Older liver grafts from donation after circulatory death are associated with impaired survival and higher incidence of biliarynon-anastomotic structure
2024-01-02TinShenShnHuZhengJunChenZhiShengZhouMengFnYngXingYnLiuJunLiChenShuSenZhengXioXu
Tin Shen ,Shn-Hu Zheng ,Jun Chen ,Zhi-Sheng Zhou ,Meng-Fn Yng ,Xing-Yn Liu ,Jun-Li Chen ,Shu-Sen Zheng ,,e,f,Xio Xu ,e,f,∗
a Department of Hepatobiliary and Pancreatic Surgery,Shulan (Hangzhou) Hospital,Hangzhou 310022,China
b Division of Hepatobiliary Pancreatic Surgery,The First Affiliated Hospital,Zhejiang University School of Medicine,Hangzhou 310 0 03,China
c Key Laboratory of Integrated Oncology and Intelligent Medicine of Zhejiang Province,Department of Hepatobiliary and Pancreatic Surgery,Affiliated Hangzhou First People’s Hospital,Zhejiang University School of Medicine,Hangzhou 310 0 06,China
d China Liver Transplant Registry,Hangzhou 310 0 03,China
e NHC Key Laboratory of Combined Multi-organ Transplantation,Hangzhou 310 0 03,China
f Institute of Organ Transplantation,Zhejiang University,Hangzhou 310 0 03,China
Keywords: Older donor Liver transplantation Survival Biliary stricture Donation after circulatory death
ABSTRACT Background: Grafts from older donors after circulatory death were associated with inferior outcome in liver transplants in the past.But it has seemed to remain controversial in the last decade,as a result of modified clinical protocols,selected recipients,and advanced technology of organ perfusion and preservation.The present study aimed to examine the impact of older donor age on complications and survival of liver transplant using grafts from donation after circulatory death (DCD).Methods: A total of 944 patients who received DCD liver transplantation from 2015 to 2020 were included and divided into two groups: using graft from older donor (aged ≥65 years,n=87) and younger donor (age <65 years,n=857).Propensity score matching (PSM) was applied to eliminate selection bias.Results: A progressively increased proportion of liver transplants with grafts from older donors was observed from 1.68% to 15.44% during the study period.The well-balanced older donor (n=79) and younger donor (n=79) were 1:1 matched.There were significantly more episodes of biliary nonanastomotic stricture (NAS) in the older donor group than the younger donor group [15/79 (19.0%) vs.6/79 (7.6%);P=0.017].The difference did not reach statistical significance regarding early allograft dysfunction (EAD) and primary non-function (PNF).Older livers had a trend toward inferior 1-,2-,3-year graft and overall survival compared with younger livers,but these differences were not statistically significant (63.1%,57.6%,57.6% vs.76.9%,70.2%,67.7%,P=0.112;64.4%,58.6%,58.6% vs.76.9%,72.2%,72.2%,P=0.064).The only risk factor for poor survival was ABO incompatible transplant (P=0.008) in the older donor group.In the subgroup of ABO incompatible cases,it demonstrated a significant difference in the rate of NAS between the older donor group and the younger donor group [6/8 (75.0%) vs.3/14(21.4%);P=0.014].Conclusions: Transplants with grafts from older donors (aged ≥65 years) after circulatory death are more frequently associated with inferior outcome compared to those from younger donors.Older grafts from DCD are more likely to develop NAS,especially in ABO incompatible cases.
Introduction
Age of donor was historically considered to be one of the main factors that negatively impact the outcome of liver transplantation,especially in terms of biliary complications,early allograft dysfunction (EAD),primary non-function (PNF),graft survival and recipient mortality [1–5].Moreover,liver grafts from older donors were potentially associated with higher discard rates in consideration of inferior outcomes in the past,especially from donation after circulatory death (DCD).
However,in recent 10 years,a previous study has shown that excellent results can be achieved with older grafts from donation after brain death (DBD),and there is virtually no upper age limit for DBD donors in liver transplantation [6].Similarly,older grafts from DCD are also not necessarily associated with poor prognosis as a result of modified clinical protocols,selected recipients,as well as advanced technology of organ perfusion and preservation.The influence of donor age on the results of DCD liver transplantation remains controversial,with several series showing a significant impact of age over 60 or 70 years on graft survival [5–9],while others failed to stratify patient and graft survival using a similar cut-off value [10,11].
In fact,grafts from older donors have been increasingly used in the past decades,due to worldwide shortage of organ and population aging.Although the definition of aged donor is different,transplants of liver grafts from older donors represented a progressively higher proportion of all available donors,especially in Europe [12].
The present study aimed to compare outcome of liver transplantation using older grafts and younger grafts from DCD donors,and analyze the risk factors associated with poor graft survival.
Methods
Patients and data collection
A total of 944 patients receiving DCD liver transplant between January 2015 and December 2020 from the First Affiliated Hospital,Zhejiang University School of Medicine and Shulan (Hangzhou)Hospital were retrospectively collected and reviewed.Liver cancer extending Hangzhou criteria,multi-organ transplants,split transplants,living-donor transplants,and pediatric transplants were excluded.DCD donors involved in the study were from Maastricht type III and IV.A clear difference of age categories between the two groups was identified as 65 years.Accordingly,the 944 patients were divided into two groups: using graft from older donor(aged ≥65 years,n=87) and younger donor (aged <65 years,n=857).As a retrospective,observational and non-interventional study,it was approved by China Liver Transplant Registry (CLTR)and by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (2013-0022) in accordance with ethical principles of theDeclarationofHelsinki.
Pathological detection of grafts
A biopsy and Hematoxylin-eosin staining for graft were performed routinely before implantation.Steatosis was defined as macrosteatosis,and the degree was measured by proportion of macrovesicular hepatic cells.
Perioperative protocols
There was no case of donor using extracorporeal membrane oxygenation (ECMO),and no case of graft using machine perfusion techniques in the study cohort either.All the patients received liver transplantation with piggyback implantation.Microsurgical anastomosis was used for hepatic artery anastomosis.
Immunosuppressive regimen consisted of basiliximab,methylprednisolone,tacrolimus and mycophenolic acid.Two dose of basiliximab (20 mg) was administered during operation and on the fourth day post-transplantation respectively.Single dose of methylprednisolone (500 mg) was administered just after reperfusion during operation intravenously.Oral mycophenolic acid was taken from the first day after transplantation and tacrolimus was taken from the fourth day after transplantation.For ABO incompatible transplants,rituximab was adopted,while plasma exchange and immunoglobulin were performed when the anti-A or anti-B antibody was higher than 1:8.
Protocol of antimicrobial prophylaxis was selected depending on preoperative infection of recipient and donor,as well as postoperative infection of recipient.Recipients with hepatitis B virus infection were given one dose of intravenous hepatitis B immunoglobulin during operation and daily oral entecavir or tenofovir,as prophylaxis against the virus recurrence.
Postoperative monitoring of complication and follow-up
Routine serological tests were performed and monitored after transplantation,including aminotransferase,bilirubin,international normalized ratio,lactate and blood gas analysis,as indexes for diagnosis of EAD,PNF and acute rejection.Liver biopsy and pathological examination were performed when acute rejection was suspected.To search for biliary complications and hepatic artery complications,Doppler ultrasound was performed,as well as enhanced computed tomography and magnetic resonance cholangiopancreatography when necessary.
Statistical analysis
Qualitative data (presented as number and frequency) were compared by Pearson Chi-square test,and quantitative data (presented as mean ± standard deviation) were compared using Student’sttest.Propensity score matching (PSM) was applied,using a caliper of 0.02 standard deviations of the log of the propensity score and at a ratio of 1:1,to reduce imbalance in measured confounders and avoid existing selection biases which could affect the survival outcome between the two groups.The graft survival and patient overall survival were calculated using Kaplan-Meier survival analysis,and log-rank test was used for survival comparison.Hazard ratio (HR) and 95% CI were evaluated by a Cox proportional-hazards regression model to identify factors associated with poor outcomes.APvalue less than 0.05 was considered statistically significant.
Results
Demographics
The percentage of transplanted grafts from older donors was less than 2% in 2015 and 2016,and a marked increase in the use of older DCD livers was observed from 2016 to 2018.As a result,the proportion then increased to 7.6% in 2017,and reached the highest level of 15.4% in 2018 (Fig.1).The age range of older donor was 65-75 years.

Fig.1.The percentage of transplanted grafts from older donors between 2015 and 2020.
Baseline characteristics before and after PSM
A total of 944 patients who met the selection criteria were included in analysis,with 87 patients using grafts from older donors (aged ≥ 65 years) and 857 using graft from younger donors (aged <65 years).Baseline characteristics which probably affect allocation of graft before matching are presented in Table 1.These variables were also adopted for performing the logistic regression necessary for calculating the PSM: donors’ characteristics [sex,body mass index (BMI),Na+,alanine aminotransferase(ALT),total bilirubin (TBil),and cause of death],grafts’ characteristics [steatosis,ABO compatibility,cold ischemia time (CIT),and warm ischemia time (WIT)],and patients’ characteristics [sex,age,model for end-stage liver disease (MELD) score,and tumor status].Among the unmatched groups,patients receiving graft from the older donor group were more likely to be older and diagnosed with hepatocellular carcinoma (HCC),and had higher MELD score.However,ischemia time and steatosis of grafts did not differ significantly between the two groups.

Table 1Baseline characteristics of recipients,grafts and donors in the older donor group and younger donor group before propensity score matching.
PSM was applied to achieve a balanced exposure groups at baseline.The well-balanced older donor (n=79) and younger donor (n=79) groups were 1:1 matched.The baseline characteristics are shown in Table 2.There were no significant differences between the matched two groups in the selected matching variables.

Table 2Baseline characteristics of recipients,grafts and donors in the older donor group and younger donor group after propensity score matching.
Complication analysis
After matching,EAD occurred in 20 (25.3%) and 17 (21.5%) cases in the older donor and younger donor groups,respectively.One case of PNF was diagnosed in each group.There were 2 cases of acute rejection within 2 months in the older donor group and 5 cases in the younger donor group.The difference did not reach statistical significance between the two groups regarding EAD,PNF,acute rejection,as well as artery complications.Non-anastomotic stricture (NAS) was observed in 21 (13.3%) cases in the matched cohort,and the majority of which presented with multifocal progressive patterns (8,38.1%) and confluence dominant disease (10,47.6%),regarding the radiological classification (Fig.2).There were significantly more episodes of biliary NAS in the recipients of older grafts (15/79,19.0%) in comparison to the recipients in the younger donor group (6/79,7.6%) (P=0.017).Four cases received re-transplantation due to PNF or biliary complications.Rates of re-transplantation were 1.3% and 3.8% for the older donor and younger donor groups,respectively (P=0.311) (Table 3).
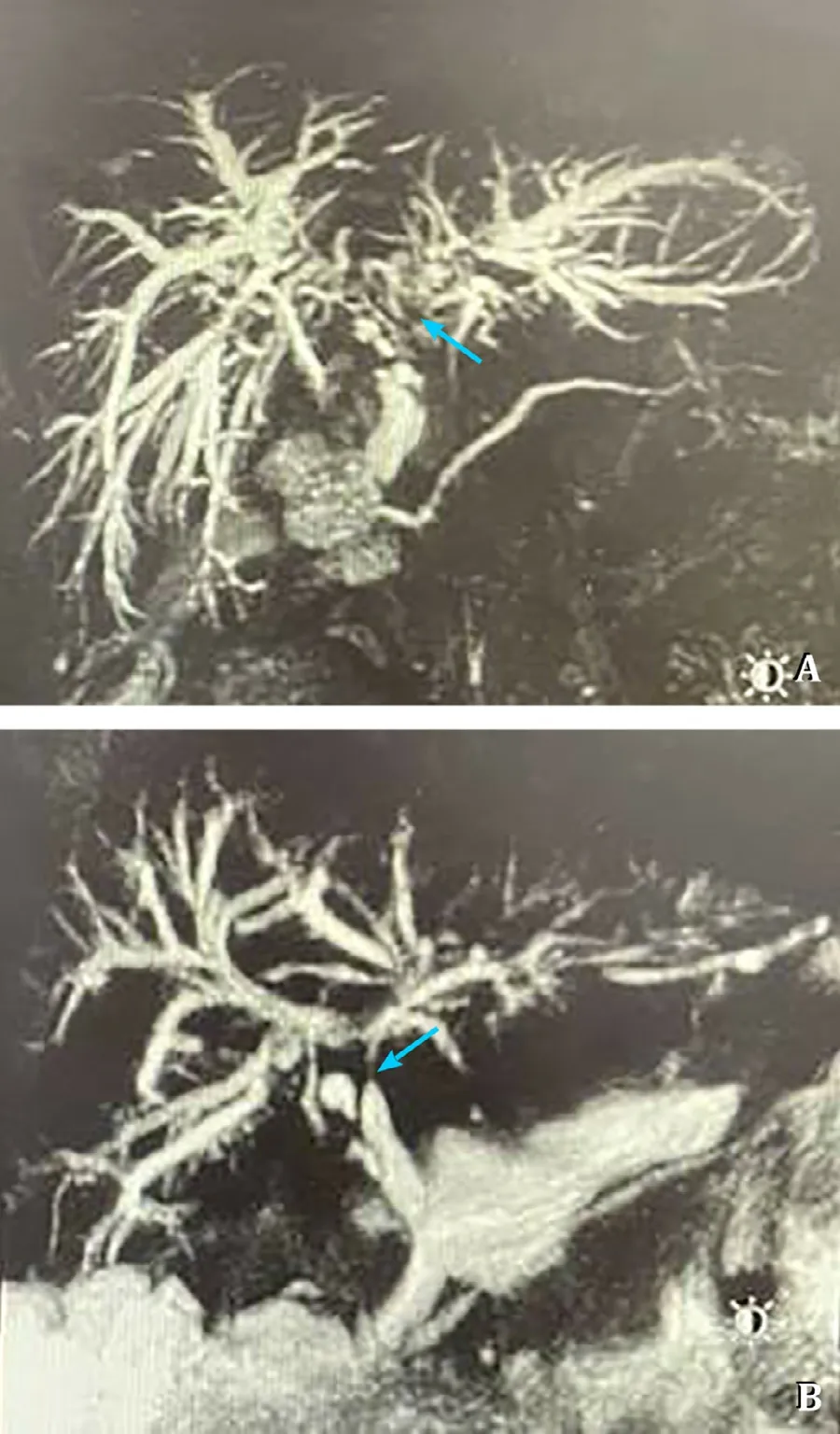
Fig.2.The majority of biliary non-anastomotic stricture cases presented with multifocal progressive patterns (8,38.1%) (A) and confluence dominant disease (10,47.6%) (B),regarding the radiological classification.
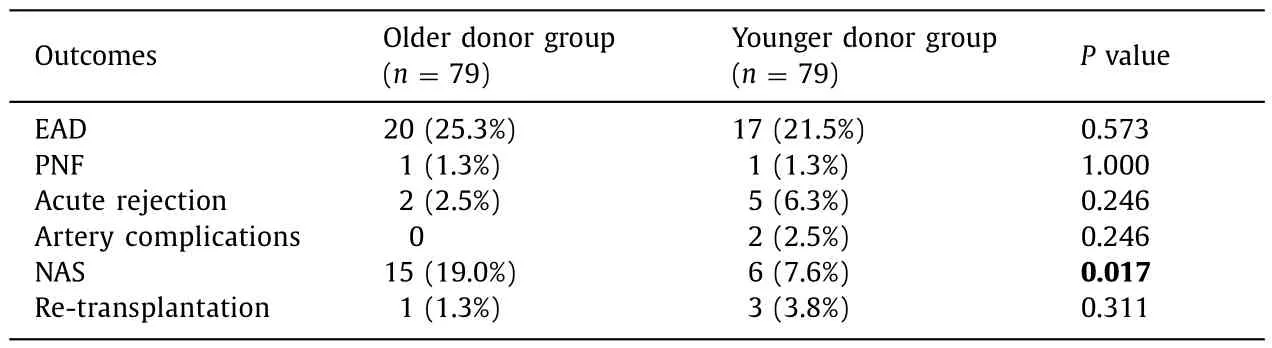
Table 3Main outcomes after LT in the older donor group and younger donor group.
Survival analysis
After matching,the median follow-up period was 57 months(range 1-82).We observed lower graft survival and overall survival in the older donor group,but there was no significant differences in 1-,2-,3-year graft survival and overall survival between the two groups (63.1%,57.6%,57.6% vs.76.9%,70.2%,67.7%,P=0.112;64.4%,58.6%,58.6% vs.76.9%,72.2%,72.2%,P=0.064) (Fig.3).
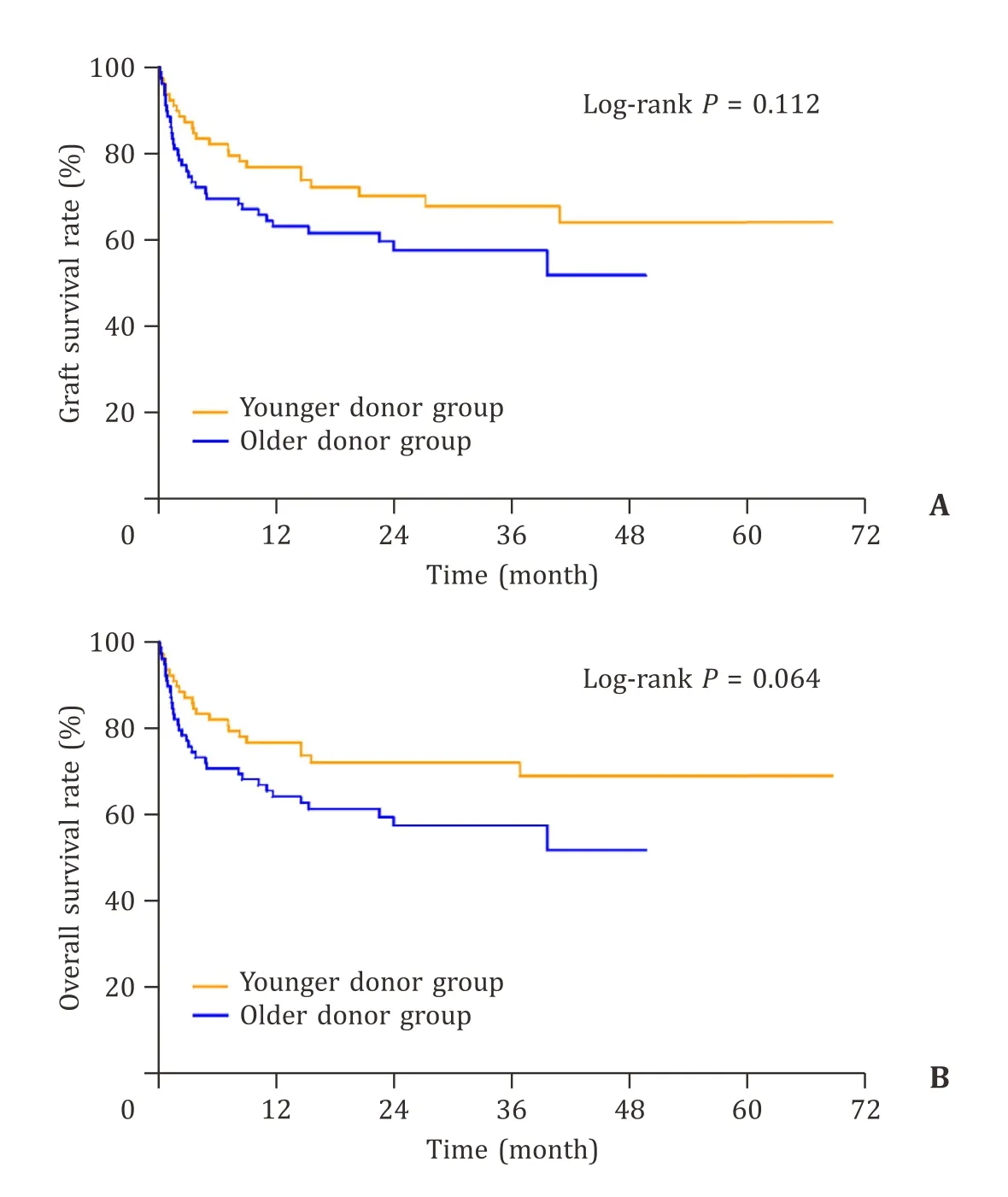
Fig.3.A: The 1-,2-,and 3-year graft survival rates between the two groups were 63.1%,57.6%,57.6% vs.76.9%,70.2%,67.7%,respectively (P=0.112).B: The 1-,2-,and 3-year overall survival rates between the two groups were 64.4%,58.6%,58.6% vs.76.9%,72.2%,72.2%,respectively (P=0.064).
Factors associated with poor survival
Considering all patients in both groups,the factors associated with poor outcomes were older donor age (HR=1.025,P=0.019),ABO incompatibility (HR=2.446,P=0.005) and HCC(HR=2.175,P=0.004),which were identified by Cox regression model (Table 4).However,to the patients who received grafts from older donors,the only risk factor for poor survival was ABO incompatible transplant (HR=3.173,P=0.008) (Table 5).

Table 4Cox regression analysis of factors associated with survival in the study cohort after propensity score matching (n=158).

Table 5Cox regression analysis of factors associated with survival in the older donor group (n=79).
Subgroup analysis based on risk factors
As noted above,15 and 6 cases of NAS occurred in the older donor and younger donor groups,respectively,of which,9 (42.9%)had ABO incompatible grafts (older donor group: 6/15,40.0%;younger donor group: 3/6,50.0%).Meanwhile,in the subgroup of ABO incompatible cases,the only significant difference was found in the rate of NAS between the older donor group and the younger donor group [6/8 (75.0%) vs.3/14 (21.4%);P=0.014](Table 6).There was no further difference between the two groups regarding ABO incompatibility.
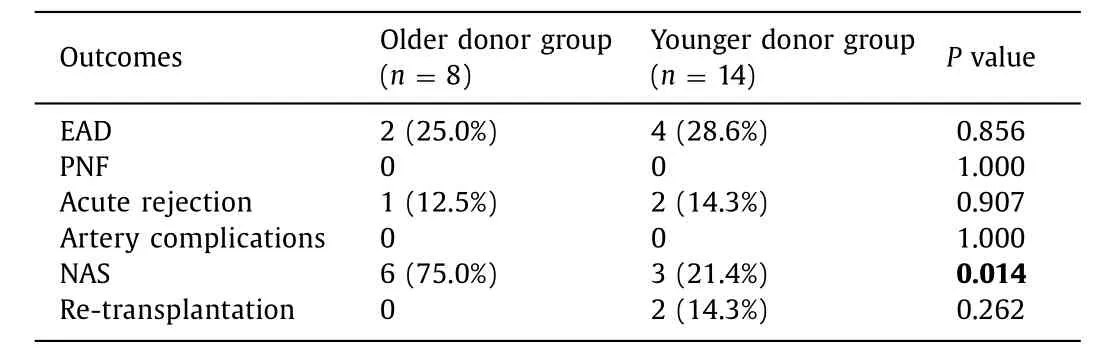
Table 6Main outcomes of recipients after LT in the subgroup of ABO incompatible grafts.
Discussion
Our study showed a progressive increased proportion of transplants with grafts from older donors between 2015 and 2020.During the study period,grafts from older donors (≥65 years)accounted for 1.68% to 15.44% of all transplanted grafts between 2015 and 2020.Whereas,there is lack of data from multicenters in China in this regard.According to research in the last decade,regional differences can be observed in discarded rate of livers from older donors,as well as the proportion of older grafts in all transplanted grafts,and the acceptance of older allografts seems to be higher in Europe than in the USA [13,14].The proportion of donors older than 70 years has increased by 132% between 1999 and 2009 in Spain [14].In France,the proportion of donors aged 65 or older has reached to 38% in 2013 [12].However,during 2005 to 2014,the percentage of DCD liver transplants with donors ≥60 years was 0.7%-4.3% in the USA [15,16].A study based on data from the Scientific Registry of Transplant Recipients represented a progressively lower proportion of liver transplants from donors older than 70 years,from 6.0% in 2003 to 3.2% in 2016 [3].
In our study,the cutoff point between older donor and younger donor was 65 years old.At present,there is no unified standard for the definition of older donor or older liver graft.Donors older than 70 years,60 years,50 years and even 40 years have all been considered “extended criteria or marginal donors”,which could be associated with poor outcome [1,2,17,18].There were studies using different cutoffs in previously reported papers,including 70 years,65 years and 60 years [8,10–12].
The age limit for DBD is less than that from circulatory death,and there is even no age limit for DBD donors [6].Single-center studies in France and the USA revealed acceptable outcomes using older DBD livers with age ≥80 and even ≥90 years [12,19].Nevertheless,the upper limit of donor age for DCD liver transplant has been remaining controversial.In China,DCDs account for the majority;as a result,we focused on the outcome with older graft from circulatory death.Although it showed no statistical difference,our study revealed an impaired 1-,2-and 3-year survival of grafts and patients in the older donor group.Recently,in several analyses of national database from the USA and the UK,donor age ≥60 years led to a significant increase of graft failure or graft loss in DCD liver transplant [7–9].In contrast,a single-center study in the UK failed to stratify patient and graft survival by donor age at the cutoff “60” in DCD transplants [10],which was reflected in another single-center report (cutoff donor age was 70 years) [11].These results emphasized the importance of well-selected older grafts and preferred recipients by taking all factors into account: lower BMI for donor,shorter WIT and CIT for graft,lower MELD score,age>45 years,BMI <35 kg/m2for recipients,and avoidance of retransplant and emergent transplant [10,11,18,19].
Before matching,patients with older age,higher MELD score and diagnosis of HCC were more likely to be selected as recipients for older graft.Considering such potential confounding factors which cause confusion,we balanced the bias through PSM.We confirmed that older donor age,ABO incompatibility and diagnosis of HCC were risk factors associated with poor graft survival in the whole cohort.And ABO incompatibility had negative influence on survival as the only independent factor in the older donor group.Involvement of ABO incompatible transplant cases resulted in relatively low survival of graft and patients.In China,the number of donated livers cannot meet the requirement of waiting recipients,due to the high incidence of hepatic failure or cirrhosis associated with hepatitis B.This results in the fact that patients with high MELD score is more likely to receive an incompatible graft.In this study,there were 14 and 8 cases of ABO incompatible transplant in the younger and donor groups respectively.Limited by the number of cases,it is difficult to make survival analysis in this subgroup.There are few reports focusing on prognosis of ABO incompatible liver transplantation using older grafts.
Despite more occurrences of EAD in the older donor group,this study represented no significant difference of rate of EAD and PNF between the two groups,as well as acute rejection,after matching.The rate of NAS in the cohort after PSM was 13.3%.And most cases presented with multifocal progressive patterns and confluence dominant disease,according to the radiological classification [20].Importantly there was a significantly higher rate(19.0%) of NAS in the older donor group.Moreover,we observed an obviously higher risk of NAS in cases using ABO incompatible older grafts (75.0%) compared to those using incompatible younger grafts(21.4%).To the best of our knowledge,liver transplant using DCD graft has been widely questioned due to the high rate of biliary complications,and the reported incidences of NAS and ischemic cholangiopathy (IC) after DCD transplant vary 30%-50% and 16%-25%,respectively [21–23].However,many reports have described IC rates as low as 5% in the DCD population [24,25].It could be improved by shortening WIT,CIT and improving machine perfusion techniques [22,26].A single-center experience in Canada revealed that the rate of IC among DCD transplant recipients was reduced from 25% to 3% by judicious donor selection with shorter procurement time [22].Meanwhile,concerns have been raised regarding older grafts in terms of susceptibility to NAS and IC [3,27],as well as older grafts from DCD donation [28].But several single-center studies reported that older grafts could be used for liver transplant with acceptable rate of IC (3.8%-13%) and favorable graft survival with a shorter CIT,especially in DBD transplant [12,29].Furthermore,association between ABO incompatibility and NAS still remains common due to immunomodulation,in spite of advanced immunosuppressive protocol for ABO incompatible transplantation.In our study,an association between higher rate of NAS and DCD transplant with older graft was observed,especially in ABO incompatible cases.In incompatible cases,more NAS was observed when using an older graft,which possibly contributed to the impaired graft survival.
In conclusion,although there is no statistical difference,the data suggested that transplants with grafts from older donors (≥65 years) after circulatory death had inferior outcome to younger donors.Relatively low survival was supposed to the involvement of ABO incompatible cases.ABO incompatibility was confirmed to be an important risk factor regarding the survival after DCD transplant with older graft.The observation that ABO incompatible DCD liver≥65 years of age was more likely to develop NAS should lead to the necessity of consideration of ABO compatibility when making decision to use a DCD liver with older age.
Acknowledgments
None.
CRediTauthorshipcontributionstatement
TianShen:Data curation,Methodology,Writing -original draft.Shan-HuaZheng:Data curation,Writing -original draft.JunChen:Data curation.Zhi-ShengZhou:Formal analysis.Meng-FanYang:Formal analysis.Xiang-YanLiu:Data curation.Jun-LiChen: Formal analysis.Shu-SenZheng:Conceptualization,Supervision,Writing -review &editing.XiaoXu: Conceptualization,Funding acquisition,Supervision,Writing -review &editing.
Funding
This study was supported by grants from the Major Research Plan of the National Natural Science Foundation of China(92159202),Key Program,National Natural Science Foundation of China (81930016),and Key R&D Program of Zhejiang (2022C03108).
Ethicalapproval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine(2013-0022).Written informed consent was obtained from all participants.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- A new prognostic model for drug-induced liver injury especially suitable for Chinese population
- Post-hepatectomy liver failure: A timeline centered review
- INSTRUCTIONS FOR AUTHORS
- Value and prognostic factors of repeat hepatectomy for recurrent colorectal liver metastasis
- Development and validation of a novel model to predict liver-related mortality in patients with idiosyncratic drug-induced liver injury
- Clinical-radiomics predictors to identify the suitability of transarterial chemoembolization treatment in intermediate-stage hepatocellular carcinoma: A multicenter study
