available for plant uptake (Luoetal.2022).Soil proteases degrade proteins into smaller molecules,providing a source of nitrogen and other nutrients for plants (Guanetal.1986).Urease facilitates the hydrolysis of urea,releasing ammonia that can be taken up by plants or converted into other forms of nitrogen by soil microorganisms (Jianetal.2016).By controlling enzyme activities,soil microorganisms play a crucial role in nutrient cycling,providing feedback for plant growth and overall ecosystem functioning (Pengetal.2021).Soil enzyme activity is influenced by various factors,including environmental and biological factors.Laboratory studies that strictly control environmental factors may not accurately reflect the changes in enzyme activities under natural conditions.Additionally,the spatial and environmental heterogeneity between sampling points during field sampling can also have a significant impact on soil enzyme activity.In this regard,controllable mesocosm manipulative studies play an important role in bridging the gap between laboratory research and field investigations (Chenetal.2023).
The composition of soil microorganisms is closely related to the structure of the plant community,forming a dynamic equilibrium (Trivedietal.2022).Plants provide carbon sources for soil organisms,which in turn regulate the decomposition of organic compounds into inorganic nutrients (Hengetal.2022).The organic compounds released by plants into the rhizosphere are known as rhizosphere sediments (Liuetal.2017).When invasive plant species alter the composition of the plant community,the rhizosphere sediments undergo changes,subsequently affecting the diversity and composition of soil microorganisms (Callawayetal.2004;Li Petal.2022).In recent years,many researchers have been investigating the impacts of alien plant invasion on soil ecosystems(Ahmadetal.2020;Woodsetal.2021;Zhangetal.2022).For example,the invasion ofHieraciumpilosellain New Zealand significantly increased soil microbial biomass,soil organic carbon and total nitrogen (Saggaretal.1999).The structure and function of microbial communities in the soil invaded byBerberisthunbergiiandMicrostegiumvimineumin New Jersey,USA,were found to differ from those in native plant soils (Kourtevetal.2002),with an increase in nitrate-nitrogen content in the invaded soil (Kourtevetal.1999).The invasion ofAgeratinaadenophorain China led to an increase in soil nitrogen content by altering the nitrogen cycle rate.Functional gene microarray analysis revealed a higher relative abundance of soil microbial genes related to nitrogen cycling in theA.adenophorainvaded soil (Zhaoetal.2019).In conclusion,soil microorganisms play a regulatory role in soil nutrient contents during alien plant invasion.
BidenspilosaL.,an annual herb belonging to the genusBidensand the family Compositae,is native to Central America.It is one of the 59 species included in the “Key List of Management of Invasive Alien Species”in China in 2023.Due to its wide ecological niche,strong adaptability,and invasiveness,B.pilosahas become widely distributed in eastern,southern,central,and southwestern China.The invasion ofB.pilosahas resulted in serious economic losses and damage to farmlands,orchards,and other ecosystems (Duetal.2007;Chauhanetal.2019).While much of the research onB.pilosahas focused on its medicinal value (Gertrudeetal.2022) and its ability to accumulate heavy metals(Lietal.2021),its potent invasive ability should not be underestimated.Studies on the invasiveness ofB.pilosaare relatively scarce,and it is generally believed that its capacity for invasion is associated with allelopathy (Taoetal.2015;Zhangetal.2016).Furthermore,the potential ofB.pilosato modify the soil environment through interactions with soil microorganisms and to enhance its competitive ability against native plants should not be overlooked.
Early researchers used denaturing gradient gel electrophoresis (DGGE) and phospholipid fatty acid(PLFA) methods to examine the effects ofB.pilosainvasion on the soil microbial community composition(Chenetal.2011;Yanetal.2016).However,these methods have a relatively low resolution for soil microorganisms.In order to overcome the spatial heterogeneity associated with field sampling,a pot experiment combined with high-throughput sequencing technology was used to analyze changes in the soil microbial community structure during different growth stages ofB.pilosa(Heetal.2013).Nonetheless,it is important to note that pot experiments may not fully capture the interactions between plants and soil microbes under natural conditions.In this study,we selectedSetariaviridis,a common and widely distributed local weed,as the native plant,and established a homogenous common-garden experiment involving monocultures and mixtures ofB.pilosaandS.viridisto simulate the invasion process ofB.pilosa.Rhizosphere and bulk soils were collected from bothB.pilosaandS.viridis.High-throughput sequencing of the 16S rRNA gene was performed to analyze the compositions of the soil microbial communities.Additionally,we measured the physicochemical properties and enzyme activities of the soil to assess any changes in soil ecological function.The main objectives of this study were to investigate the impact of invasiveB.pilosaon soil microbial community compositions,elucidate the underlying mechanisms,and identify the key microbes involved in the invasion process.Furthermore,we aimed to uncover the potential ofB.pilosato enhance its competitive growth by influencing the soil microbiota.These findings will contribute to a better understanding of the soil microbial mechanisms underlyingB.pilosainvasion and provide new insights into the role of soil microbiota in the successful invasion of alien plant species.
2.Materials and methods
2.1.Site description
We designed a field experiment at the Langfang Scientific Research Pilot Base of the Chinese Academy of Agricultural Sciences (CAAS),Hebei,China (39°30´42´´N,116°36´07´´E),which has been ongoing since 2012.The climatic conditions at the experimental site are representative of those in northern China and suitable for crop cultivation.Before conducting the field experiment,the site was mainly farmland with planted crops such as wheat,corn,and cotton.The soil at the study site was sandy clay loam (FAO/UNESCO Classification).In 2012,the initial soil organic matter,total nitrogen,total phosphorus,and total potassium contents of the experimental site were 12.24±0.95,0.94±0.05,0.74±0.05,and 16.99±0.71 g kg-1,respectively.Soil and plant samples were collected in October 2018.The average annual temperature in 2018 was 12.90°C and the annual rainfall was 570.00 mm based on data from the China Meteorological Data Service Center (Appendix A).The average temperature and mean annual rainfall from 2012 to 2018 were 13.21°C and 554.29 mm,respectively(Appendix A).
2.2.Experimental design
Experimental plots were established to simulate the invasion of the invasive plantB.pilosa,andS.viridis,a widely distributed annual weed in the invasion area,was selected as the native plant in this experiment.The area of each plot was 3 m×2 m and plots were separated using a 1 m wide buffer strip.Three treatments were designed for the experiment: a monoculture ofB.pilosa,a mixture ofB.pilosaandS.viridis(1:1) and a monoculture ofS.viridis.Each treatment had three replicates,and the plots had a completely randomized block design.In May 2012,a total of 100 seeds were sown in five rows with a 40 cm width in each plot.In the mixture plantation plots,50 seeds of each plant were randomly sown into five rows.After sowing,drip irrigation was used to ensure that the seeds could germinate.Subsequently,the plants were left to grow naturally without further watering.No fertilizer was applied during the experiments.The composition of the plants in each plot was maintained by manually removing other weeds every two weeks.Wilted plants were removed and discarded in the following spring.Additional seeds were not sown unless they were insufficient to maintain the plant composition produced in the previous year.Soil samples were taken after six years of natural growth in each treatment.
2.3.Plant biomass measurement
For each plot,three (0.5 m×0.5 m) quadrats were randomly selected,all plants within each quadrat were uprooted,and the soil on their roots was shaken off.In each quadrat,individual plant species were separated and placed into individual paper bags.The plant samples were then dried at 80°C for 72 h until they reached a constant weight.Subsequently,the dried plant samples were weighed to determine their biomass.The average biomass of each plant species in the three quadrats of each plot was calculated as the final biomass of each species in the plot.As plant samples from 2018 were no longer available,we measured the total carbon and nitrogen contents of the roots and shoots of the plants in the corresponding monoculture plots in 2021.
2.4.Soil sampling
Two types of soil (rhizosphere and bulk) were collected for each treatment,yielding the following sample scheme: rhizosphere (BR) and bulk (B) soil ofB.pilosain monoculture treatment ofB.pilosa;rhizosphere (SR)and bulk (S) soil ofS.viridisin monoculture treatment ofS.viridis;and rhizosphere soil fromB.pilosa(BSR) orS.viridis(SBR) and bulk soil (BS) in the mixture treatment ofB.pilosaandS.viridis.
Before collecting soil from each plot,we randomly selected five cores (20 cm deep and 30 cm wide) and removed 1 cm of litter from the soil surface.We then collected soil samples from the cores,avoiding the roots.In monoculture plots,exotic and native plants were pulled up separately,and the roots were shaken violentlyinsituuntil approximately 80% of the soil was removed from the roots.The soil that remained on the roots and had fallen off were collected as the rhizosphere (BR and SR)and bulk (B and S) soils.In the mixture plots,the roots ofB.pilosaandS.viridiswere carefully separated after being pulled up and shaken,as described above.Soil retained on the invasive plant and native plant roots was collected as two kinds of rhizosphere soils (BSR and SBR),and the soil that had dropped off was mixed and collected as the bulk soil (BS).The soils were then sieved through a 20-mesh sieve to remove impurities and completely homogenized to form one sample.Twentyone soil samples were collected in total.All soil samples were divided into two parts: One part was stored at -80°C for 16S rRNA sequencing,and the other part was used for measuring soil properties and soil enzyme activities.
2.5.Soil properties
The physicochemical properties of each soil sample were determined using the method described by Lu(1999).Soil moisture content was determined using the oven-drying method and expressed as the weight loss percentage after drying the fresh soil at 105°C for 24 h to a constant weight.The soil pH was determined by the electrode method and obtained by reading the pH meter value (Mettler Toledo Instruments,Shanghai,China)from a suspension with a soil-water ratio of 1:2.5 (w:v).Soil organic matter and plant total carbon contents were determined using potassium dichromate oxidation and an external heating method (Nelson and Sommers 1983).The total nitrogen (TN) contents of the soil and plants were determined using the Kjeldahl method (Nelson and Sommers 1973).The total phosphorus (TP) content was determined using the molybdenum-antimony resistance colorimetric method with sodium hydroxide melting(Olsenetal.1954).Total potassium (TK) content in the soil was determined using the flame photometer method(Isaac and Johnson 1983).The available phosphorus(AP) content in the soil was determined using the molybdenum-antimony resistance colorimetric method after leaching with a sodium bicarbonate solution (Olsenetal.1954).The available potassium (AK) content in the soil was determined using ammonium acetate extraction and flame photometry (Tan 1995).Ammonium nitrogen (NH4+) and nitrate nitrogen (NO3-) contents were determined using ultraviolet spectrophotometry with a potassium chloride extraction (Bremner 1965).
2.6.Soil enzyme activities
We measured the activities of several enzymes that play important roles in soil biogeochemical cycling,including acid phosphatase,alkaline phosphatase,sucrase,acidic protease,neutral protease,alkaline protease,and urease.These enzyme activities of fresh soil (0.1 g)were measured according to the instruction manual of the corresponding enzyme activity detection kit (Cohesion Biosciences LIMITED,London,UK).
2.7.Soil microbial community
DNA extraction and purificationTotal DNA was extracted from a soil sample (5 g) using a MoBio PowerSoil DNA isolation kit (MoBio Laboratories,Carlsbad,CA,USA).Total DNA was purified using a MoBio PowerSoil DNA isolation kit (MoBio Laboratories,Carlsbad,CA,USA).The 260/230 and 260/280 nm ratios of the purified product were determined using a Thermo NanoDrop One (Thermo Fisher Scientific,Wilmington,DE,USA) to evaluate the DNA quality.
PCR amplification and 16S rRNA gene sequencingThe 16S rRNA gene was amplified and sequenced as described by Caporasoetal.(2011,2012).The V4 region of the bacterial 16S ribosomal RNA (16S rRNA)gene was amplified using primers F515-R806.The PCR amplification system consisted of 5 U of DNA polymerase (Sigma,St.Louis,USA),2.5 μL reaction buffer containing 100 μmol L-1dNTPs and 0.1 μmol L-1for each primer,10 ng genomic DNA was added to each amplification system,and the final PCR volume was 25 μL.The PCR conditions were as follows:Initial denaturation at 94°C for 1 min,followed by 35 cycles at 94°C for 20 s,50°C for 25 s,72°C for 45 s,and extension at 72°C for 10 min.Each sample was replicated three to five times as described above.The PCR products were mixed together and purified using the QIAquick Gel Extraction Kit (Qiagen,Chatsworth,CA,USA) and quantified using PicoGreen (BMG Labtech,Jena,Germany).Finally,the PCR products were denatured and run on MiSeq (Illumina Inc.,San Diego,CA,USA) for paired ends with 150 bp reads according to the manufacturer’s instructions.
Data processingTo reduce the impact of random sequencing errors,we removed the sequences with (i) a Q score<20;(ii) a read length<140 bp after the proximal PCR primer;and (iii) possible chimeras analyzed by comparing U-Chime (Edgaretal.2011).The sequences were resampled to 54,490 sequences per sample after the removal of singleton operational taxonomic units(OTUs).The UCLUST algorithm (Edgar 2010) was used to cluster the qualified sequences with 97% similarity to the OTU datasets.To ensure the reliability of the data,any OTU that appeared only once in the OTU dataset of the three duplicate samples was deleted.Finally,the OTU data were normalized to the relative abundance for the subsequent data analysis.
2.8.Statistical analyses
We calculated Simpson’s reciprocal index (1/D) to characterize the α diversity of the microbial communities using R software version 4.0.3 (R Foundation for Statistical Computing,Vienna,Austria).The β-diversity index of the soil microbial communities was determined by the Bray-Curtis distance calculated using R software,and the complementary result was defined as the similarity index.The similarity index values ranging from 0 (dissimilar) to 1 (similar) were used to analyze the similarities of the soil microbial community compositions among replicates or between treatments.Principal component analysis (PCA)was performed in R software using the “vegan” package to examine the differences among microbial communities.R software was used to screen variable factors of the soil properties with variance inflation factors (VIF)<20,and the canonical correspondence analysis (CCA) was performed with the soil microbial community data.Variance partitioning analysis (VPA) was used to determine the proportion of variance in the soil microbial community explained by the selected environmental factors using R software.Further,the Mantel test was used to calculate the correlations between soil characteristics and microbial community composition using R software.The “rfPermute”and “randomForest” packages in R software were used to perform Random Forest analysis in order to determine the importance of significant environmental factors on the soil microbial community.SPSS (version 19.0;IBM,Armonk,NY,USA) was used to conduct a one-way analysis of variance (ANOVA) at the 95% confidence interval to analyze the significance of differences.The normality tests were performed before ANOVA,and a logarithmic transformation was performed for data that did not meet the assumption.Multiple comparisons between the two groups were performed using the LSD method.Differences in plant biomass between the monoculture and mixture,the C and N contents between plant roots and shoots,and the C and N contents between roots or shoots of the two species were tested using Student’st-test in SPSS.Similarity percentages (SIMPER) analysis in R software was used to test the contribution rate of each microbial genus to differences between microbial communities in the different planting regimes.The online LDA effect size (LEfSe) project (http://huttenhower.sph.harvard.edu/galaxy/) (Segataetal.2011) was used to identify the biomarkers with significant differences(LDA>2.0) betweenB.pilosaandS.viridis.Further,we conducted a correlation analysis between environmental factors and genera that were significantly different in the soils of invasive and native plants,using Pearson’s correlation analysis in SPSS software (two-tailed test).The Benjamini-Hochberg (BH) method (Benjamini and Hochberg 1995) was adopted to adjust theP-values of the correlation to control the false discovery rate (FDR).
3.Results
3.1.Effects of competition on soil microbial communities
Diversity of soil microbial communitiesWe analyzed the microbial communities in the bulk and rhizosphere soils ofB.pilosaandS.viridiswhen they were in either a monoculture or mixture.Simpson’s reciprocal index was used to characterize the diversity of the soil microbial communities,and the diversities in BR and BSR were significantly higher (P<0.05) than those in SR and SBR (Fig.1).Similarly,the microbial diversity in the rhizosphere soil ofS.viridisin SBR was significantly higher (P<0.05) than in SR (Fig.1).Under monoculture conditions,the microbial diversity in BR was higher than in B,whereas it was the opposite (P<0.05) inS.viridissoils.

Fig.1 Simpson’s reciprocal index of the microbial communities in different soils (mean±SE,n=3).B,bulk soil in monoculture treatment of B. pilosa;BS,bulk soil in mixture treatment of B. pilosa and S. viridis;S,bulk soil in monoculture treatment of S. viridis;BR,rhizosphere soil of B.pilosa in monoculture treatment of B. pilosa;BSR,rhizosphere soil of B.pilosa in mixture treatment of B. pilosa and S. viridis;SR,rhizosphere soil of S.viridis in monoculture treatment of S. viridis;SBR,rhizosphere soil of S.viridis in mixture treatment of B. pilosa and S. viridis.Different letters indicate significant differences among samples (P<0.05),tested by one-way ANOVA.
Differences in soil microbial community compositionPCA was used to analyze the differences in microbial community composition among the different treatment soils.First,we analyzed the microbial community compositions between 2012 and 2018.The differences in microbial community compositions between the soil samples collected in 2012 and 2018 were significant(Appendix B).We further analyzed the microbial community composition in the soil of each treatment in 2018 (Appendix B).The PCA results revealed that the microbial compositions in the rhizosphere soils were distinctly different from those in the bulk soils.In the rhizosphere soils,the microbial compositions ofS.viridisandB.pilosawere also different (Appendix B).We further calculated the similarity between microbial communities based on the Bray-Curtis distance.The community similarities between SR and the other soils were found to be significantly lower (P<0.05,Appendix C),particularly in the BR and BSR.This indicates that the invasion ofB.pilosaled to a noticeable and significant alteration in the soil microbial community.
3.2.Specific taxonomic groups
According to the 16S rRNA gene sequencing,a total of 54,490 OTUs were identified and classified into 42 phyla and 806 genera.Specifically,40 phyla and 669 genera were identified in the bulk soil,while 39 phyla and 730 genera were identified in the rhizosphere soil.
At the phylum levelFourteen phyla had a relative abundance of >1%.In the bulk soils,Acidobacteria exhibited the highest relative abundance,ranging from 19.30 to 21.76%.In the rhizosphere soil of the native plant (SR),Acidobacteria was also the dominant phylum with a relative abundance of 26.90%.On the other hand,in the rhizosphere soils of BR,BSR,and SBR,Actinobacteria was the dominant phylum with relative abundances ranging from 21.43 to 22.74% (Appendix D).
The relative abundances of different phyla in the rhizosphere soil changed significantly with the invasion ofB.pilosa.For the native plantS.viridis,the relative abundances of Actinobacteria and Alphaproteobacteria in the SBR increased 0.39-0.50 folds (P<0.05)compared with those in the SR.In contrast,the relative abundances of Acidobacteria,Planctomycetes,Verrucomicrobia,Chloroflexi,Armatimonadetes and Woesearchaeota decreased significantly in SBR(P<0.05).Under monoculture,the relative abundances of Actinobacteria,Alphaproteobacteria,Betaproteobacteria,Deltaproteobacteria,and Chlamydiae were significantly higher (P<0.05) in the BR than in the SR.However,the relative abundances of Acidobacteria,Planctomycetes,Verrucomicrobia,Chloroflexi,Armatimonadetes,Woesearchaeota,Thaumarchaeota,and Euryarchaeota were reversed (P<0.05) (Appendix E).
At the genus levelEighteen genera had a relative abundance of >1%.TheGp6of Acidobacteria had the highest relative abundance in the bulk and rhizosphere soils,except in the SR,where theGp4of Acidobacteria was the dominant genus (Appendix F).
The invasion ofB.pilosacaused increases in the abundances of certain genera in the bulk soil (B) compared with the native plant soil (S),includingSkermanella,Blastocatella,Pseudolabrys,Kribbella,Reyranella,Variovorax,Streptophyta,andPromicromonospora(P<0.05).Conversely,the relative abundances ofSubdivision3generaincertae sedis,Methanomassiliicoccus,andLysinibacilluswere significantly reduced (P<0.05) in the bulk soil ofB.pilosa.These genera accounted for 7.70% of the dissimilarity in microbial communities between B and S (Table 1).
In the rhizosphere soil,the relative abundances ofSolirubrobacter,Arthrobacter,Skermanella,Blastococcus,Nocardioides,Aeromicrobium,Agromyces,Marmoricola,Pseudolabrys,Kribbella,Bradyrhizobium,Minicystis,Reyranella,Vasilyevaea,Pseudonocardia,Modestobacter,Ilumatobacter,Variovorax,Geodermatophilus,andPromicromonosporain SR were significantly increased (P<0.05) when competing for growth withB.pilosa(SBR).The relative abundances of these genera in the BR were significantly higher(P<0.05) than in the SR (Appendix G).However,the relative abundances ofGp6,Gp4,Subdivision3genera incertaesedis,Pirellula,Gemmata,Gp25,Thermogutta,Tepidisphaera,Chthonomonas/Armatimonadetesgp3,andGp18in SR were significantly reduced (P<0.05)when competing for growth withB.pilosa(SBR),and significantly lower (P<0.05) in BR than in SR (Appendix G).These genera together contributed 46.40% (SR and SBR)and 42.10% (SR and BR) to the microbial community dissimilarity (Table 1).

Table 1 Contribution rate of each genus between the soils tested using similarity percentage (SIMPER) analysis1)
Biomarkers between B. pilosa and S. viridis rhizosphere or bulk soilsWe compared the microbial communities with a relative abundance greater than 0.1%betweenB.pilosaandS.viridisin the rhizosphere or bulk soils using the LEfSe method.LEfSe analysis identified 72 and 175 biomarkers with significant differences(LDA>2.0) in the bulk and rhizosphere soil,respectively(Fig.2;Appendix H).In bulk soils,the relative abundances of six genera of Actinobacteria (LDA>3.01) and eight genera of Proteobacteria (LDA>2.99) were significantly higher inB.pilosasoli (B) compared toS.viridissoil (S) under monoculture.Additionally,only a few microorganisms significantly aggregated in S (Fig.2-A;Appendix H).However,in the rhizosphere,there was a significant increase in the number of microbial species exhibiting significant differences in relative abundance between the soils of the two plants.For example,the relative abundances of Actinobacteria(LDA=4.61),Cyanobacteria/Chloroplast (LDA=3.20),Gemmatimonadetes (LDA=3.71),and Proteobacteria(LDA=4.00) increased significantly in BR,with 47 genera accumulating (LDA>2.92).Additionally,nine genera from Acidobacteria,Bacteroidetes,Planctomycetes,and Verrucomicrobia in BR were also significantly increased(LDA>2.91) (Fig.2-B;Appendix H).InS.viridissoil,the five phyla of Acidobacteria (LDA=4.63),Armatimonadetes(LDA=3.27),Euryarchaeota (LDA=3.04),Thaumarchaeota(LDA=4.60),and Verrucomicrobia (LDA=3.94) were enriched (Fig.2-B;Appendix H).The relative abundances of eight genera in these five phyla and five genera from Planctomycetes and Proteobacteria increased significantly(LDA>2.96) (Fig.2-B;Appendix H).

Fig.2 Cladogram based on LEfSe analysis showing the differences in the relative abundances of taxa (>0.1%) at six levels between Bidens pilosa and Setaria viridis in the bulk (A) and rhizosphere (B) soils based on an LDA score >2.0.B,bulk soil in monoculture treatment of B. pilosa;S,bulk soil in monoculture treatment of S. viridis;BR,rhizosphere soil of B.pilosa in monoculture treatment of B. pilosa;SR,rhizosphere soil of S.viridis in monoculture treatment of S. viridis.The outermost ring represents the phylum level and the innermost ring represents the genus level.Each node represents a member within that level.The red and green nodes indicate that the taxa in the B.pilosa and S.viridis soils showed differences in relative abundance,and the yellow nodes indicate non-significant differences.The full names of species at each taxonomic level,represented by the letters in the figure,are presented in Appendix H.
3.3.Relationships between soil microbial communities and environmental variables
Effects of B. pilosa invasion on environmental variablesWe measured the total biomass of each plant species per unit area (0.5 m×0.5 m) in both the monoculture and mixture treatments (Appendix I).The changes in the biomasses of the two plant species during the competition were the opposite.During the competition,the biomass ofB.pilosaincreased,whereas that ofS.viridisdecreased by 66.56% (P<0.05).We also measured the total carbon and nitrogen contents in the roots and shoots of both plant species (Appendix J).The shoot nitrogen content ofB.pilosawas the highest and almost twice the root (P<0.05) and shoot nitrogen contents ofS.viridis(P<0.05).The total carbon contents in the shoots of both plant species were significantly higher than in the roots (P<0.05).However,the total carbon content in the roots ofB.pilosawas significantly higher than in the roots of the native plants (P<0.05),whereas the opposite was observed in the shoots (P<0.05).
The invasion ofB.pilosasignificantly increased the activities of acid phosphatase,alkaline phosphatase,and urease in the rhizosphere soil by 1.98-2.25 folds compared to the rhizosphere soil of the native plantS.viridis(P<0.05;Appendix K).Acid phosphatase activity in BR was significantly higher (P<0.05) than in B.An increase in enzyme activity is beneficial for the transformation of soil nutrients and increases the contents of available nutrients in the soil.The organic matter contents inB.pilosasoils (BR and B) were approximately 1.30 folds (P<0.05) higher than inS.viridissoils (SR and S).The TN (P<0.05) and NO3-(P<0.05) contents in BR were significantly higher (1.30-2.71 folds) than those in SR.In addition,soil moisture in BR was 1.59 folds higher(P<0.05) than in SR.
Relationships between environmental variables and microbial communitiesWe analyzed the relationships between soil microbial communities and the environmental variables using canonical correspondence analysis (CCA).A total of 13 environmental variables,biomass,root N,root C,pH,TN,NO3-,NH4+,TP,AP,TK,AK,moisture,and organic matter,were selected based on variance inflation factors (VIF)<20 and used to construct a CCA model(Fig.3-A).The first two axes accounted for 22.89%of the microbial community variations.These results showed that soil pH and plant biomass were significantly related with the microbial communities (Appendix L).The selected factors were then divided into three groups (N group,soil organic matter,TN,NO3-,NH4+,TP,AP,TK,and AK contents;S group,soil pH and moisture;P group,plant biomass,root N,and root C) for VPA analysis to determine their proportions in explaining the variation of the soil microbial community (Fig.3-B).The contribution of group N was 36.25%,the contribution of group S was 8.22%,and the contribution of group P was 13.34%.Together these environmental variables explained 68.60%of the variation in the soil microbial community,with soil nutrient content explaining the highest proportion of the variation.The Mantel test further verified that plant biomass (r=0.27,P<0.05),soil pH (r=0.36,P<0.05),organic matter (r=0.68,P<0.05),TN (r=0.39,P<0.05),NO3-(r=0.48,P<0.05),NH4+(r=0.14,P<0.05),AP (r=0.45,P<0.05),and AK (r=0.29,P<0.05) were significant variables for shaping the soil microbial communities(Table 2).For these significant variables,the Random Forest method was used to determine their relative importance in affecting the soil microbial community.These variables were found to collectively explain 77.43%of the variation in soil microbial community.Among these variables,organic matter had the highest explanatory value of 15.36%,followed by AP with 10.83%,NO3-with 9.39%,TN with 7.98% and pH with 5.57% (Fig.4).

Fig.3 The relationship between environmental factors and soil microbial community analyzed by canonical correspondence analysis (CCA) (A) and variance partitioning analysis (VPA) (B).B,bulk soil in monoculture treatment of B. pilosa;BS,bulk soil in mixture treatment of B. pilosa and S. viridis;S,bulk soil in monoculture treatment of S. viridis;BR,rhizosphere soil of B.pilosa in monoculture treatment of B. pilosa;BSR,rhizosphere soil of B.pilosa in mixture treatment of B. pilosa and S. viridis;SR,rhizosphere soil of S.viridis in treatment S;SBR,rhizosphere soil S.viridis in mixture treatment of B. pilosa and S. viridis.Root N,total nitrogen in root;Root C,total carbon in root;OM,organic matter;TN,total nitrogen;TP,total phosphorus;AP,available phosphorus;TK,total potassium;AK,available potassium.Environmental factors with variance inflation factor values smaller than 20 were selected.These factors were divided into three groups (N group,soil organic matter,TN,NO3-,NH4+,TP,AP,TK,and AK contents;S group,soil pH and moisture;and P group,plant biomass,root N,and root C) for VPA.The values in the circle represent the proportion of environmental factor groups explaining soil microbial community variation.
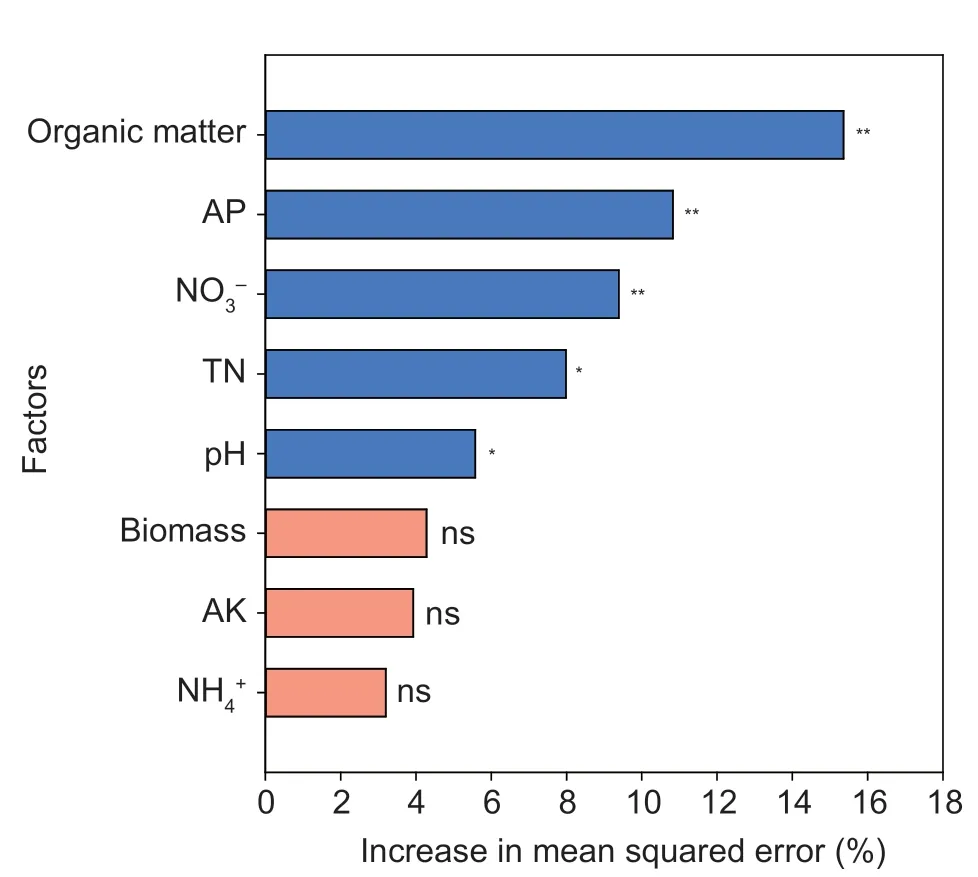
Fig.4 Importance of environmental factors in shaping soil microbial communities based on the Random Forest model.The environment variables explained 77.43% of the total variance.The asterisks indicate significant effects (*,P<0.05;**,P<0.01).AP,available phosphorus;TN,total nitrogen;AK,available potassium.
In addition,we analyzed the correlations between the dominant microbial genera inB.pilosaandS.viridissoils and environmental variables using Pearson’s correlationanalysis (two-tailed test) (Fig.5).Most of the genera enriched in the rhizosphere and bulk soils ofB.pilosawere significantly positively correlated with the activities of phosphatases and proteases (r=0.50-0.78,P<0.05).Additionally,these genera exhibited significant positive correlations with organic matter,TN,NO3-,AP and TP(r=0.51-0.90,P<0.05),and plant biomass (r=0.52-0.67,P<0.05).However,they were significantly negatively correlated with pH (r=-0.69 to -0.52,P<0.05).The microorganisms enriched inS.viridissoils were negatively correlated with soil enzyme activity and physicochemical properties (P<0.05).

Table 2 Correlations between environmental factors and microbial communities of the different source soils as determined by the Mantel test1)

Fig.5 Pearson’s correlations between the relative abundances of soil microbial genera and environmental factors.Root N,total nitrogen in root;Shoot N,total nitrogen in shoot;Root C,total carbon in root;Shoot C,total carbon in shoot;TN,total nitrogen;TP,total phosphorus;AP,available phosphorus;TK,total potassium;AK,available potassium.The P-values of correlations were adjusted using the Benjamini-Hochberg (BH) method.*,P<0.05,**,P<0.01,***,P<0.001.
4.Discussion
4.1.Bidens pilosa invasion alters the soil nutrient environment
Increased resource availability is one of the drivers of the successful invasion of alien plant species (Wei and Chen 2023).Soil is an important part of ecosystems,and it has a complex relationship with plants.Any influence of invasive alien plant species on soil properties may cause drastic changes in plant community composition and structure (Zhangetal.2017).Exotic plants can successfully invade an area by changing the soil nutrient environment (Ehrenfeld 2003).For example,the invasion ofHalogetonglomeratussignificantly increases the nitrate and phosphorus contents in the soil (Dudaetal.2003).The invasion ofA.adenophoraincreases the soil nitrogen cycling rate and results in the accumulation of nitrate and ammonia in the rhizosphere soil (Li Qetal.2022).Mikaniamicranthaincreases the available nitrogen content of the soil,allowing it to absorb more nitrogen and subdue native species (Yuetal.2021).Nitrogen is one of the main nutrients that limit plant growth,and increasing nitrogen availability can benefit the utilization and competitiveness of invasive plants (Hierroetal.2005).According to our results,the TN and NO3-contents in the rhizosphere soil invaded byB.pilosawere significantly increased (Appendix K).They were found to be the main factors in shaping the soil microbial community (Fig.4).Bidenspilosaexhibited a significantly higher biomass compared to the native plant.Additionally,the TN content in the shoots ofB.pilosawas nearly twice that of the native plant.These findings suggest thatB.pilosahas a higher nitrogen use efficiency.Previous studies have shown thatB.pilosatends to use more NO3-(Chen and Chen 2019),which may be one of the reasons for its successful invasion.Saggaretal.(1999) demonstrated that invasive plants increase the soil organic carbon content.During theB.pilosainvasion,the organic matter contents in the rhizosphere and bulk soil were significantly higher than in the native plant soil (Appendix K).This result is consistent with the results of an the early pot experiment (Heetal.2013).Soil organic matter provides a food source for soil microorganisms,and these microorganisms play a crucial role in breaking down organic matter into its component nutrients and making them available for plant uptake (Coonanetal.2020).Soil organic matter explained 15.36% of the variation in soil microbial composition and was the most important factor influencing the soil microbial community (Fig.4).
Soil enzymes can promote the conversion of soil nutrients,and their activities are closely related to the soil nutrient content.A meta-analysis showed that the activities of N-and P-related enzymes in invasive soils were significantly higher than those in non-invasive soils (Zhou and Staver 2019).The improvements in soil enzyme activities accelerate soil nutrient cycling,thereby creating a nutrient-rich soil environment.Soil urease can convert organic nitrogen into inorganic nitrogen,and its activity is often used to characterize the intensity of soil nitrogen supply.In this study,the variation was the same as that of the soil nitrate content (Appendix K).Phosphatase can promote the decomposition of phosphorus compounds in the soil (Margalefetal.2017).In this study,the activities of acid phosphatase and alkaline phosphatase in the rhizosphere soil ofB.pilosaincreased significantly by 0.66-1.70 folds.However,there was no significant increase in the soil AP content(Appendix K).This difference may exist becauseB.pilosatransferred the absorbed phosphorus to the fruits for reproduction during the sampling period (Heetal.2013).
4.2.Bidens pilosa invasion alters the soil microbial composition
The soil microbial community is closely related to the composition of the plant community,and they influence each other (Trivedietal.2022).Exotic plants tend to be tall,have well-developed roots,and have high amounts of biomass and litter.These characteristics can result in various environmental changes at invasion sites.In our study,plant biomass was one of the primary factors responsible for microbial changes (Fig.3).
The successful invasion of alien plants affects soil microbial diversity and function through changes in root exudates,litter,and leachates (Vogelsang and Bever 2009;Faheyetal.2020).Rice (1983) defined this phenomenon as an example of allelopathy.Allelochemicals secreted by the roots of invasive plants into the rhizosphere soil have a screening effect on soil microorganisms and may lead to the formation of microbial communities that differ from those of native plants (Marilleyetal.1998).For example,B.thunbergiiandM.vimineum,which invaded the eastern United States,can rapidly change the soil characteristics,soil microbial community structure and enzyme activities(Kourtevetal.2003).Dudaetal.(2003) used the Biolog EcoPlate approach and found that the functional diversity of soil bacteria in the soil ofH.glomeratuswas significantly higher than that of indigenous plants.Our results showed that the microbial diversity in the rhizosphere soil of invasive plants was significantly higher than in the rhizosphere soil of native plants(Fig.1).Additionally,soil microorganisms are highly sensitive to plant root exudates.The types of root exudates change with changes in the plant communities caused by alien plant invasion,and microorganisms in the rhizosphere are affected more directly.This explains why the microbial community composition in the rhizosphere soil of the invasive plants was significantly different from that of the native plant (Appendices B and C).This is also why more microorganisms varied significantly between the different plant rhizosphere soils(Fig.2).
The rhizosphere is the interface between root systems and the soil,where millions of microorganisms live,and it serves as a hub for nutrient exchange and element cycling.Plants provide carbon sources for soil microorganisms in the form of rhizosphere sediments(Li Petal.2022).These carbon sources stimulate the growth of microorganisms,which in turn release enzymes that break down organic matter and make more nutrients available for plant uptake.Additionally,root exudates can also affect the growth and activity of soil microorganisms by altering the physical and chemical properties of the rhizosphere microenvironment,such as pH.In summary,root exudates have a multifaceted impact on soil microbial communities.They not only provide nutrients but also regulate the distribution and activity of microorganisms by modulating the rhizosphere microenvironment.The soil microbial community is highly sensitive to pH,and a small change in pH has a significant impact on soil microbial composition (Rousketal.2010;Wangetal.2017;Lietal.2020).In this study,pH was the main environmental factor responsible for the differences between microbial communities in the rhizosphere and bulk soils (Figs.3 and 4;Table 2).The rhizosphere is more conducive to soil microbial growth than the bulk environment.Bidenspilosacan produce nearly 200 natural secondary metabolites (Silvaetal.2011),which can provide more nutrients for soil microorganisms.In this study,the microbial diversity in the rhizosphere soil ofB.pilosawas higher than that in the bulk soil (Fig.1),which is consistent with the results of a previous study onA.adenophora(Li Qetal.2022).Higher rhizosphere microbial diversity is crucial for increasing nutrient cycling and promoting plant growth(Lingetal.2022).
Soil biotic feedback is another mechanism used by invasive alien plant species.The alien plants often benefit from symbiosis with soil microbes in invaded areas(Richardsonetal.2000;Reinhart and Callaway 2006).In this study,many microbial species from Actinobacteria and Proteobacteria were enriched in the soil invaded byB.pilosa(Fig.2;Appendices E and G).Actinobacteria has vast metabolic diversity,which plays an important role in the cycles of organic compounds (Becarellietal.2021) and is related to the generation of organic matter(Suela Silvaetal.2013).Alphaproteobacteria includes abundant nitrogen-fixing bacteria (Koxetal.2018).In addition,some genera of the phylum Actinobacteria,such asArthrobacter,Nocardioides,Pseudonocardia,andSolirubrobacter,have the potential to biodegrade toxic substances (Makondeetal.2015;Wangetal.2016).These soil microbes might be conducive to the adaptability ofB.pilosato complex environments.It should be noted that soil microorganisms play a crucial role in ecosystems,but their functions are influenced by various environmental factors.Currently,our understanding of microbial functions is primarily based on laboratory studies that isolate and identify different microorganisms.However,the interactions and mutual influences among microorganisms in the natural environment are highly complex,and there are still many unknown factors (Chenetal.2023).Therefore,we can only make “inferences” about the potential functional changes in our research based on the relative abundances of these microorganisms,without direct evidence.Further experimental verification is necessary to determine whether they assist in the colonization and spread of invasive plants.
4.3.Uncertainties and limitations
The soil microbial community composition is affected by many factors,such as climatic factors,plant photosynthetic capacity,and root exudates.This study was conducted in a homogeneous common garden,which made the growth environment essentially uniform for all the samples.Therefore,it was impossible to analyze the impacts of climatic factors over one year.In further research,the impacts of temporal changes in climatic factors on soil microbial community compositions could be studied.
In addition,soil microorganisms can produce many extracellular enzymes,and several related to carbon,nitrogen and phosphorus cycling were detected in this study.In future studies,the combination of metagenomic and metabolomic approaches can be used to comprehensively analyze the whole process by which soil microorganisms affect soil nutrient cycling.
Furthermore,our conclusions were drawn based on one invasive and one native species of plants.To make the conclusions sound,we can expand the common garden to include multiple plants to obtain more universal results.
5.Conclusion
The results of this study showed that the invasion ofB.pilosahad a significant impact on the composition of the soil microbial community,particularly in the rhizosphere soil.The presence ofB.pilosaresulted in an enrichment of bacterial genera belonging to Actinobacteria and Proteobacteria,which play vital roles in organic matter formation and protein decomposition.Consequently,the activities of the corresponding enzymes involved in these processes,as well as the organic matter and nitrogen content in the invading soil,showed significant increases.Biomass,soil pH,and soil nutrient contents were identified as the main factors contributing to the soil microbial differences.Furthermore,correlation analysis revealed positive relationships between the soil microorganisms selectively associated withB.pilosainvasion and soil nutrients,as well as soil enzyme activities.These results indicate thatB.pilosahas the potential to modulate soil ecological functions by changing the soil microbial composition.This,in turn,leads to increased soil nutrient content and promotes competitive growth.
Acknowledgements
This work was funded by the National Key R&D Program of China (2022YFC2601100,2021YFD1400100 and 2021YFC2600400),and the National Natural Science Foundation of China (42207162).
Declaration of competing interests
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.07.025
杂志排行
Journal of Integrative Agriculture的其它文章
- Emergence of highly pathogenic avian influenza A (H5N8) clade 2.3.4.4b viruses in grebes in lnner Mongolia and Ningxia,China,in 2021
- Can whole steps of grain production be outsourced? Empirical analysis based on the three provinces of Jiangsu,Jilin,and Sichuan in China
- Promoting grain production through high-standard farmland construction: Evidence in China
- The protective effect of cyclodextrin on the color quality and stability of Cabernet Sauvignon red wine
- Quantification of the adulteration concentration of palm kernel oil in virgin coconut oil using near-infrared hyperspectral imaging
- Quantifying the agreement and accuracy characteristics of four satellite-based LULC products for cropland classification in China
