“Parenchyma transection-first” strategy is superior to “tunnel-first”strategy in robotic spleen-preserving distal pancreatectomy with conservation of splenic vessels
2024-01-02MengYngLiHoZheCuiJiNingHoBinXuEnLiZhngZhuZengYinZhiMingZho
Meng-Yng Li ,Ho-Zhe Cui ,Ji-Ning Ho ,D-Bin Xu ,En-Li Zhng ,Zhu-Zeng Yin ,Zhi-Ming Zho ,
a Faculty of Hepato-Pancreato-Biliary Surgery,The First Medical Center,Chinese PLA General Hospital,Beijing 100853,China
b School of Medicine,Nankai University,Tianjin 30 0 071,China
Keywords: Pancreatic parenchyma transection-first strategy Kimura’s procedure Splenic vessel preservation Minimally invasive surgery
ABSTRACT Background: Creating a tunnel between the pancreas and splenic vessels followed by pancreatic parenchyma transection (“tunnel-first” strategy) has long been used in spleen-preserving distal pancreatectomy (SPDP) with splenic vessel preservation (Kimura’s procedure).However,the operation space is limited in the tunnel,leading to the risks of bleeding and difficulties in suturing.We adopted the pancreatic “parenchyma transection-first” strategy to optimize Kimura’s procedure.Methods: The clinical data of consecutive patients who underwent robotic SPDP with Kimura’s procedure between January 2017 and September 2022 at our center were retrieved.The cohort was classified into a“parenchyma transection-first” strategy (P-F) group and a “tunnel-first” strategy (T-F) group and analyzed.Results: A total of 91 patients were enrolled in this cohort,with 49 in the T-F group and 42 in the P-F group.Compared with the T-F group,the P-F group had significantly shorter operative time(146.1 ± 39.2 min vs.174.9 ± 46.6 min,P <0.01) and lower estimated blood loss [40.0 (20.0–55.0) mL vs.50.0 (20.0–100.0) mL,P=0.03].Failure of splenic vessel preservation occurred in 10.2% patients in the TF group and 2.4% in the P-F group (P=0.14).The grade 3/4 complications were similar between the two groups (P=0.57).No differences in postoperative pancreatic fistula,abdominal infection or hemorrhage were observed between the two groups.Conclusions: The pancreatic “parenchyma transection-first” strategy is safe and feasible compared with traditional “tunnel-first strategy” in SPDP with Kimura’s procedure.
Introduction
Since 1996,when Kimura introduced his novel technique of conserving the splenic artery and vein in a spleen-preserving distal pancreatectomy (SPDP),known as Kimura’s procedure [1],this technique has been widely accepted and performed [2].In recent years,minimally invasive surgery has been proven to be safe and feasible in pancreatic surgery,especially in distal pancreatectomy [3].For benign and low-grade malignant tumors,minimally invasive SPDP with Kimura’s procedure has been a promising treatment option [3,4].Robotic surgery has advantages in flexibility of surgical arms and stability of surgical views.Several studies suggested that robotic SPDP has higher spleen-preserving rate and lower conversion to laparotomy rate than laparoscopic SPDP [5,6].
However,even for robotic surgery,SPDP is much more complex than traditional distal pancreatectomy with splenectomy [7].Traditionally,this procedure required identifying the splenic vessel branches and creating a tunnel between the pancreatic body and splenic vessels for further transection of the pancreatic parenchyma [8–10].We named this the “tunnel-first” strategy.This step was challenging,especially when the tunnel was not in front of the superior mesenteric vein.Limited space during the process increased the risk of incidental tearing of vein branches [11].Additionally,suturing the bleeding site was fairly difficult owing to the presence of splenic vein and pancreas.Therefore,we introduced our approach to overcome these limitations,which we named the pancreatic “parenchyma transection-first” strategy.By transecting pancreatic parenchyma without creating the tunnel,this surgical approach avoided disturbance mentioned above.
In this study,we summarized our data on these two SPDP strategies to compare the perioperative outcomes and evaluated the feasibility of the “parenchyma transection-first” strategy.
Patients and methods
Patients and study design
Patients who received robotic SPDP with Kimura’s procedure in our institution from January 2017 to September 2022 were enrolled.The flow-chart of selection procedure was shown in Fig.1.Surgical methods selection was made by the senior surgeon based on the preoperative discussion and intraoperative exploration.The data were retrieved from an electronic medical record system.All the surgeries were performed by the same surgical team.The surgical team had passed the learning curve of robotic distal pancreatectomy and robotic pancreaticoduodenectomy before January 2017 [12].
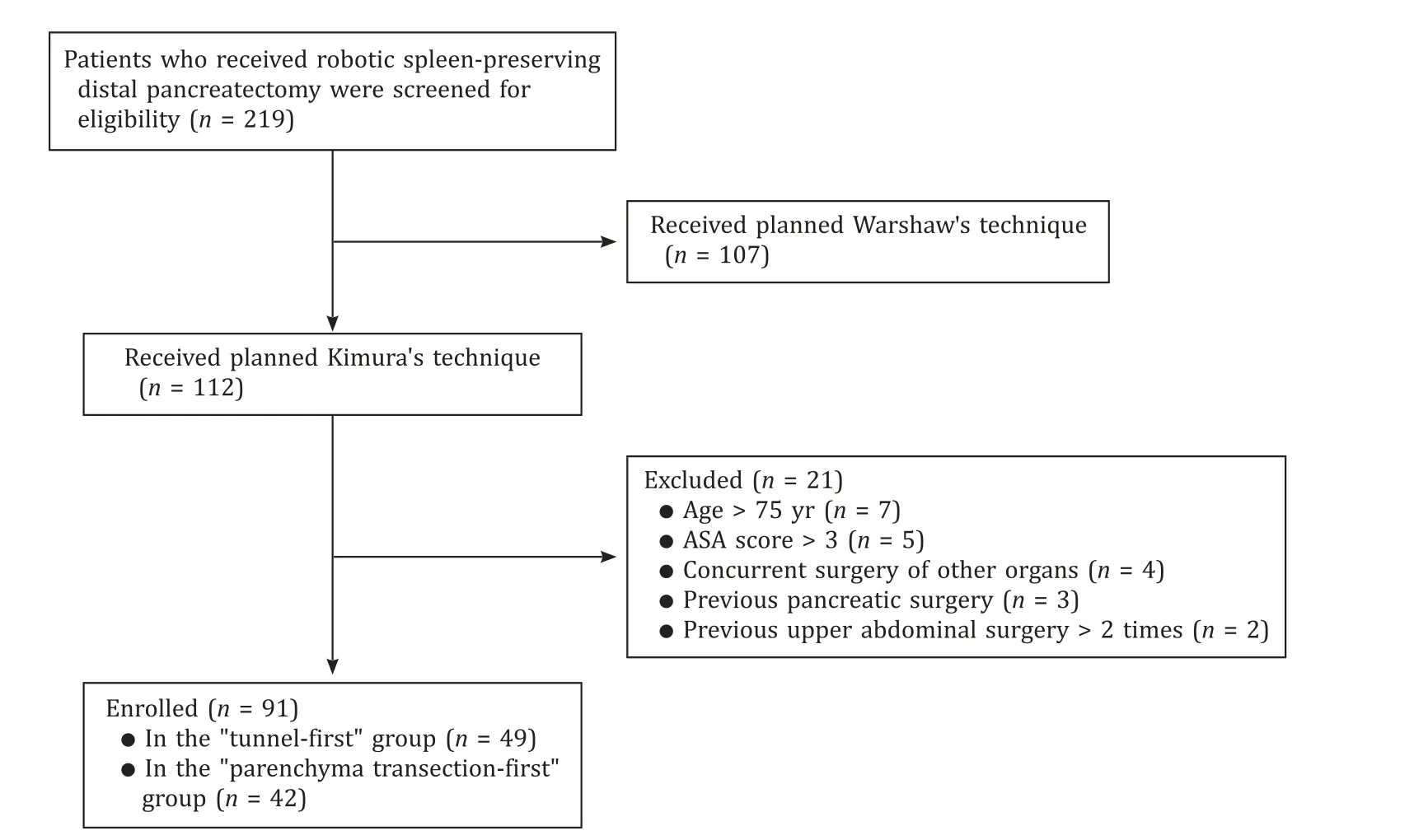
Fig.1.Flow-chart of patient selection.ASA: American Society of Anesthesiologists.
The inclusion criteria were: (1) age ≤75 years;(2) patients diagnosed with benign or low-grade malignant pancreatic tumors;(3) tumor located in the pancreatic body/tail;and (4) no other surgical contradictions.
The exclusion criteria were: (1) an American Society of Anesthesiologists (ASA) score >3;(2) a history of previous pancreatic surgery;(3) previous upper abdominal surgery >2 times;(4) concurrent surgery of other organs;and (5) distant metastasis.
All the surgeries were performed on the da Vinci Si or da Vinci Xi Surgical System (Intuitive Surgical Inc.,Sunnyvale,CA,USA).This study was approved by the Ethics Committee of our hospital (S2022–530–01).All patients signed the preoperative informed consent with the agreement for perioperative data collection.
Outcome variables
Demographic data and pathological characteristics were retrieved,including age,sex,body mass index (BMI),ASA score,history of previous upper abdominal surgery and pancreatitis,tumor type,tumor location,and tumor size.
Perioperative data were collected as follows: operative time,estimated blood loss,blood transfusion,conversion to open,conversion to Warshaw’s procedure,pancreatic parenchyma transection methods,remnant management,perioperative complications,postoperative hospital stay,90-day mortality,and 30-day readmission.Operative time was calculated from skin incision to abdominal closure,with robotic docking time included.Estimated blood loss was calculated by visual estimation [13,14].Postoperative pancreatic fistula (POPF) and hemorrhage were defined according to the International Study Group of Pancreatic Surgery (ISGPS) [15].The severity of complications was classified based on the Clavien-Dindo grading system [16].
Surgical procedure and “parenchyma transection-first” strategy
The patient was placed in a supine position with a slight right tilt under general anesthesia.The pneumoperitoneum was created with 12 mmHg of pressure.Trocars shared the same position with robotic distal pancreatectomy,as previously reported [17].Exploration was performed to exclude metastasis.The gastrocolic ligament was divided along the greater curve of the stomach to the hilum of the spleen to expose the pancreas.The tumor size and location were rechecked.The inferior border of the pancreas from the neck to the tail was dissected,and the splenic vein was identified.
For the “tunnel-first” strategy (Fig.2),the pancreas was elevated,and the splenic vein was carefully separated from the pancreatic parenchyma right to the tumor edge.Subsequently,the splenic artery was also isolated from the superior edge of the pancreas.After that,the pancreatic parenchyma was transected with a stapler or ultrasonic scalpel.For the “parenchyma transection-first”strategy (Fig.3),after the pancreas was ablated from the retroperitoneum and the splenic vein was uncovered from the Toldt fascia,adhesion and extrusion of the splenic vein with pancreas and tumor were observed,and pancreatic parenchyma was directly transected with ultrasonic scalpel at the right edge of the tumor from inferior to superior and from anterior to posterior without splenic vein isolation.The splenic vein and artery were isolated during the transection,and their branches were carefully ligated and divided [9].

Fig.2.The “tunnel-first” strategy.A : Dissection of SPV and SPA;B : ligation of SPA branch;C : isolation of SPA in the inferior border of pancreas;D : transection of pancreatic parenchyma with stapler.SPV: splenic vein;SPA: splenic artery.

Fig.3. Pancreatic “parenchyma transection-first” strategy.A : Adhesion of SPV with pancreas and tumor;B : direct transection of pancreatic parenchyma,exposing SPV and its branches;C : transection plane with exposed SPV and SPA;D : dissection of vessel branches with distal pancreas lifted.SPV: splenic vein;SPA: splenic artery;T: tumor.
After the transection of the pancreas,the distal pancreas was elevated,and the splenic vessels were separated from medial to distal.Branches of vessels were divided by clips or an ultrasonic scalpel.The splenic artery was temporarily occluded,if required [18,19].After resection of the pancreas,the splenic vessels were re-examined,and potential bleeding sites were reinforced by sutures.The proximal pancreas stump was sutured and the pancreatic duct ligated.
Statistical analysis
Statistical analyses were performed using SPSS version 25.0(SPSS Inc.,Chicago,IL,USA).Normally distributed continuous variable data were presented as mean ± standard deviation (SD) and compared using Student’st-test.Non-normally distributed data were reported as median (interquartile range) and analyzed with Mann-WhitneyUtest.Categorical variables were presented as number (percent) and compared using Chi-square test or Fisher’s exact test,as indicated.APvalue <0.05 was considered statistically significant.
Results
Demographic and pathologic characteristics
A total of 91 consecutive patients received robotic SPDP with Kimura’s procedure.Among them,49 adopted the traditional “tunnel-first” strategy (T-F group),while 42 received the“parenchyma transection-first” strategy (P-F group).The demographic and pathologic data were listed in Table 1.The two groups were comparable in age,sex,BMI,ASA score,history of upper abdominal surgery,and pancreatitis.As for pathological features,no difference was observed in the tumor location,tumor size,or tumor types between the two groups.

Table 1Demographic information and pathological characteristics.
Perioperative outcomes
The decision to preserve splenic vessels was made preoperatively and confirmed after intraoperative exploration.The perioperative data of the cohort were shown in Table 2.Compared with the T-F group,the P-F group had significantly shorter operative time(146.1 ± 39.2 min vs.174.9 ± 46.6 min,P<0.01) and lower estimated blood loss [40.0 (20.0–55.0) mL vs.50.0 (20.0–100.0) mL,P=0.03].Subgroup analysis on tumor location revealed that the PF group had significantly shorter operative time than the T-F group for tumor located in pancreatic body,with no significant difference in tumor located in the tail.In addition,estimated blood loss was comparable between the P-F and T-F groups in tumor locationbased subgroup analysis.One patient in the T-F group received blood transfusion because of intraoperative bleeding.Approximate 10.2% (5/49) patients in the T-F group converted to Warshaw,compared with 2.4% (1/42) in the P-F group (P=0.14).Of the six patients converted to Warshaw,three in the T-F group underwent emergency conversion owing to intraoperative bleeding,while others underwent elective conversion owing to adhesion of splenic vessels.There was no conversion to open surgery in the cohort.As for the parenchyma transection method,11 patients received stapler transection in the T-F group,and all other patients in the two groups received ultrasonic scalpel transection.Suture closure or reinforcement of the proximal pancreatic remnant was performed in all patients except for seven who had stapled closure.

Table 2Perioperative outcomes.
Postoperative outcomes
The grade 3/4 complications were similar in the two groups(P=0.57).Different transection methods and pancreatic remnant management did not result in a difference in grades B/C POPF(P=0.61).One patient in the T-F group died within 90-day after operation.Besides,the abdominal infection,postoperative hemorrhage and 30-day readmission were comparable in the two groups.No reoperation was needed.Median postoperative hospital stay was 6.0 d (5.0–9.0) in the T-F group,and 6.0 d (5.0–8.0) in the P-F group (P=0.72).
Discussion
The “tunnel-first” strategy was widely applied in the preservation of splenic vessels,such as in central pancreatectomy and SPDP with Kimura’s procedure [20,21].While tumor extrusion and local adhesion make it challenging to dissect the splenic vein and artery branches,separation itself would cause massive bleeding or even tumor rupture [22].As shown in Fig.2,creating the tunnel limited the operation space and surgical view.Especially in emergent bleeding,there was not enough space and proper direction between the splenic vessels and pancreatic parenchyma for sutures [19].
The pancreatic “parenchyma transection-first” strategy was initially used in central pancreatectomy in our center and was expanded to Kimura’s procedure.As shown in Fig.3,by transecting the parenchyma inferiorly to superiorly and anteriorly to posteriorly,the pancreas was divided,creating more space and better surgical view.In the “tunnel-first” technique,branches were much less common in the vertical transection plane than in the horizontal plane along the vessels.Furthermore,suturing the bleeding site was easier with a lateral view when lifting the distal pancreas after transection.
Splenic vessel preservation is the hotspot of SPDP studies [22].A meta-analysis involving 14 studies and 945 patients concluded that Kimura’s procedure had a lower incidence of postoperative complications including gastric varices and spleen infarctions compared with Warshaw surgery (P<0.001) [23].Another metaanalysis also confirmed this conclusion [7].While Kimura’s technique is technically challenging,it leads to a longer operative time(P<0.001) and more estimated blood loss (P=0.006) [23].Robotic surgery was an independent predictor for splenic vessel preservation,possibly owing to the high-quality surgical view and precise manipulation of the robotic system [24,25].Even though it was not significant,our data showed that the rate of conversion to Warshaw was relatively lower in the P-F group,and the reason may be that “transection-first” strategy provided a better space for branches identification and suturing which decreased the incidence of vein tearing and bleeding.Emergency bleeding was believed to be an important reason for the failure of splenic vessel preservation [19].Totally,the “parenchyma transection-first” strategy revealed an advantage in operative time and estimate blood loss.However,further subgroup analysis revealed that “parenchyma transection-first” strategy may have better performance in tumor located in pancreatic body,considering the operative time.No difference was observed in estimated blood loss in subgroup analysis,possibly due to the insufficient case number (type II error).
POPF was one of the major complications in distal pancreatectomy [26].The mortality case of our cohort was due to POPFrelated hemorrhage.The treatment of pancreas remnants is determined by the method of transection used: stapling or ultrasonic scalpel.Only the latter was capable for “parenchyma transectionfirst” strategy,followed by suture closure.While for the “tunnelfirst” strategy,a stapler can be used.However,recent ISGPS expert consensus stated that there is no difference in the POPF rate after left pancreatectomy between the suture closure and stapler techniques [27,28].Besides,the misoperation of the stapler may lead to the tearing of splenic vessels and branches.
“Parenchyma transection-first” strategy also can be applied in pancreaticoduodenectomy.The traditional surgical procedure was dissection of the common hepatic artery,dissection and ligation of the gastroduodenal artery,followed by transection of the pancreatic neck [29].In minimally invasive surgery,pancreas may disturb the operation because of the bottom-up surgical view and operation procedure,while according to our experience,transecting the pancreatic neck followed by the isolation of the common hepatic artery and ligation of the gastroduodenal artery will shorten the operative time without increasing the risk of bleeding (data unpublished).
There were also some limitations to this study.Firstly,this was a retrospective cohort study,leading to inevitable selection bias.Further randomized controlled trials are needed to validate the conclusion.Secondly,we noticed that the “parenchyma transection-first” strategy tended to have a lower conversion to Warshaw but did not reach significance,which may be due to the relatively insufficient cases.Thirdly,this cohort only included robotic surgery.Whether this strategy is suitable for laparoscopic surgery needs further study.
In conclusion,the pancreatic “parenchyma transection-first”strategy is safe and feasible compared with traditional “tunnel-first strategy” in SPDP with Kimura’s procedure.
Acknowledgments
None.CRediTauthorshipcontributionstatement
Meng-YangLi:Conceptualization,Data curation,Formal analysis,Writing– original draft.Hao-ZheCui:Conceptualization,Data curation,Formal analysis,Writing– original draft.Jia-NingHao:Conceptualization,Data curation,Writing– original draft.Da-BinXu:Data curation.En-LiZhang:Data curation.Zhu-ZengYin:Data curation.Zhi-MingZhao:Conceptualization,Investigation,Methodology,Project administration,Resources,Writing– review&editing.
Funding
None.
Ethicalapproval
This study was approved by the Ethics Committee of Chinese PLA General Hospital (S2022-530-01).All patients signed the preoperative informed consent with the agreement for perioperative data collection.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- A new prognostic model for drug-induced liver injury especially suitable for Chinese population
- Post-hepatectomy liver failure: A timeline centered review
- INSTRUCTIONS FOR AUTHORS
- Value and prognostic factors of repeat hepatectomy for recurrent colorectal liver metastasis
- Older liver grafts from donation after circulatory death are associated with impaired survival and higher incidence of biliarynon-anastomotic structure
- Development and validation of a novel model to predict liver-related mortality in patients with idiosyncratic drug-induced liver injury
