Development of therapeutic cancer vaccines using nanomicellar preparations
2024-01-13YanQinWenFengZengWeiLiang
Yan Qin,Wen-Feng Zeng,c,Wei Liang,c,
Abstract Cancer treatment is a multifaceted challenge,and therapeutic vaccines have emerged as a promising approach.The micellar preparation efficiently encapsulates antigen polypeptides and enhances antigen presentation through the major histocompatibility class I pathway,promoting cytotoxic T lymphocyte immune responses.Moreover,it enables codelivery of both antigen and adjuvant to the same target antigen-presenting cells.Combining the micellar vaccine with traditional cancer treatments(such as chemotherapy,radiotherapy,and surgery) has demonstrated improved efficacy in murine tumor models. Overall, the polyethylene glycol-phosphatidylethanolamine micelle-based vaccine presents a promising platform for cancer therapeutic vaccines.By leveraging the strengths of various treatment modalities,this innovative vaccine approach holds the potential to revolutionize cancer therapy and bring new possibilities for cancer patients.
Keywords:Intracellular codelivery;Lymph node targeting;PEG-PE micelle;Therapeutic cancer vaccine
1. Introduction
One of the primary goals of our study was to develop therapeutic cancer vaccines using biodegradable and biocompatible micelles.Therapeutic vaccines using micellar vectors offer several key advantages over traditional vaccines.These advantages include(i)cytosolic antigen delivery,which favors the processing and cross-presentation of exogenous antigens through the major histocompatibility class I(MHC-I) pathway, and (ii) simultaneous induction of appropriate activation of dendritic cells(DCs)for efficient antigen presentation and costimulation of CD8+T cells, which are crucial factors for achieving effective antitumor immunity.[1]Here,we discuss the progress in cancer vaccines and highlight the benefits of micellar vaccines as a promising approach for therapeutic cancer vaccines.
2. The history of nanomicellar cancer vaccines
Cancer remains a challenging health issue and is the second leading cause of death in China.[2]Over the past decade,the morbidity and mortality rates of various cancer types have increased slightly. Although developed countries have seen a minor reduction in morbidity and mortality,this is believed to be primarily due to a combination of preventive measures and improved early detection, rather than significant advancements in treatment effectiveness.[3]Conventional tumor treatments involve surgery and radiation therapy to remove the primary solid tumor combined with chemotherapy to completely eliminate the tumor cells and prevent recurrence and metastasis.However,these approaches have certain limitations.Surgery and radiation cannot address tumor metastasis, and chemotherapy often faces challenges,such as highly toxic adverse effects and developing of multidrug resistance in tumor cells.Therefore,there is a need for improved therapies that can reduce the recurrence rate in patients with cancer during treatment and ultimately extend their survival.Therefore, cancer vaccines offer a promising therapeutic approach.They have shown good tolerance in patients,causing rare serious adverse effects while effectively stimulating a tumor-specific immune response that activates cytotoxic T lymphocytes(CTLs).[4,5]These CTLs not only have the potential to eliminate existing tumor cells but also provide immune memory to prevent tumor recurrence.
Over a century ago,Coley demonstrated the potential role of the immune system in combating cancer.[6]Since then,significant progress has been made in understanding tumor immunology, such as the expression of tumor antigens by tumors, activation of tumor antigen-specific T cells in the host,and the role of immunosuppression,particularly in the tumor microenvironment,in inhibiting antitumor immunity.The primary purpose of a cancer vaccine is to alert the patient's immune system to the presence of cancer and activate effective T cell-mediated tumor-specific attacks.An effective cancer vaccine aims to alert the patient's immune system to the presence of cancer and trigger a potent T cell-mediated attack against the tumor. To achieve this, antigenic substances must be delivered in a granular form rather than in a soluble form,promoting antigen uptake and cross-presentation and enhancing the CTL response.[7]In addition, codelivering antigens with adjuvants that promote DC maturation and Th1-type immune responses is vital for more effective T-cell activation and interferon γ production.[8,9]Biodegradable particles,such as polymeric particles,have emerged as potential carriers in cancer vaccine development studies.[10,11]In addition to serving as vaccine vectors,biodegradable particles have great potential to enhance vaccine strategies.One of their applications involves delivering tumor antigens in a granular form rather than in a soluble form, allowing the formation of hybrid cell-particle structures.Within these structures,granules can carry various immune stimulants,thereby enhancing antitumor potential and improving the delivery of tumor antigens to stimulated DCs in vivo.Clinical and preclinical studies have shown promising results using biodegradable polymeric particles, liposomes, and virus-like particles as cancer vaccine vectors.[12]Using particles as delivery vehicles offers several advantages over nongranular vaccine systems.First,it protects the vaccine payload from premature degradation. Second, it enhances the intracellular delivery of the loaded material.[13,14]Third,it provides sustained release of the vaccine payload.[15]Finally,it enables the codelivery of multiple components, such as antigens and adjuvants,to enhance the overall immune response.Furthermore,using biodegradable particles, as opposed to nonbiodegradable ones,has an additional advantage in eliminating the need for carrier removal.[16]Typically,biodegradable particles are less toxic than nonbiodegradable particles, making them safer and more suitable for biomedical applications. Biodegradable polymer particles serve as versatile delivery systems and can be made from various polymers,such as polylactic acid-glycolic acid,polylactic acid,polyanhydride,and chitosan.[12,17]This versatility allows researchers to tailor the properties of the particles to suit specific vaccine requirements,making them attractive options for advancing vaccine development and delivery strategies.
3. The advantages and limitations of polyethylene glycol-phosphatidylethanolamine-based cancer vaccine
We developed a micellar preparation using polyethylene glycolphosphatidylethanolamine (PEG-PE) as the structural unit. Polyethylene glycol-phosphatidylethanolamine comprises hydrophobic phosphatidylethanolamine and hydrophilic polyethylene glycol.Initially, we used this micellar preparation to encapsulate various small-molecule drugs,such as doxorubicin hydrochloride,vinorelbine,and alprostadil.[18-20]The results showed that the micellar formulation had significant advantages over the original drug in terms of reduced toxicity,altered distribution,and improved metabolism in vivo.Building upon these findings,we investigated the potential of micellar preparations to encapsulate tumor-associated antigens and immune adjuvants such as monophosphoryl lipid A (MPLA),effectively transforming them into cancer vaccine preparations.Our studies revealed that micellar preparations significantly improved the efficiency of delivering encapsulated antigen polypeptides into the cytoplasm, promoting antigen presentation via the MHC-I pathway,and activating CTL immune responses.[1]Unlike cell-penetrating peptides,micelles facilitate the delivery of peptides to the cell membrane without accompanying them to the cytosol,thus preventing unwanted intracellular effects in target cells.Moreover, we observed that PEG-PE micelles enhanced the activity of MPLA approximately 100-fold,as demonstrated by increased production of tumor necrosis factor α, interleukin 6, and interleukin 12.In addition,the expression of costimulatory molecules was significantly higher in micellized MPLA than in nonmicellized MPLA.This enhanced activity could be attributed to the monomerization of MPLA by PEG-PE micelles and the characteristics of membrane fusion,which assisted the formation of the MPLA-Toll-like receptor 4-Myeloid differentiation factor 2 complex for rapid signaling.[21]Micellar vaccines offer distinct advantages over other therapeutic vaccines that use biodegradable nanoparticles. Unlike larger granular liposomes(>100 nm in diameter),which tend to remain at the site of inoculation after immunization,[22]micellar preparations (10-20 nm in diameter)rapidly spread to the lymph nodes, including both draining and distal lymph nodes,following subcutaneous inoculation.Within these lymph nodes,specific CD8α+DCs efficiently take up,process,and present antigens from the micelles,leading to the generation of high-quality,specific T lymphocytes capable of targeting and eliminating tumor cells.[1,23]The micelles were foundtohave extensive contact with resident CD8α+DCs in the lymph nodes and delivered more antigens to these DCs compared with their free form.This effective antigen delivery into CD8α+DCs promoted increased cross-presentation of antigens and enhanced the generation of effector CD8+T-cell immune responses(Figure 1).These findings underscore the critical role of CD8α+DCs in cytotoxic T-cell immunity triggered by PEG-PE micelle-based vaccines, providing a valuable approach for generating T cellmediated immunity.Furthermore,the PEG-PE micelle vaccine allows simultaneous antigen and MPLA adjuvant codelivery to the same target antigen-presenting cells.Notably,the individual encapsulation and delivery of PEG-PE micelles significantly reduced the induction of the CTL response.In our mouse model,micelles coloaded with the HPV16 E7 antigen and synthetic adjuvant MPLA were prepared and tested as vaccines. Vaccination with these micelles increased the number of E7-specific CD8+cells.Compared with mice inoculated with soluble HPV16 E7 antigen and MPLA,[1]those receiving micelle-based vaccines exhibited slower tumor progression,better survival rates,and superior protection against repeated tumor challenges.Similar positive outcomes have been observed in other tumor models.
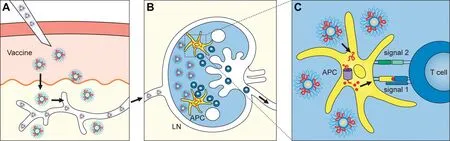
Figure 1. Micellar vaccines offer several advantages because of their appropriate size and design. A, When administered through subcutaneous injection,micellar vaccines can rapidly spread into lymph nodes owing to their proper size.B,In the lymph nodes,the micelles establish extensive contact with the resident CD8α+ DCs, facilitating the delivery of a higher quantity of antigens compared with their free form. C, Another significant benefit of micellar vaccines is their ability to deliver antigens and adjuvants simultaneously to antigen-presenting cells.This dual delivery system enhances the effectiveness of immune adjuvants and improves antigen presentation through the MHC-I pathway.
This kind of cancer vaccine has its limitations.First,it is difficult to mass produce,has a short shelf life,and has no guarantee of uniform size. Second, to adapt to rapid preparation and encapsulate multiple antigen sources,such as DNA,RNA,and subunit antigens,the use of nanoscale materials(including,but not limited to PEG-PE)to facilitate vaccine development must be evaluated.Novel nanoscale materials must be developed if the existing ones are not suitable.Third, deeper mechanistic investigations are needed to determine how nanoscale properties such as size,shape,geometry,and surface functionalization contribute to an effective immune response.
4. The future perspective of cancer vaccine development
Cancer treatment is a complex and multifaceted endeavor,and cancer therapeutic vaccines have emerged as promising options for the fight against cancer. Although relatively new, these methods offer hope for improving cancer treatment outcomes. However, it is essential to acknowledge that cancer therapeutic vaccines also have some limitations.[24]Therefore, combining these methods with existing treatment methods could potentially lead to more satisfactory therapeutic outcomes.Our research in this area yielded promising results.In the mice experiments,we observed that the micellar cancer vaccine effectively suppressed and regressed tumors when used alone. However, its effectiveness was further enhanced when combined with standard tumor treatments,such as surgery,chemotherapy,and radiation.For instance,when chemotherapy alone was used, tumors initially decreased or disappeared; however, they recurred after stopping treatment, and subsequent chemotherapy had reduced efficacy.In contrast,when the tumor vaccine was administered after chemotherapy,the recurrence rate was significantly reduced, and more than half of the experimental animals experienced complete tumor regression and were cured. Similarly, when combined with surgical treatment,the micellar vaccine prevented tumor recurrence in most experimental animals, whereas surgery alone led to tumor recurrence in approximately half of the cases.The absence of tumor recurrence and the specific immune response observed in animals treated with the combined tumor therapy vaccine after surgery demonstrated the effectiveness of this approach(Figure 2).Furthermore,we investigated the combination of a micellar vaccine and traditional radiation therapy for solid malignant tumors. The results showed that the order of treatment was crucial for therapeutic effectiveness.Administering the vaccine before radiotherapy yields a satisfactory therapeutic effect,whereas subsequent administration results in poor treatment outcomes.This difference in efficacy was attributed to the presence of adequate quantity and quality of CTLs after radiotherapy to infiltrate the damaged tumor tissue and clear the tumor cells before immunosuppression caused by the repair process began.[25]Thus,by combining it with other standard cancer treatments, we can enhance overall therapeutic efficacy and potentially improve the outcomes of patients with cancer.

Figure 2. Micellar vaccines synergize with surgical operations for enhanced tumor control.[1]Schematic outline of the experiment designs for combinational therapy of micellar vaccine with surgery.A,One hundred days postsurgery,the mice were immunized with the micellar vaccine(red line),normal saline(NS,black line),or other controls and then boosted twice at 7-day intervals.B,Tumor relapse curve was shown(n=9).Subsequently, the tumor-free mice were rechallenged with 2 × 105 and 5 × 105 TC-1 cells at days 42 and 132, respectively. C, The tumor-free percentage was shown(n=6 for NS control;n=9 for micellar vaccine).****P <0.0001.
Overall, the PEG-PE micelle-based vaccine has the potential to combine multiple desirable features of a therapeutic vaccine into a single formulation.This serves as a unique platform for developing effective cancer vaccines. In addition, PEG-PE micelle-based vaccines also hold the potential for developing personalized vaccines,which will ensure broader, longer-term protection and deal with the heterogeneity of cancers.
Acknowledgments
The authors thank Prof Yangxin Fu of Tsinghua University for constructive advice.
Financial support and sponsorship
This study was supported by a grant from the Strategic Priority Research Program of the Chinese Academy of Sciences(no.XDA09030303).
Conflicts of interest statement
The authors declare that they have no conflict of interest with regard to the content of this report.
Author contributions
Y.Q.,W.Z.,and W.L.composed and wrote the manuscript.
Data availability statement
Not applicable.
Ethical approval
Not applicable.
杂志排行
Oncology and Translational Medicine的其它文章
- Xiao-Ping Chen:the “Master of the Scalpel” who saves lives by entering the forbidden territory of hepatopancreatobiliary surgery
- Chinese expert consensus on laparoscopic hepatic segmentectomy and subsegmentectomy navigated by augmented-and mixed-reality technology combined with indocyanine green fluorescence imaging
- Proposal of a modified classification for hilar cholangiocarcinoma
- Immunotherapy for mucosal melanoma
- Pancreatic cystic neoplasms:a comprehensive approach to diagnosis and management
- FGF2 promotes the chemotherapy resistance in colon cancer cells through activating PI3K/Akt signaling pathway
