The efficacy and safety of endoscopic ultrasound-guided fine-needle biopsy in gallbladder masses
2024-01-02TingTongLiTianMinZiDengXueJieChenTianFuKeJiaMaJiaHaoXuXiaoYanWang
Ting Tong,Li Tian,Min-Zi Deng,Xue-Jie Chen,Tian Fu,Ke-Jia Ma,Jia-Hao Xu,Xiao-Yan Wang
Endoscopic Center,Department of Gastroenterology,the Third Xiangya Hospital,Central South University,No.138 Tongzipo Road Yuelu District,Changsha 410013,China
Keywords: Adverse events Diagnostic yields Endoscopic ultrasound-guided fine-needle biopsy Gallbladder masses Specimen adequacy
ABSTRACT Background: Endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) is a widely used modality for acquiring various target samples,but its efficacy in gallbladder masses is unknown.The aim of this retrospective study was to evaluate the efficacy and safety of EUS-FNB in patients with gallbladder masses.Methods: The study samples were composed of patients from March 2015 to July 2019 who needed to identify the nature of gallbladder masses through EUS-FNB.The outcomes of this study were the adequacy of specimens,diagnostic yields,technical feasibility,and adverse events of the EUS-FNB in gallbladder masses.Results: A total of 27 consecutive patients with a median age of 58 years were included in this study.The 22-gauge FNB needle was feasible in all lesions.The median follow-up period of the patients was 294 days.The specimens sufficient for diagnosis account for 89% (24/27) and 93% (25/27) in cytology and histology,respectively.The overall diagnostic yields for malignancy showed the sensitivity,specificity,positive predictive value,negative predictive value,and accuracy were 95.45% [95% confidence interval(CI): 75.12%-99.76%],100% (95% CI: 46.29%-10 0%),10 0% (95% CI: 80.76%-100%),83.33% (95% CI: 36.48%-99.12%),and 96.30% (95% CI: 80.20%-99.99%),respectively.The subgroup analysis revealed that FNB could obtain sufficient specimens and high diagnostic yields in both gallbladder mass <20.5 mm group and ≥20.5 mm group.One patient experienced mild abdominal pain after the procedure and recovered within one day.Conclusions: EUS-FNB is a reasonable diagnostic tool for the pretreatment diagnosis of patients with gallbladder masses,especially for patients who may miss the opportunity of surgery and need sufficient specimens to identify the pathological type so as to determine chemotherapy regimens.Further largescale studies are needed to confirm our conclusion.
Introduction
Gallbladder carcinoma (GBC) is a significant health problem worldwide and its management presents a great challenge for the biliary specialists [1].These tumors,usually detected at advanced stage,are highly malignant and have a poor prognosis [2].Even those patients with tumors confined to the gallbladder wall (stage I disease) only have a median survival time of 19 months [3,4].Normally,GBC is diagnosed by means of transabdominal ultrasound(TUS),computed tomography (CT) and magnetic resonance imaging (MRI).However,imaging alone cannot conclusively define or confirm the malignant potential of these lesions.For example,malignant melanoma,lymphoma,and metastatic tumors cannot be distinguished.Besides,imaging patterns of lesions such as xanthogranulomatous cholecystitis and adenoma are quite similar to those of malignancies.Thus,it is essential to confirm the tumor properties or pathological type of gallbladder masses with other methods.
In the last decade,the endoscopic retrograde cholangiography (ERC) drainage [5]and endoscopic transpapillary gallbladder drainage (ETGD) [6]are the conventional cytological sampling methods for GBC.However,their results are not satisfying [7-9],since the accuracy of ERC-based sampling in the gallbladder mass was ranging from 46% to 88% [5,6].In addition,there have been instances of adverse events following this procedure,such as post-ERCP pancreatitis,cholangitis,and sepsis [6].Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a useful and less-invasive diagnostic method for obtaining pathological specimens from diverse types of lesions,especially pancreatic tumors,abdominal or mediastinal lymph nodes [10].Although the current guideline does not include GBC in the indications for fineneedle aspiration or biopsy [11],EUS-FNA for gallbladder tumors has been demonstrated to be relatively safe and reliable in several reports [2-6,12-16].
Recently,increasing reports have mentioned endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) in obtaining specimens from intra-or extraluminal lesions [17].Compared with EUS-FNA,EUS-FNB could provide higher diagnostic yield and diagnostic accuracy with fewer needle passes [18].No significant difference was found in adverse events between the two techniques [18].Despite the wide use of EUS-FNB in diagnosing various pancreaticobiliary lesions,its role in investigating gallbladder masses remains scarce.The aim of this study was to retrospectively evaluate the efficacy and safety of EUS-FNB with gallbladder lesions suspected of being GBC,in a small cohort of patients from a single center.
Methods
Study design
This study was conducted at the Third Xiangya Hospital,Central South University.Patients with gallbladder masses who underwent EUS-FNB from March 2015 to July 2019 were reviewed and consecutive patients who were ≥18 years of age and received EUS-FNB using a 22-gauge (g) needle were included in the study.
Ethics
Each patient provided informed consent for EUS-FNB before undergoing the procedure.The original identification number of each patient was encrypted for privacy.The study was approved by the Institutional Review Board of the Third Xiangya Hospital,Central South University (2020-S021).
EUS-FNB procedure
EUS was performed by two endosonographers with experience of over 100 EUS-guided tissue acquisition procedures.Patients received oxygen and were sedated using propofol or midazolam according to the liver function of patients.Their vitals were monitored throughout the procedure by an experienced anesthetist.Convex linear array echoendoscopy (EG-580UT,Fujifilm,Tokyo,Japan;GF-UCT260,Olympus,Tokyo,Japan) and an ultrasonography apparatus (SU-90 0 0,Fujifilm,Tokyo,Japan or EU-ME2,Olympus,Tokyo,Japan) were used for the EUS-FNB with a 22-g reverse bevel needle (EchoTip ProCore;Cook Medical,Limerick,Ireland).The gallbladder,liver,portal vein,common bile duct,pancreas,and spleen were scanned using a combination of B-mode and color Doppler EUS imaging,operating at 7.5 MHz.Once the target lesion was identified and no underlying vessels in the path of the needle were ensured,the FNB needle was advanced within the lesion.Slow-pull or standard negative suction with 5 mL pre-vacuum was applied.The needle was moved back and forth within the target lesion 15-20 times,and a total of two to four needle passes were performed.Then,the needle was withdrawn.
The specimen was expelled onto a glass slide using a 10-mL air-filled syringe until no further material could be expressed,and then,we reinserted the stylet into the needle for removing remnant materials.The adequacy of the specimens was preliminarily estimated by the macroscopic on-site evaluation(MOSE).The macroscopic visible core (MVC),which was defined as whitish or yellowish pieces of tissue with an apparent bulk,not including paste-like or liquid-like specimens,was carefully examined [10].Needle passes would be performed until the specimens were enough at the operator’s discretion.Then,the intact specimens were transferred into the bottle containing 10% formalin for histological evaluation,and the fragments smeared for cytological evaluation were placed into an alcohol bottle.
Specimens analysis
The specimens were then delivered to the pathology department and interpreted by two experienced pathologists.The adequacy and blood contamination of the specimens were assessed based on the consensus of two pathologists.Each specimen was evaluated using a 4-point scale (0=no significant specimen for diagnosis,1=not enough specimen to diagnose,2=moderate specimen but still diagnosable,3=sufficient specimen for diagnosis) for specimen adequacy and graded on a 4-point scale (0=free of blood,1=sparseness of erythrocytes,2=moderate bloodiness,and 3=blood clots present) for contamination with blood [19-21].Histology and cytology samples were classified into three grades:malignant (including positive and suspicious for malignancy),negative (including atypia and negative for malignancy) and inconclusive (due to lack of core tissue) [19,20].
Evaluation of adverse events
After the procedure,the patients were moved to the recovery room for at least 2 h,where their vital signs (e.g.,pulse,blood pressure,blood oxygen levels) were closely monitored as the effects of anesthesia wore off.Then they were transferred to the ward for treatments.Simultaneously,the patients were monitored for electrocardiogram,blood pressure,heart rate,oxyhemoglobin saturation,and temperature for 24 h as a precautionary measure.During the clinical observation,the level of serum amylase,C-reactive protein and hematologic profiles were measured to monitor the adverse events (such as massive bleeding,significant bile peritonitis,post-procedural pancreatitis,shock or iatrogenic infections).During the follow-up period,the patients returned to the hospital for reexaminations (laboratory and imaging examinations) when they had any discomfort or 3 months after the operation.The tumor seeding associating with EUS-FNB procedures was evaluated during the follow-up by the ascites cytology examination or imaging modalities (e.g.,CT,MRI,EUS,positron emission tomography-computed tomography).If the imaging result suggested possible metastasis,an appropriate biopsy method would be used to obtain the pathological confirmation [22].
Final diagnosis
The diagnostic criteria are based on the following: (1) for patients who received surgical resection,the diagnosis was based on pathological diagnosis of the resected specimen;(2) for patients who received no surgical resection,the diagnosis was based on the clinical follow-up (by imaging and phone) for at least 12 months or until the patient died [19,20].Malignancy diagnosis was recorded if (1) the lesion showed enlargement or metastasis,or (2) the patient presented malignant symptoms such as anemia,weight loss,or died of tumor adverse events during the follow-up.A benign diagnosis was recorded if (1) the lesion spontaneously resolved or(2) had no sign of deterioration in follow-up imaging [19,20].
Statistical analysis
The main outcomes of this study were (1) the specimen adequacy for diagnosis,(2) the diagnostic yield for malignancy,including sensitivity,specificity,positive predictive value (PPV),negative predictive value (NPV),and accuracy,(3) the technical feasibility,and (4) the incidence of adverse events of EUS-FNB in gallbladder masses.
Descriptive statistics were summarized,including age,sex,initial symptoms,location and size of lesions,puncture path,followup duration and diagnosis of biopsy specimens.We used frequency,proportion and medians,inter-quartile range (IQR) to describe categorical variables and continuous variables,respectively.The true positive (TP) was defined as a consistent malignant result of EUS-FNB with the final malignant diagnosis,and the false positive (FP) was defined as a lesion diagnosed to be malignant by EUS-FNB but turned out to be benign.Likewise,the true negative (TN) was defined as agreeing benign diagnosis by both EUSFNB and final diagnosis,and the false negative (FN) was initially categorized as benign by EUS-FNB but finally diagnosed as malignant [13].The sensitivity is equal to TP/(TP+FN).The specificity is equal to TN/(TN+FP).The PPV is equal to TP/(TP+FP).The NPV is equal to TN/(TN+FN).Diagnostic accuracy is equal to(TP+TN)/(TP+FP+TN+FN) [23].Statistical analysis was conducted by using SPSS software version 13.0 (SPSS Inc.,Chicago,IL,USA).
Results
Baseline characteristics
A total of 27 patients (27 lesions) with suspected GBC found by various imaging modalities underwent EUS-FNB.The patients included 11 females (41%) and 16 males (59%),with a median age of 58 years (IQR: 52-70).The patient’s baseline and lesion characteristics are listed in Table 1.Five patients (19%) obtained the diagnosis from surgical resection specimens,and the remaining 22 patients (81%) obtained the diagnosis through clinical follow-up with a median follow-up period of 294 days (IQR: 104-430).Of the 27 patients who underwent EUS-FNB,18 (67%) were diagnosed as malignant,4 (15%) were suspicious,4 (15%) were atypia,and 1 (4%)was benign.Among the 4 patients of atypia,1 (4%) was false negative based on a compatible clinical course.

Table 1Demographics and baseline characteristics of 27 patients undergoing FNB(n=27).
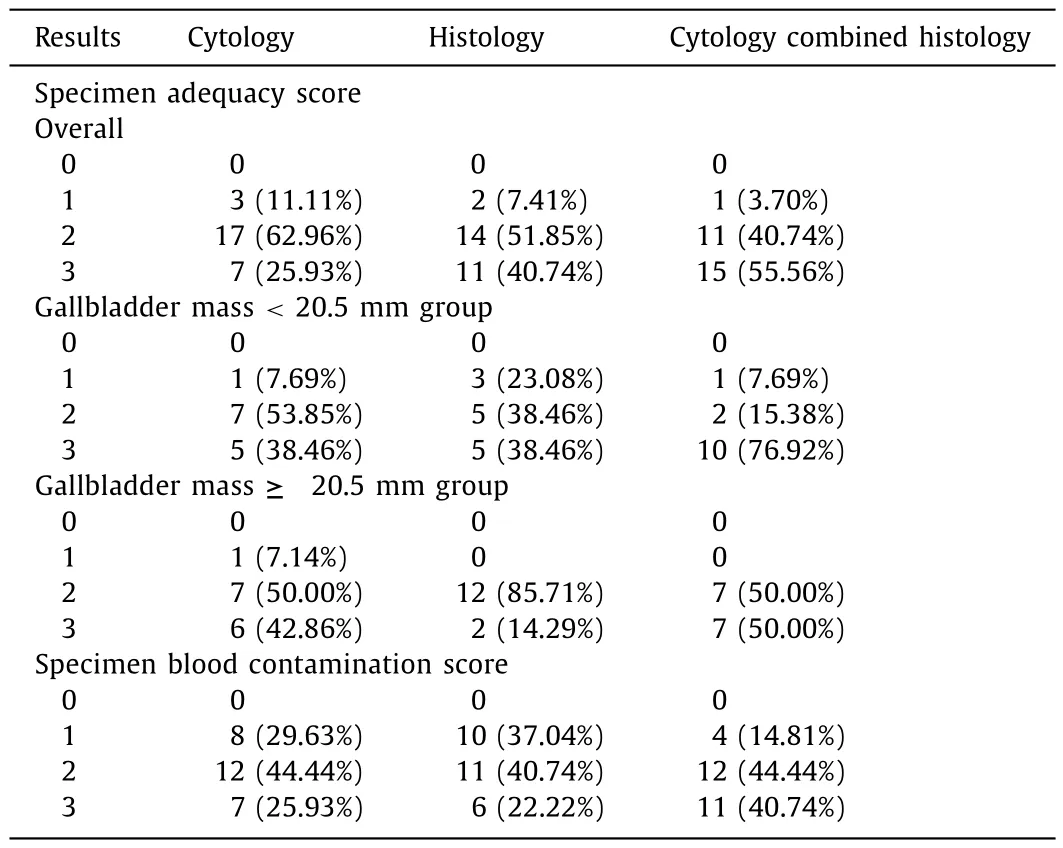
Table2Results of specimen adequacy and blood contamination of EUS-FNB in gallbladder masses.
Adequacy of specimens
The MVC was observed in 99% (82/83) passes.The only pass had no MVC was performed in the gallbladder neck.Pathologists considered that 24 (89%) and 25 (93%) specimens were sufficient(scores 2 and 3) for cytological and histological diagnosis,respectively (Table 2).The samples which were insufficient (scores 0 and 1) to reach both the cytological and histological diagnosis were procured from the same patient who had no MVC.The other specimens which could not reach the cytological diagnosis were also acquired from the gallbladder neck.
We divided the patients into two subgroups based on the median of gallbladder mass size.There were 12 (12/13,92%) versus 14 (14/14,100%) specimens sufficient (scores 2 and 3) for cytology combined histology diagnosis in gallbladder mass <20.5 mm group and ≥20.5 mm group,respectively.The detailed evaluation of adequacy of specimens were listed in Table 2.
Diagnostic yields of EUS-FNB
Overall,the diagnostic yields for malignancy in EUS-FNB using a 22-g needle showed sensitivity,specificity,PPV,NPV,and accuracy of 95.45% [95% confidence interval (CI): 75.12%-99.76%],100%(95% CI: 46.29%-100%),100% (95% CI: 80.76%-100%),83.33% (95%CI: 36.48%-99.12%),and 96.30% (95% CI: 80.20%-99.99%),respectively.The diagnostic sensitivity,specificity,and accuracy for cytology and histology were 90.91% (95% CI: 69.38%-98.41%) vs.95.45%(95% CI: 75.12%-99.76%),100% (95% CI: 46.29%-100%) vs.100% (95%CI: 46.29%-100%),and 92.59% (95% CI: 75.53%-99.04%) vs.96.30%(95% CI: 80.20%-99.99%),respectively.The diagnostic accuracy of cytology,histology,and the two combined in malignant and benign lesions were 90.91% (95% CI: 71.00%-98.66%) vs 100% (51.09%-100%),95.45% (76.49%-99.99%) vs.100% (51.09%-100%),and 95.45%(76.49%-99.99%) vs.100% (51.09%-100%),respectively (Table 3).
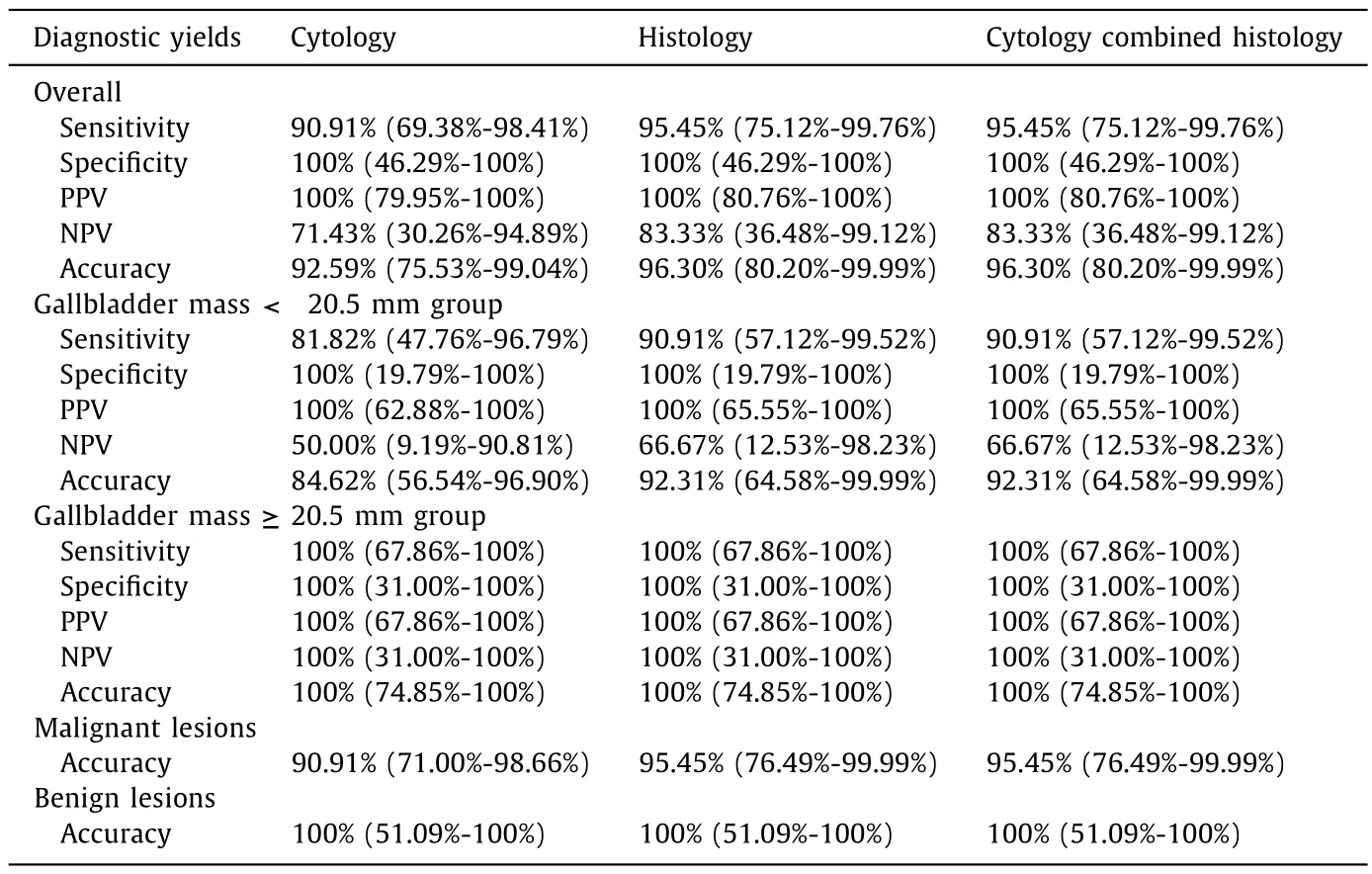
Table 3Results of diagnostic yields of EUS-FNB in gallbladder masses.
We also conducted a subgroup analysis for the diagnostic yields.The overall diagnostic yields showed sensitivity,specificity,PPV,NPV,and accuracy in gallbladder mass <20.5 mm group and≥20.5 mm group were 90.91% (95% CI: 57.12%-99.52%) vs.100%(67.86%-100%),100% (95% CI: 19.79%-100%) vs.100% (31.00%-100%),100% (95% CI: 65.55%-10 0%) vs.10 0% (67.86%-10 0%),66.67% (95%CI: 12.53%-98.23%) vs.100% (31.0 0%-10 0%),and 92.31% (95% CI:64.58%-99.99%) vs.100% (74.85%-100%),respectively.The detailed diagnostic yields for cytology,histology were listed in Table 3.
Immunohistochemical staining was performed in two patients,of which one was diagnosed as adenocarcinoma,and the other was neuroendocrine carcinoma (Figs.1,2).
Blood contamination,technical feasibility and adverse events
Regarding the cytological and histological assessment,30%(8/27) and 37% (10/27) of the passes had a low degree of blood contamination (score 0 or 1) (Table 2).
Only one patient encountered mildly abdominal pain within 24 h after the EUS-FNB procedure but remitted within one day without any special treatment.No serious procedure-related adverse events occurred with any of the 27 patients within the initial 2 h and following 24 h.Moreover,there was no apparent tumor seeding during our follow-up periods through imaging examination.
Discussion
GBC is a fast-growing tumor with a high mortality rate [24].Although surgical resection is the optimal method for treatment,not all patients need or are suitable for surgery because they have benign lesions,or are in poor health or in an advanced stage of cancer.The pathological type,grade,and stage of the disease are considered vital for characterizing the lesion [25].In addition,any therapeutic regimens with pathological evidence are thought to be ethically and medically important.Thus,obtaining pathological evidence of gallbladder masses prior to resection or chemotherapy is necessary for both operable and non-operable cases.
Over the recent years,EUS has been accepted as an important tool in preoperative staging.Besides its capacity to delineate tumor location and size,it presents a good accuracy for detecting lymph node metastasis,vascular invasion and predicting resectability [26].Particularly,when combined with tissue acquisition,it has shown increased pretreatment diagnostic yield and staging accuracy [11,26,27].Owing to the advantage of providing core tissue with preserved integral architecture which is essential for histological diagnosis,immunochemical and/or biomolecular testing of masses,EUS-FNB could obtain more specimens for an accurate diagnosis without increasing the number of needle passes or the presence of an on-site pathologist [18,28].Nevertheless,the use of EUS-FNB in the gallbladder is still uncommon and has been reported in only a few publications [12,29,30].Not only did these studies not specifically focus on the application of EUSFNB in gallbladder masses,but also were limited to 10 cases in total (Franseen needle in 1,Trucut needle in 1,and reverse bevel needle in 8) [12,29,30].Thus,they could not provide adequate information in gallbladder masses sampling using EUSFNB.The current study was the first dedicated study to identify the efficacy and safety of EUS-FNB in gallbladder mass lesions,and suggested that EUS-FNB with 22-g needle was feasible and performed proficiently in specimen quality and diagnosis yields without increasing the adverse events in gallbladder mass lesions.
Generally,EUS-FNB could obtain more specimens.The amount of the specimens was essential for an accurate diagnosis.In our study,most specimens were adequate for cytological and histological diagnosis,even in the gallbladder mass <20.5 mm group.One patient obtained a false negative diagnosis in both histological and cytological evaluations.This may be due to the limited size,fibrosis of the lesion,or incorrect MOSE evaluation.Another patient with a false negative cytological diagnosis (neuroendocrine tumor)obtained the correct diagnosis (neuroendocrine carcinoma) after it was combined with the results of immunohistochemistry using histological specimens.This confirmed that the adequate specimens are necessary for the pathological diagnosis in the EUSguided biopsy,especially when cytology results do not match the clinical course and where the treatment pathway is altered for patients who may miss the opportunity of surgery and referred for chemotherapy.
In addition,the adequate specimens are helpful in precision medicine.It was reported that EUS-FNB is competent in procuring adequate specimens for genomic profiling [31].Moreover,EUS-FNB specimens had a strong correlation with the surgical specimens,which represented the original tumor [32],therefore could be used for creating human-derived organoids,xenografts,and cell lines successfully,which could recapitulate the original tumors and were useful in screening target therapies for patients [33-35].Therefore,EUS-FNB performed in gallbladder masses may not only increase the amount of tissue acquisition,but also play an important role in precision medicine.

Fig.1.Example images acquired throughout the diagnostic pathway of a single patient,EUS-FNB,cytopathological and histopathological findings of the EUS-FNB specimen from a single patient.A : Contrast enhanced CT of the abdomen showing an irregular thickening of the gallbladder wall (red arrow).B : EUS image of the gallbladder tumor during the acquisition of the tissue sample using a 22-gauge FNB needle.C : High grade dysplastic glands could be seen in inflammatory fibrous effusions,and canceration could not be ruled out (original magnification × 400).D : Some epithelial with large and deep stained nuclei were heteromorphic and clustered,and they were considered as tumor cells and tended to become cancerous (original magnification × 400).CT: computed tomography;EUS-FNB: endoscopic ultrasound-guided fine-needle biopsy.
From our study,we obtained the satisfactory diagnostic yields in both subgroups,which were comparable to the yields reported in the review by Li et al.[36]and we believed that EUS-FNB was competent in gallbladder lesions.However,in the study of Paik et al.[30]gallbladder lesions showed the lowest adequacy in EUS-FNB compared with the other lesions.Meanwhile,diagnostic accuracy increased from 38% to 62% when combined with cytology findings [30].Therefore,they believed that EUS-FNA might be more appropriate than EUS-FNB in gallbladder lesions.However,this study was limited to the sampling of 8 gallbladder mass lesions,comparing with 27 in our study.Further,a comparison between EUS-FNA and EUS-FNB sampling of gallbladder masses would provide useful information to the community.
According to a systematic review and meta-analysis of 51 EUSFNB studies [36],adverse events including bleeding,acute pancreatitis,abdominal or chest pain,perforation,and fever were related to the FNB procedure.The total EUS-FNB related adverse event rate was 0.59%,and no FNB-related deaths were reported [36].Compared with the FNA-specific morbidity,the overall rate of FNBrelated adverse events was lower (0.98% vs.0.59%) [36,37].Needle tract seeding is a rare adverse event that is possible during EUS-guided sampling.Based on large retrospective surveys,its frequency was estimated at 0.003% to 0.009% for EUS-FNB [38].European Society of Gastrointestinal Endoscopy (ESGE) reported that there was no increased risk of peritoneal seeding,gastric wall metastasis,or postoperative recurrence whether preoperative EUSguided sampling had been performed or not for pancreatic cancer,intraductal papillary mucinous neoplasm,or cholangiocarcinoma [11].To date,as there is no dedicated research on adverse events of EUS-FNB in GBC,there is no data for reference.In our study,each patient was reviewed by imaging at least once during the follow-up period,and there was no procedure-related adverse event or needle tract seeding,except one experienced mildly abdominal pain.The low adverse event rate was likely because we only collected the gallbladder mass on the side of the gastrointestinal tract,and the needle did not pierce through the lesions to reach into the cavity of the gallbladder.Additionally,the thick elastic fibrotic wall of the gallbladder contracted after piercing.
There were some limitations in our study.First,the rapid onsite evaluation (ROSE),a method to observe through a microscope whether a smear air dried and stained with Diff-Quik contains sufficient cells [39],may improve the specimen adequacy.Although it is unavailable in our center,the endoscopists were trained for MOSE,an alternative to ROSE,to improve tissue acquisition and diagnostic accuracy.Second,the small sample size and single-center nature of this study may weaken the reference value of our center’s experience.Next,we plan to conduct a prospective,largescale study of the application of EUS-FNB in gallbladder mass lesions to further provide evidence for the efficacy and safety of EUSFNB in gallbladder masses.
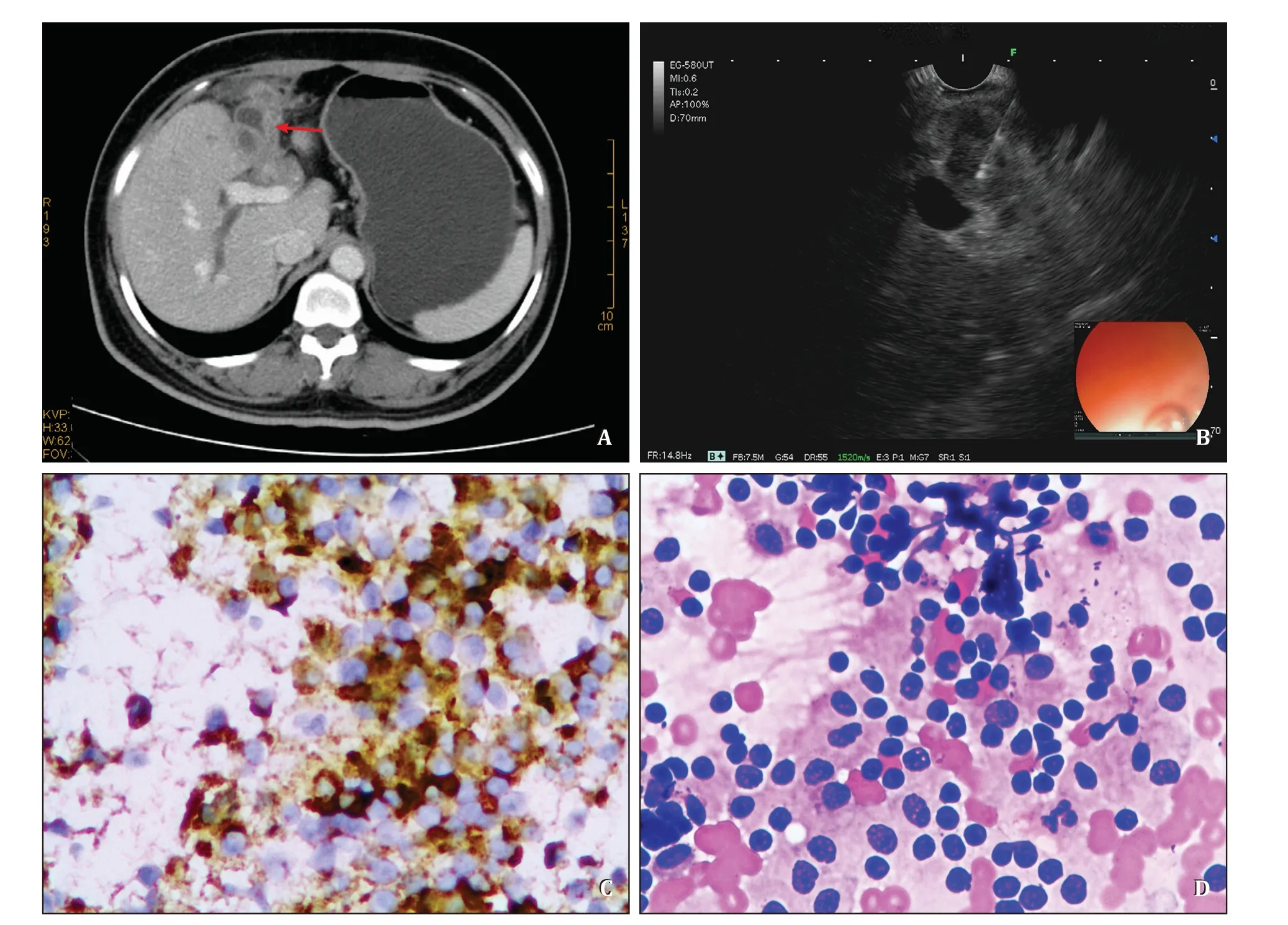
Fig.2.The case that final diagnosis based on the immunohistochemistry.A : Contrast enhanced CT of the abdomen showing an irregular thickening of the gallbladder wall and irregular gallbladder contour (red arrow).B : EUS image of the gallbladder tumor during the acquisition of the tissue sample using a 22-gauge FNB needle.C : Based on the result of histology combined with immunohistochemistry,this case was considered neuroendocrine carcinoma.CgA (+),Syn (+),CD56 (+) [The image showed the CgA(+)](original magnification × 400).D : Duct epithelial cells and acinar cells were observed,and a few small round cells were distributed in patches,with mild and consistent morphology (original magnification × 400).EUS: endoscopic ultrasound-guided fine-needle biopsy;CT: computed tomography.
In summary,our data suggested that EUS-FNB with a 22-g needle is suitable for diagnosing the gallbladder masses suspected malignancy before treatment,especially for patients who may miss the opportunity of surgery and need sufficient specimens to identify the pathological type to determine chemotherapy regimens.However,the prospective,large-scale studies are needed to provide further evidence for the efficacy and safety of EUS-FNB in gallbladder masses.
Acknowledgments
We thank to the pathologists,including Yang Liu,Zhi-Jiao Zhou,and Ye-Ning Yang,for their help in selecting the appropriate figures.
CRediTauthorshipcontributionstatement
TingTong:Conceptualization,Data curation,Formal analysis,Investigation,Methodology,Project administration,Writing– original draft.LiTian:Conceptualization,Methodology,Project administration,Writing– review &editing.Min-ZiDeng:Conceptualization,Data curation,Formal analysis,Project administration.Xue-JieChen:Investigation,Project administration.TianFu:Investigation,Project administration.Ke-JiaMa:Investigation,Project administration.Jia-HaoXu:Investigation,Project administration.Xiao-YanWang:Conceptualization,Funding acquisition,Methodology,Project administration,Writing– review &editing.
Funding
The study was supported by a grant from the National Major Diseases Multidisciplinary Cooperative Diagnosis and Treatment Project—Gastrointestinal Cancer MDT Diagnosis and Treatment Project.
Ethicalapproval
The study was approved by the Institutional Review Board of the Third Xiangya Hospital,Central South University (2020-S021).Written informed consent was obtained from all participants.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- A new prognostic model for drug-induced liver injury especially suitable for Chinese population
- Post-hepatectomy liver failure: A timeline centered review
- INSTRUCTIONS FOR AUTHORS
- Value and prognostic factors of repeat hepatectomy for recurrent colorectal liver metastasis
- Older liver grafts from donation after circulatory death are associated with impaired survival and higher incidence of biliarynon-anastomotic structure
- Development and validation of a novel model to predict liver-related mortality in patients with idiosyncratic drug-induced liver injury
