Immunotherapy for mucosal melanoma
2024-01-13YuDuXueBaiLuSi
Yu Du,Xue Bai,Lu Si
Abstract Mucosal melanoma(MM)is extremely rare in Caucasians,whereas it is the second predominant melanoma subtype in Asian and other non-Caucasian populations.Distinct from cutaneous melanoma in terms of epidemiology,biology,and molecular characteristics,MM is characterized by more aggressive biological behavior,lower mutational burden,more chromosomal structure variants,and poorer prognosis.Because of the rarity of MM,its biological features are not fully understood,and potential novel therapies are less well depicted.Whereas immunotherapy has shown encouraging efficacy for cutaneous melanoma,its efficacy in MM is unclear due to limited sample sizes in clinical trials.Thus,in this review,we describe the epidemiological,clinical,and molecular features of MM and summarize the efficacies of different immunotherapies for MM,including immune checkpoint inhibitors,vaccines,oncolytic virus therapy,adoptive T-cell therapy,and various combination therapies.
Key words: Antiangiogenic therapy;Combination therapy;Immune checkpoint inhibitors;Immunotherapy;Mucosal melanoma
1.Introduction
Mucosal melanoma (MM) is a subtype of melanoma occurring in mucosal membranes at various anatomic sites, including the head and neck regions,gastrointestinal,genitourinary tracts,and so on.Unlike cutaneous melanoma (CM) showing a high tumor mutational burden (TMB) with UV signature, MM typically presents a low TMB and high copy number and structural variations.[1-3]Apart from tolerogenic immune microenvironment, MM tends to be more aggressive.Because of the occult sites of origin and no obvious early symptoms,MM is often diagnosed late with metastatic spread through the lymphatic and hematologic vascular networks,with a 5-year survival rate of approximately 25%.[4-6]With the advent of immunotherapy (Figure 1), the 5-year survival rate has increased to 30%to 50%in melanoma.[7,8]However,MM is less responsive to anti-programmed cell death 1 (PD-1) and anticytotoxic T-lymphocyte-associated protein 4(CTLA-4)therapies than CM.Because of its relative rarity,our understanding of MM remains limited, and the biological and biochemical behavior of MM is still obscure.In this review,we provide an updated overview of MM with its epidemiology,clinical and molecular features,and response to different types of immunotherapies including immune checkpoint inhibitors(ICIs),vaccines,oncolytic virus therapy,and adoptive T-cell therapy(ACT).
Figure 1. Timeline of the primary immunotherapies for mucosal melanoma.
2.Epidemiology and clinical features
Mucosal melanoma is a rare subtype of melanoma in Caucasian populations, composing approximately 1.3% of all melanomas in the United States.[9]Although the incidence of melanoma is increasing, MM is estimated to have a stable incidence.[10,11]Occurring with similar frequency across all races,MM makes up a higher proportion of melanomas in Hispanics(3.6%-4%),non-Hispanic Blacks(9%-10.3%),and Asians(14.8%-27%)than Caucasians,[12-18]as it is less diluted due to the lower incidence of CM in these populations with darker skin color.
The incidence of MM varies in different anatomical sites(Table 1). In Western countries, the most common primary sites are head and neck (30.9%-55.4%), anal/rectal (21.8%-23.8%),and female genital tract(18%-30.4%).[9,11,19]Lian et al[6]discovered that the lower gastrointestinal tract was the most common anatomic site of primary lesions(26.5%)in China,followed by the nasal cavity and paranasal sinuses(23%),gynecological sites(22.5%),and oral cavity (15%). Notably,no survival differences have been observed across different anatomic sites of MM.[6,19,20]Based on these findings in Asian populations,Cui et al[21]proposed a unified staging system for MM regardless of the primary anatomic site.

Table 1Incidences of mucosal melanoma in different primary sites
Typically, MM is more common in individuals older than 60 years and more frequent in women than in men.[11,21,22]Compared with CM,MM is usually characterized by an aggressive clinical behavior and a worse prognosis,with more advanced stage at diagnosis and higher recurrence rate after surgery.[6]The clinical presentation of MM depends mainly on the location of the lesions.In a retrospective analysis, the most common metastatic sites of MM were regional lymph nodes (21.5%), lung (21%), liver(18.5%), and distant lymph nodes (9%).[6]Mucosal melanoma is often associated with a deeper thickness and more involved with ulceration than CM, whereas tumor thickness and ulceration have been found with little or no prognostic significance in multiple studies.[5,19,20,23]In contrast,patients with primary MM penetrated to the muscularis propria layer or deeper structures have worse survival than those with MM limited to mucosal or submucosal layer only.[21]Besides, the number of regional lymph node metastases and site of distant metastases are both independently associated with overall survival(OS).[5,19-21]Similar to CM,MM patients with elevated serum lactate dehydrogenase levels at the time of staging have a poorer prognosis.[21]
3.Molecular features
Genetically,MM is recognized by the presence of unique molecular features compared with CM,namely,a generally lower point mutational burden yet a larger amount of chromosomal structure aberrations.[1-3,24-27]
Generally, MM has an average of 8000 single-nucleotide variants per tumor, which is more than 10 times lower than that in CM (>80,000 single-nucleotide variants).[28]Although BRAF and NRAS genes are predominantly mutated in CM,they present with a lower mutation rate in MM.[29-31]Newell et al[1]conducted the largest genomic analysis of MMs(n=112)from China,Australia,United States, and Europe and reported that significantly mutated genes were NRAS (17.9%), BRAF (16.4%), NF1 (16.4%), KIT(14.9%),SF3B1(11.9%),TP53(9.0%),SPRED1(7.5%),ATRX(6.0%),HLA-A(6.0%),and CHD8(4.5%).A systematic review indicated that the most frequent mutant genes from analyses of more than 100 MM samples were KIT, BRAF, NRAS, NF1, SF3B1,TP53,MTOR,ATRX,BAP1,and TERT.[32]In an analysis of 284 MM patients,GNAQ and GNA11 mutations were found in 4.6%and 4.9% patients, suggestive of a poor prognosis.[33]Without UV radiation as the primary carcinogenic factor,MMs tend to have more heterogeneous pathogenic sequence variations in the BRAF and NRAS genes.Despite a markedly high prevalence of mutations at the V600 codon of BRAF in CM,codons D594,G469,and K601 are frequently altered in MM.[1,30]Similarly, whereas mutations at the Q61 codon of the NRAS exon 3 are most common in CM,the G12 and G13 codons in exon 2 are highly involved in MM.[25,32,34,35]
Differences were observed in the spectrum and distribution of the driver genes among different anatomical sites (Table 2). UV radiation-related mutations are predominant in the majority of conjunctival melanoma samples.[36]BRAF and NRAS occur more frequently in the mucosae of the head and neck district.[35]Conversely,KIT mutation presents a higher prevalence in the genitourinary tract and anorectal sites compared with the head and neck sites.[32,37,38]Moreover, ATRX and SF3B1 mutations were more frequently observed in melanomas located in the lower anatomy,whereas CTNNB1 mutations were more common in the upper anatomy.[32,34]

Table 2Incidences of significantly mutated genes across different anatomical sites in mucosal melanoma
Furthermore, copy number variations and structural variants are more common in MM.Similar to the discoveries in CM,MM presents with frequent amplification of TERT,CCND1,KIT,MITF,CCND1,MDM2, CDK4, and NOTCH2, but copy loss of NF1, PTEN,CDKN2A,ATM,and ARID1B.[1,34]In addition,CDK4 and MDM2 genes are commonly coamplified on chromosome 12,simultaneously with TERT on chromosome 5 via linking translocations.[1,24]An analysis of 213 Chinese MM samples indicated that the amplification rates of CDK4 and CCND1 were 47.0% and 27.7%, respectively,whereas the deletion rate of P16INK4awas 57.7%,and CDK4/6 inhibitors significantly suppressed the PDX tumor growth with abnormal CDK4 pathway.[39]PTEN loss,amplification of MAPK and CDK4/6 pathways,and WNT-β catenin dysregulation are associated with immunosuppression in CM,[40]whereas their specific role in MM is still unknown,deserving for further researches.
4.Overview of therapeutic strategies of MM
Because of the rarity of MM and scarcity of clinical trials,standard treatment for MM has not yet been established.Therefore,the treatment of MM often draws on the experience of CM.Complete surgical resection is still the only potential venue of cure for resectable MM,but the recurrence rate is extremely high.[18]The role of immunotherapy for MM in neoadjuvant and adjuvant settings has been evaluated in an effort to reduce recurrence and improve survival(Figure 2).Whereas targeted therapy and immunotherapy have significantly changed the treatment landscape of CM,advanced MM tends to be less responsive to immunotherapy.Combination therapies based on immunotherapy provide new opportunities for the treatment of advanced MM.

Figure 2. Various immunotherapies for mucosal melanoma.
5.Immunotherapy for metastatic/unresectable MM
5.1.Immune checkpoint inhibitor monotherapy
Immune checkpoint inhibitors have revolutionized the treatment of melanoma and brought significant survival benefits,which are currently the standard treatments for CM.However,ICIs seem less effective in MM than CM,with limited knowledge deriving from retrospective studies or subset analyses of prospective trials.
For MM patients,the efficacy of ipilimumab has been proven less favorable,with objective response rate(ORR)ranging from 3.9%to 12%, median progression-free survival (PFS) of 2.7 to 4.3 months,and median OS of 6.4 to 12 months.[41-45]As for anti-PD-1 antibodies,the effectiveness varies from different studies as a result of study designs with various sample sizes as well as different anti-PD-1 antibodies,including nivolumab,pembrolizumab,and toripalimab.
Clinical trials suggest that Caucasian patients with MM are also less likely to respond to anti-PD-1 antibodies compared with those with CM. In CheckMate 172 study involving 63 MM patients treated with nivolumab after failure of ipilimumab, a median OS of 11.5 months was achieved.[46]A pooled analysis of CA209-003 and CA209-038 and CheckMate 066,037,and 067 demonstrated an ORR of 23.3%and a median PFS of 3.0 months in MM patients treated with nivolumab.[43]In addition, a post hoc analysis of KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 involving 1567 patients treated with pembrolizumab reported that the ORR in 84 MM patients was 19%, the median PFS was 2.8 months,and median OS was 11.3 months.[47]
As for studies conducted mainly in Asian populations,a phase IB study(KEYNOTE-151)reported an ORR of 13.3%in 15 Chinese MM patients treated with pembrolizumab as a second-line treatment.[48,49]The POLARIS-01 study demonstrated that in 22 Chinese MM patients, toripalimab provided a very poor ORR of0%,amedianPFSof1.9months,andamedianOSof10.3months.[50]A Japanese phase II study evaluated the clinical efficacy of nivolumab in 17 Japanese MM patients,reporting an ORR of 23.5%,a median PFS of 1.4 months, and a median OS of 12 months.[51]Another postmarketing observational study involving 208 Japanese MM patients reported a median OS of 11.3 months for nivolumab administered as second-or later-line treatment.[52]Moreover,several multicenter retrospective studies have reported that anti-PD-1 monotherapy yielded an ORR of 20% to 29%, a median PFS of 3.9 to 6.2 months, and a median OS of 12.4 to 20.4 months in MM patients.[44,53-56]
5.2.Anti-PD-1 antibody plus other ICIs
Several studies have investigated whether anti-PD-1/anti-CTLA-4 combination could improve efficacy compared with anti-PD-1 monotherapy in MM. A pooled analysis conducted by D'Angelo et al[43]compared the combination of nivolumab and ipilimumab with nivolumab alone.The combination therapy resulted in a better ORR(37.1%vs 23.3%)and median PFS(5.9 vs 3.0 months)compared with nivolumab monotherapy in MM,although the efficacy was markedly inferior to that observed in CM.A single-arm,phase II study involving 12 patients treated with nivolumab plus ipilimumab demonstrated an ORR of 33.3%, 1-year PFS rate of 54.7%, and 1-year OS rate of 75%.[57,58]However,the anti-PD-1/anti-CTLA-4 combination has not provided clinical benefit over anti-PD-1 blockade alone in some studies.[55,56]In a large-sample-size international multicenter retrospective study including 197 MM patients treated with anti-PD-1 antibody (nivolumab or pembrolizumab) plus ipilimumab, the clinical efficacy between these combinations and anti-PD-1 monotherapy were compared.[56]The study showed no remarkable difference in ORR(31%vs 29%),PFS(4 vs 5 months),and OS(21 vs 19 months)between these 2 groups.
As an inhibitory immune checkpoint, lymphocyte-activation gene 3(LAG-3)has been found upregulated in many neoplasms,including melanoma.[59]Preclinical models have demonstrated that dual inhibition of LAG-3 and PD-1 exhibits synergistic antitumor activity.[60]Relatlimab is a first-in-class human immunoglobulin G4 anti-LAG-3 antibody.In a phase 2-3,global,randomized trial,Tawbi et al[61]evaluated the efficacy of relatlimab plus nivolumab versus nivolumab alone in patients with untreated advanced melanoma.The trial recruited 51 MM patients including 23 cases treated with relatlimab-nivolumab and 28 with nivolumab alone, and no significant difference in PFS was observed.However,given the small sample size and rarity of MM,further studies are necessary to fully comprehend the efficacy of the relatlimab-nivolumab combination in patient with MM.
5.3.Anti-PD-1/programmed cell death 1 ligand antibody plus antiangiogenic therapy
New combination therapies are being explored for MM and demonstrate promising clinical efficacy.Studies have shown that vascular endothelial growth factor(VEGF)is strongly expressed in MM and contributes to both disease progression and immunosuppression,[62-64]and the combination of immune checkpoint blockade with VEGF receptor inhibitor has garnered significant interest(Table 3).[70]Sheng et al[65]conducted a phase Ib trial of 33 patients with advanced MM to evaluate the combination of axitinib with toripalimab. The combination yielded exciting results (ORR,48.3%;median PFS,7.5 months;median OS,20.7 months).Interestingly, no association was found between survival benefits and programmed cell death 1 ligand(PD-L1)expression or TMB level,whereas a 12-gene expression profile signature was significantly correlated with improved PFS and OS.[71]A real-world study investigating the combination of axitinib with anti-PD-1 antibody(toripalimab or pembrolizumab)was conducted later,reporting an ORR of 24.5%and a median OS of 11.1 months,which is slightly lower than that reported in the previous phase Ib trial.[66]In a phase I study, the combination of LBL-007 (an anti-LAG3 antibody),toripalimab,and axitinib resulted in an ORR of 45.4%and median PFS of 5.5 months in 11 patients(10 mucosal,1 acral).[67]A phase II study of vorolanib combined with toripalimab in MM patients with no prior systemic therapy demonstrated an ORR of 22.2%and median PFS of 5.7 months in the 150 mg vorolanib group.[68]The LEAP-004 study evaluated the efficacy of lenvatinib plus pembrolizumab in 103 patients with advanced melanoma,demonstrating median PFS of 4.2 months and OS of 14.0 months.[72]Whereas ORR was 21.4% in the total population, it reached 33.3% in the 30 patients with PD on prior anti-PD-1 plus anti-CTLA-4 therapy.However,an international multicenter,randomized phase III trial (LEAP-003) evaluating this combination in patients with metastatic acral melanoma and MM was discontinued as the OS end point was not reached.The regimen of anti-PD-L1 inhibitor plus VEGF receptor inhibitor was also assessed.Mao et al[69]reported a multicenter, phase II study including 43 MM patients treated with first-line therapy of atezolizumab plus bevacizumab.Objective response rate was 45.0%, median PFS was 8.2 months,and 1-year OS rate was 76.0%. Overall, the combination of antiangiogenic therapies with ICIs demonstrates impressive clinical efficacy in MM,warranting larger prospective randomized studies to further validate these results.
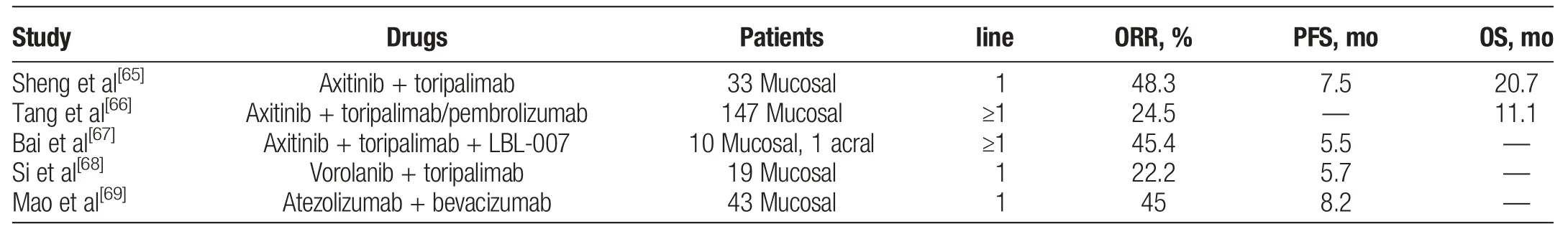
Table 3Anti-programmed cell death 1/programmed cell death 1 ligand antibody plus antiangiogenic therapy for advanced mucosal melanoma
5.4.Anti-PD-1/PD-L1 antibody plus other agents
Although BRAF mutations are infrequent in MM,the combination of BRAF inhibitor and MEK inhibitor should be considered for MM patients with BRAF V600E mutation.[18]In COMBI-i study,spartalizumab plus dabrafenib and trametinib did not improve PFS in patients with BRAF V600-mutant melanoma compared with placebo plus dabrafenib and trametinib.[73]KIT aberrations were relatively common in MM.KIT-targeted therapies such as imatinib or nilotinib have demonstrated clinical benefit in some MM patients with KIT aberrations.[74,75]In addition,the preliminary results of a phase II study have demonstrated that imatinib plus toripalimab was a promising therapy for patients with advanced melanoma harboring C-KIT aberrations (ORR, 55.6%).[76]However, whether imatinib plus toripalimab could provide clinical benefit for MM remains unclear.Clinical trials are underway to investigate other potential agents,including CDK inhibitors,MDM2 inhibitors,CD40 antibodies,and TLR agonists,for combination with ICIs(Table 4).
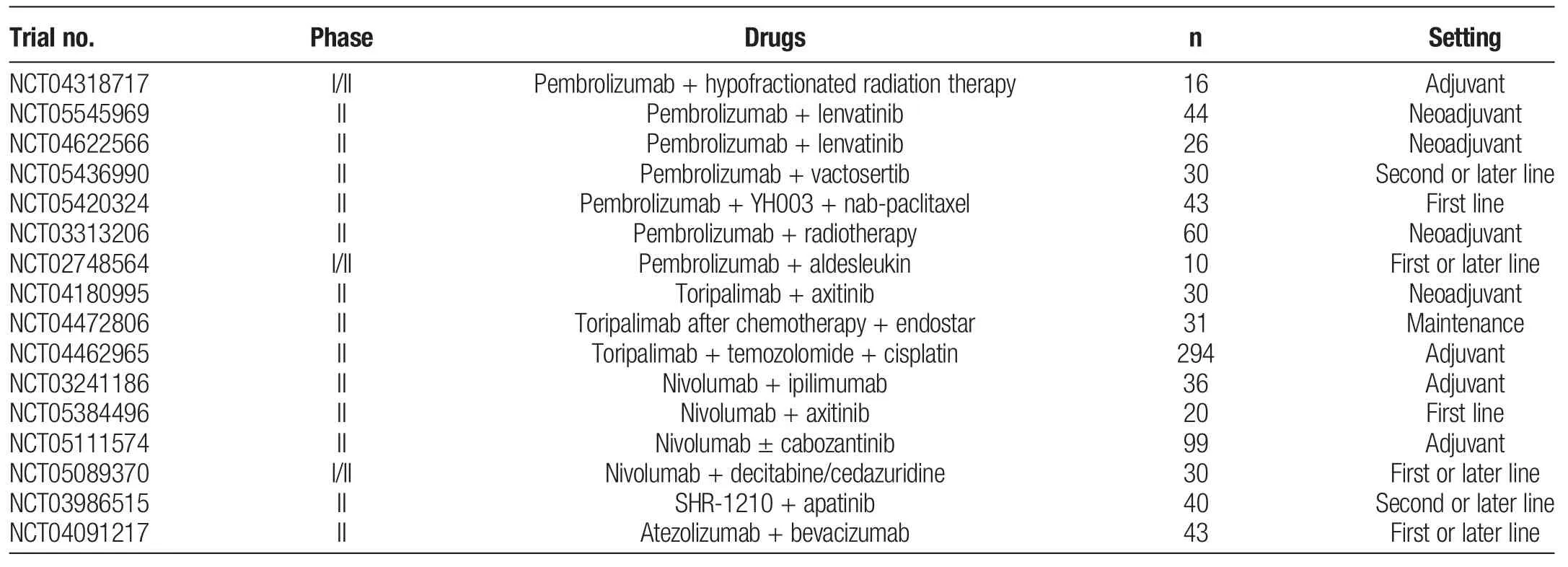
Table 4Ongoing clinical trials of combined immune checkpoint inhibitor therapies for mucosal melanoma
5.5.Cytokines
Interleukin 2 is the first approved immunotherapy that can stimulate natural killer cells and CD8+T cells to attack tumors. A novel engineered interleukin 2 receptor agonist nemvaleukin alfa has been discovered to selectively induce expansion of immune effector cells,especially CD8+T and natural killer cells with minimal effects on regulatory T cells.[77]In a phase I/II ARTISTRY-1 trial,the efficacy of nemvaleukin alfa alone and with pembrolizumab was tested in patients with a range of solid tumors.[78]For 46 patients with advanced melanoma,ORR was 13%,whereas durable antitumor activity was observed in 2 of 6 MM patients (ORR, 33.3%).[78,79]Nemvaleukin alfa has been granted orphan drug designation for the treatment of MM.
5.6.Vaccines
Cancer vaccines targeting tumor-associated or tumor-specific antigens aim to stimulate the immune system and eradicate tumor lesions. However, most melanoma vaccines so far have been determined to be ineffective in large clinical trials. In the E1694 study,880 patients with stage IIB/III melanoma were randomly assigned(1:1)to receive high-dose interferon α2b(HDI)therapy or vaccination with GM2 conjugated to keyhole limpet hemocyanin and administered with QS-21,and HDI demonstrated improved relapse-free survival(RFS)and OS.[80]Similarly,recombinant MAGE-A3 with AS15 immunostimulant failed to improve disease-free survival in the DERMA study.[81]Furthermore, the response rates of various vaccines were reported to be 2.6%to 3.8%in patients with multiple cancer types including melanoma.[82]FixVac is an intravenously administered liposomal RNA vaccine targeting 4 tumor-associated antigens(NY-ESO-1,MAGE-A3,tyrosinase,and TPTE).In a phase I trial,the ORR was 16%in the FixVac monotherapy group(n=25)and 35%in the FixVac/anti-PD-1 combination group(n=17),indicating the potential of liposomal RNA vaccine in patients with checkpoint inhibitor-experienced melanoma.[83]
With the development of DNA and RNA sequencing,as well as computational approaches to predict tumor antigens,personalized cancer vaccine approaches have been investigated and tested in clinical trials.NeoVax was deemed safe and could generated long-term antigen-specific immune responses in a phase I trial.[84]The response rate was 59%in patients with unresectable stage III or stage IV melanoma who received NeoVax plus nivolumab in a phase Ib trial.[85]RO7198457 is a liposomal RNA vaccine that contains up to 20 tumor-specific neoepitopes.In a phase 1 study of patients with advanced solid tumors receiving RO7198457 and atezolizumab,responses were seen in 3 patients in a cohort of 10 patients with untreated advanced melanoma.[86]In KEYNOTE-942 trial,mRNA-4157(a novel mRNA-based personalized cancer vaccine)plus pembrolizumab as adjuvant therapy significantly improved RFS and distant metastasisfree survival in patients with resected stage IIIB/IIIC/IIID and IV melanoma compared with pembrolizumab alone, with a reduction in the risk of recurrence or death by 44%.[87,88]
Little is known about the efficacy of cancer vaccines in MM.In a phase II study,16 patients with advanced melanoma(10 cutaneous,1 mucosal,5 uveal)were enrolled to receive the combination of dendritic cell vaccines targeting tumor blood vessel antigens and dasatinib.[89]Of 13 evaluable patients, the ORR was 46%. With further exploration of cancer vaccine, it may bring clinical benefit for MM in the future.
5.7.Oncolytic virus therapy
Oncolytic virus therapy is an emerging class of cancer therapeutics depending on selectively killing cancer cells without harming the normal tissues by genetically engineered or naturally occurring virus. Data on clinical experience of patients receiving various oncolytic virus therapies are now becoming available in many cancers,including melanoma.
Talimogene laherparepvec(T-VEC),an engineered oncolytic herpes simplex virus type 1,is the only oncolytic virus approved by the US Food and Drug Administration. Compared with granulocyte macrophage colony-stimulating factor, intratumoral injection of T-VEC in a phase 3 trial resulted in a higher durable response rate and improvements in PFS and OS in patients with advanced melanoma with good safety profile, leading its approval in 2015.[90]A number of other oncolytic viruses including ONCOS-102,coxsackievirus A21, PVSRIPO, RP1, and OrienX010 have also shown promising oncolytic activities in melanoma.[91-95]To optimize the clinical efficacy of oncolytic virus therapy,the use of oncolytic virus therapy in combination with other promising emerging therapies is of great interest, especially checkpoint inhibitors. Chesney et al[96]evaluated the combination of T-VEC and ipilimumab versus ipilimumab alone in a phase II trial.Combination therapy achieved a significantly higher response rate (39% vs 18%), and responses were observed in both injected and noninjected tumors including visceral lesions. Talimogene laherparepvec plus pembrolizumab was also explored in a phase 3 study.Although combination therapy did not improve PFS or OS compared with pembrolizumab,T-VEC plus pembrolizumab was well tolerated.[97]The ORR of 35%to 50%was observed in other combinations of an oncolytic virus and a checkpoint inhibitor, including ONCOS-102 plus pembrolizumab, coxsackievirus A21, and ipilimumab.[98,99]Overall, these data suggested potential synergistic therapeutic activity of oncolytic viruses and checkpoint inhibitors in melanoma.
Many researches above have excluded MM patients. Fröhlich et al[100]reported a patient with MM of the urethra who developed disease progression after pembrolizumab monotherapy.The patient was then treated with T-VEC in intravaginal mucosal metastases and experienced a partial response, suggesting the potential of T-VEC in anti-PD-1 refractory melanoma.In another report,a patient with MM of the left maxillary sinus received T-VEC injections after recurrence of maxillary sinus during the treatment of nivolumab and ipilimumab.[101]The recurrent tumor of left maxillary experienced a significant shrinkage,and the patient stayed stable disease for over 1 year. In a phase Ib trial, toripalimab plus intralesional injection of OrienX010 was evaluated in 15 patients with melanoma with liver metastases including 9 MM patients.[102]Whereas the ORR was 13.3%,the response rate was 40%for injection lesions. Although scanty information is available in clinical data, oncolytic virus therapy demonstrates preliminary efficacy and represents a therapeutic option for MM.
5.8.Adoptive T-cell therapy
Adoptive T-cell therapy is a personalized treatment that involves the isolation,engineering,and expansion of T cells and subsequent reinfusion to the host,which has shown clinical advances in nonhematologic solid tumors,including melanoma.In particular,ACT with tumor-infiltrating lymphocytes(TILs)has demonstrated remarkable antitumor activity in a series of phase 1-2 trials,with response rates of approximately 50%and durable complete response rate of 20%mostly in patients who had not received anti-PD-1 therapy.[103-105]Most recently,an autologous TIL therapy lifileucel was explored in patients with advanced melanoma who had been previously treated with checkpoint inhibitors and BRAF±MEK inhibitors.Objective response rate of 36%and disease control rate of 80%were achieved,indicating the potential of lifileucel after failure of previous immune checkpoint inhibition.[106]In a phase 3 study,168 patients with stage IIIC or IV melanoma were enrolled to compare the efficacy of TIL therapy with ipilimumab. Median PFS in the TIL group was more than twice as long as in the ipilimumab group (7.2 vs 3.1 months,P <0.001). The ORRs were 49% and 21% in the TIL and ipilimumab group, respectively. Notably, among these patients,86% received previous anti-PD-1 treatment.[107]These findings showed TIL therapy was a promising first-or second-line treatment in patients with melanoma,even among those who were primary refractory to checkpoint inhibitors.Neoantigen-driven recognition by TILs has been found as the major role in tumor clearance following TIL therapy. Kristensen et al[108]found that the frequency of neoantigen-specific CD8+T cells in TIL infusion products correlated with increased survival in patients with melanoma. Neoantigenspecific T-cell responses were found increased in the peripheral blood,and neoantigen-specific T cells were detectable for up to 3 years after TIL infusion.[105]Interestingly, BRAF V600E-specific CD4+T cells were identified from a patient with stage IV acral melanoma who achieved a complete response following TIL infusion and persisted long-term in the peripheral blood,suggesting their contribution to effective antitumor immune response.[109]Therefore, exploring novel strategies to enhance the potency of neoantigen-specific T cells might improve the efficacy of TIL therapy in melanoma.[110]
Engineered T-cell receptor(TCR)and chimeric antigen receptor(CAR)T-cell therapies have also been investigated as new therapeutic strategies for melanoma in preclinical and clinical trials. T-cell receptor-engineered T-cell therapy is on the basis of transducing host T cells with antigen-specific TCRα/β chains to create high-affinity T cells that target tumor-associated antigens.T-cell receptor T-cell therapies targeting MART-1 or tyrosinase seem to be less effective than TIL therapy.[111,112]However,impressive clinical responses were seen in melanoma with NY-ESO-1-specified TCR T-cell therapy. In a clinical trial, 5 of 11 patients with metastatic melanoma achieved objective clinical responses,and 2 patients kept complete remission for more than 1 year.[113]In a phase 3 trial,tebentafusp, a gp100-targeting TCR/anti-CD3-bispecific fusion protein, demonstrated a higher PFS (31% vs 19% at 6 months)and OS(73%vs 59%at 1 year)in patients with uveal melanoma compared with those receiving pembrolizumab, ipilimumab, or dacarbazine.[114]Although CAR T-cell therapy has shown striking potency in treating advanced hematological cancers, evidence on its efficacy in melanoma is very limited.Chimeric antigen receptor T-cell therapies targeting different antigens including CD16,CD70, B7-H3, HER2, VEGFR-2, CSPG4, GD2, GD3, gp100,and NY-ESO-1 have displayed satisfactory antitumor effects in many preclinical studies, leading to further exploration in clinical trials.[115-123]In a clinical trial, 24 patients with metastatic cancer including metastatic melanoma were enrolled to receive anti-VEGFR-2 CAR T-cell therapy,but no response was observed along with severe adverse effects, which led to termination of the trial.[123,124]Multiple clinical trials testing the efficacy of CAR T-cell therapy are still underway.Currently,no CAR T-cell therapy has been proven effective in melanoma in clinics, but CAR T-cell therapy is still in an early stage of development,and more exploration may bring promising results in the future.
Limited researches were conducted to explore the efficacy of ACT in MM because of its rarity.Zhang et al[125]investigated the combination therapy of cytokine injection, cryosurgery, and transfer of cytokine-induced killer cells in treating 10 patients with oral MM.The results were encouraging that all patients experienced an objective response including 7 cases of continuing complete remission.[125]In another study,1 patient with rectal MM who received TIL infusion achieved stable disease for 9.3 months after failure of both PD-1 and CTLA-4 blockade monotherapies.[126]Nevertheless,evidence of ACT for MM is limited;further investigation is needed to improve the efficacy of genetically modified T cells in the treatment of MM.
6.Adjuvant immunotherapy after definitive surgery
Although ICIs have become standard adjuvant therapies for CM,their roles in MM are currently unclear.The CheckMate 238 study recruited 906 patients with stage IIIB-C or IV melanoma to receive nivolumab or ipilimumab as adjuvant therapy.[127,128]Among 29 MM patients,the 4-year RFS rate in the ipilimumab group seemed better than that in the nivolumab group, whereas the difference was not significant(18.8%vs 46.2%;hazard ratio,1.71;95%confidence interval,0.68-4.29).In an open-label,phase II randomized trial, 145 MM patients were enrolled to compare the efficacy of toripalimab versus HDI as adjuvant therapy.[129]Toripalimab and HDI showed similar efficacies(median RFS,13.6 vs 13.9 months),whereas toripalimab demonstrated a more favorable safety profile,indicating that toripalimab might be a better choice.Previous studies have revealed that temozolomide plus cisplatin is superior to both HDI and observation.[130,131]Lian et al[132]retrospectively compared temozolomide plus cisplatin with toripalimab as adjuvant therapy for 247 MM patients and discovered that adjuvant temozolomide plus cisplatin shows remarkably longer RFS and OS than toripalimab (median RFS, 28.2 vs 12.0 months; OS, 93.4 vs 39.3 months). However, it remains unclear whether the combination of temozolomide-based chemotherapy and a PD-1 blockade confers additional survival benefits. An ongoing phase II clinical trial(NCT04462965)may provide evidence for these questions in the future.
7.Neoadjuvant immunotherapy
Mucosal melanoma is often diagnosed at an advanced stage because of inconspicuous clinical manifestations. Achieving wide negative margins in MM is challenging,which renders a significant number of patients experiencing postoperative local recurrence. Neoadjuvant therapy may improve resectability and long-term outcomes of MM.Ho et al[133]conducted an analysis of 36 patients with resectable MM treated with neoadjuvant anti-PD-1± anti-CTLA-4 immunotherapy. The results suggested that ORR was 47%, pathologic response rate was 35%, event-free survival was 9.2 months,and 3-year OS rate was 55%. Interestingly, an improvement in event-free survival and OS was observed in patients with pathologic response, highlighting the necessity for further investigation. Recently, a phase II trial including 313 patients with resectable stage IIIB to IV melanoma provided an event-free survival benefit in the neoadjuvant-adjuvant pembrolizumab group compared with the adjuvant pembrolizumab group.[134]Moreover, 4 MM patients were involved in the neoadjuvant-adjuvant pembrolizumab group and were alive at the last follow-up. The combination of antiangiogenic therapies with ICIs has also been investigated in MM in neoadjuvant therapy.A phase II study included patients with resectable MM to receive neoadjuvant toripalimab/axitinib for 8 weeks before surgery followed by adjuvant toripalimab.[135,136]Up to the cutoff date of April 2022, the pathologic response rate was 27.6% (8/29), including 4 with pathologic complete response and 4 with pathologic partial response.[136]The median RFS was 11.7 months.Notably,CD3+and CD3+CD8+T cells were increased in tumor samples after the neoadjuvant therapy. Neoadjuvant lenvatinib and pembrolizumab are also being evaluated in resectable MM.The preliminary results suggested that the pathologic response rate was 40%(6/15).[137]Nevertheless,the role of neoadjuvant immunotherapy in MM still needs to be further explored.
8.Biomarkers for immunotherapy
Yet,there is still a lack of predictive and prognostic biomarkers for immunotherapy in MM.In a study of 152 Asian patients with advanced melanoma (31% with MM) receiving anti-PD-1 therapy,high neutrophil-to-lymphocyte ratio was associated with poor PFS,suggesting its potential predictive value of response to immunotherapy.[138]Mucosal melanoma often expresses lower levels of PD-L1 than CM.[139]The predictive value of PD-L1 expression for immunotherapy response is also being explored in MM.A pooled analysis reported that PD-L1 expression ≥5%was associated with a higher ORR in MM patients regardless of immunotherapy groups.[43]However, no difference was observed between ORR among PD-L1-positive and PD-L1-negative patients in another study.[65]Intriguingly,in a study involving 10 MM patients treated with combinational ipilimumab/nivolumab immunotherapy,MAGE-A3, CTAG1B (NY-ESO-1), and MAGE-A4 seemed to be the genes with the greatest difference between responders and nonresponders. Moreover, immunohistochemical expression of NY-ESO-1, MAGE-A3, and MAGE-A4 was significantly higher in responders,and the positivity of all 3 markers is associated with response significantly.[140]Tumor mutational burden is often used as a predictive biomarker for immunotherapy.[141]However,MM is typically associated with a low TMB. Sheng et al[65]discovered that MM patients with TMB of greater than 6 mutations/Mbp had a better ORR to the combination of axitinib and toripalimab than those with TMB of less than 6 mutations/Mbp (83.3% vs 45.5%),but the difference was not statistically significant. Similar results were observed in another observational study that the nonprogressor group tended to have a higher proportion of TMB-high tumors as compared with the immunotherapy-resistant group.[142]Besides,a study evaluated indoleamine 2,3-dioxygenase and PD-L1 expression in 32 Japanese patients with acral melanoma and MM treated with anti-PD-1 antibody.[143]Low expression of indoleamine 2,3-dioxygenase in tumors was associated with poor PFS and poor response to anti-PD-1 therapy, whereas PD-L1 expression was not associated with PFS.Recently,eIF4F,the eukaryotic translation initiation complex,has been found to regulate the response to ICIs by regulating the interferon γ-inducible PD-L1 expression.[144,145]An analysis of 23 MM patients treated with ICIs demonstrated that eIF4F complex in the tumor was significantly associated with response to ICIs.[146]With the development of next-generation technology,distinct potential markers including 12-gene expression signature of genes related to immune regulation, inflammation, and angiogenesis and 18-gene T cell-inflamed gene expression profile have been found to be associated with response to ICIs in MM.[71,142]
9.Conclusion
Mucosal melanoma is an aggressive melanoma subtype that is often diagnosed at an advanced stage with lymph node and distant metastases.Distinct from CM,MM is usually associated with a lower TMB,different frequency of driver mutations,and higher chromosomal structure aberrations.Despite recent remarkable advances of immunotherapy and targeted therapy in the treatment of melanoma,there is still a lack of standardized and effective interventions for MM patients.Patients with MM tend to respond less well to current immunotherapies as compared with CM.The most promising therapy for MM seems to be the combination of antiangiogenic agents and ICIs.Other immunotherapies including vaccines, oncolytic virus therapy, and ACT have also demonstrated preliminary efficacy in some MM patients.However,most studies about MM are small-cohort clinical trials and respective studies because of its rarity.Large randomized controlled trials are warranted to understand the biology and optimize combination therapies.
Acknowledgments
Not applicable.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China(82272676,82073011,81972562,81972566),Beijing Medical Award Foundation (YXJL-2020-0889-0106), Beijing Municipal Administration of Hospitals' Ascent Plan (DFL20220901),and Beijing Xisike Clinical Oncology Research Foundation(Y-Roche2019/2-0076).
Conflicts of interest statement
The authors declare that they have no conflict of interest with regard to the content of this report.
Author contributions
L.S.contributed to the conceptualization.Y.D.and X.B.wrote the first edition of the manuscript.All authors reviewed and approved the final version of this article.
Data availability statement
Not applicable.
Ethical approval
Not applicable.
杂志排行
Oncology and Translational Medicine的其它文章
- Xiao-Ping Chen:the “Master of the Scalpel” who saves lives by entering the forbidden territory of hepatopancreatobiliary surgery
- Chinese expert consensus on laparoscopic hepatic segmentectomy and subsegmentectomy navigated by augmented-and mixed-reality technology combined with indocyanine green fluorescence imaging
- Proposal of a modified classification for hilar cholangiocarcinoma
- Development of therapeutic cancer vaccines using nanomicellar preparations
- Pancreatic cystic neoplasms:a comprehensive approach to diagnosis and management
- FGF2 promotes the chemotherapy resistance in colon cancer cells through activating PI3K/Akt signaling pathway
