光强对苗期大豆叶片水力导度及叶脉性状的影响
2023-12-29高静陈吉玉谭先明吴雨珊杨文钰杨峰
高静,陈吉玉,谭先明,吴雨珊,杨文钰,杨峰
光强对苗期大豆叶片水力导度及叶脉性状的影响
高静,陈吉玉,谭先明,吴雨珊,杨文钰,杨峰
四川农业大学农学院/农业农村部西南作物生理生态与耕作重点实验室/四川省作物带状复合种植工程技术研究中心,成都 611130
【目的】探究光强对苗期大豆叶片水力导度、光合特性和叶水势的影响,分析叶脉性状对不同生长光强的适应机制,为提高大豆光能利用提供理论支撑。【方法】选用强耐荫型的大豆品种南豆12和弱耐荫型的大豆品种桂夏7为试验材料,在人工气候室进行盆栽试验,设置高光强((424.47±12.32)µmol·m-2·s-1,HL)、中光强((162.52±20.31)µmol·m-2·s-1,ML)和低光强((93.93±9.87)µmol·m-2·s-1,LL)处理。在处理20 d后研究不同生长光强对苗期大豆叶片水力导度、光合参数、叶片水势及叶脉性状的影响。【结果】相对于高光强处理,低光强处理下南豆12和桂夏7的叶片水力导度显著降低,南豆12的叶片水力导度在3个处理下均显著高于桂夏7。与高光强处理相比,在中、低光强处理下南豆12的叶片水力导度分别降低7.56%和21.24%,气孔导度分别降低43.96%和58.89%,净光合速率分别降低29.44%和46.49%。同样,桂夏7的叶片水力导度分别降低42.16%和23.71%,气孔导度分别降低54.55%和45.79%,净光合速率分别降低37.03%和42.06%。南豆12和桂夏7的叶片水势在处理间均无显著差异。大豆的叶片水力导度与气孔导度在3个光强处理下均达到极显著正相关(<0.01),随着光强的降低,叶片水力导度与净光合速率呈显著正相关(<0.05),与气孔导度呈极显著正相关(<0.01)。对于叶脉结构,与高光强相比,中、低光强处理下两个大豆品种的小叶脉密度以及主叶脉和小叶脉的木质部导管面积均显著降低,且南豆12的小叶脉密度和主叶脉木质部导管面积在中、低光强处理下均显著高于桂夏7。南豆12的主叶脉密度在处理间无显著变化,桂夏7的主叶脉密度在中、低光强处理下较高光强显著降低11.4%和15.0%。光强降低显著增长了叶脉到气孔的距离。南豆12在中、低光强处理下叶脉到气孔的距离较高光强增长21.33%和60.01%,桂夏7叶脉到气孔的距离增长31.50%和53.59%。相关性分析表明,大豆叶片水力导度与小叶脉密度、主叶脉和小叶脉的木质部导管面积呈显著正相关(<0.05),与叶脉到气孔的距离呈极显著负相关(<0.01)。【结论】光强会通过调控大豆叶脉结构影响叶片水力导度,弱光降低大豆叶片水力导度,但叶片水力导度和气孔导度保持协调,维持叶片水分供需平衡。弱光下具有较高的叶脉密度能够缩短水分运输的距离,保证较好的叶片水分供应能力,从而有利于CO2的扩散和光合作用,这是耐荫型大豆适应弱光环境的又一策略。
大豆;叶片水力导度;气孔导度;光强;叶脉
0 引言
【研究意义】大豆是我国重要的粮、油、饲兼用作物,营养价值高。近年来大豆消费呈刚性增长,但大豆种植面积和产量已连续多年下降,供需严重失衡[1]。在农业生产中常采用密植或间套作提高大豆种植面积,但不同种植模式直接影响大豆冠层光照强度。弱光下大豆的形态建成和光合能力均会受到抑制,极大地限制了产量增加和品质提升[2]。光合作用是干物质积累和形态建成的基础生理过程[3]。在C3植物中,CO2的扩散已被证明是非生物胁迫下降低光合速率主要的限制因子[4-6]。叶片水力导度(leaf)是指液态水通过叶片运输的效率,是光合速率的重要制约因素。当叶片张开气孔以吸收CO2进行光合作用时,不可避免地引起水分的散失导致叶肉细胞的干燥。植物面临着平衡最大限度吸收CO2和最小限度散失水分的挑战。水分由叶脉运输浇灌容易干燥的叶肉细胞,较好的水分供给能力能够保持气孔最大程度的开放,确保胞间CO2的稳定供应[7]。这表明气孔保持开放进行光合作用的能力取决于叶片中水分供给能力,也就是leaf。气孔导度(s)和leaf的协同对维持叶片水分的供需平衡,保证叶片组织进行正常的生理生化功能具有重要意义[8]。【前人研究进展】光强已被证明会影响大豆光合特性和leaf。弱光会导致大豆净光合速率和气孔导度不同程度的降低、改变栅栏细胞排列和形状、增加类囊体的垛叠程度从而增加光捕获[9-10]。由黑暗到光亮的转变增加了核桃、向日葵和水稻的leaf[11-13]。但是红橡树和银杏的leaf对光强的变化不敏感[13-14]。在杉树中,冠层上部的叶片比下部的叶片具有更高的leaf和s[15]。与喜荫树种相比,喜阳树种具有较高的leaf[16-17]。leaf和光合作用的关系也是近几年的研究热点。在高温、钾肥和干旱处理下均观察到leaf和s之间良好的相关性[18-20]。叶脉性状与植物的光合碳固定、水分运输直接或间接相关,是植物适应逆境胁迫的关键因素[21-22]。已有的研究揭示了高温和干旱下叶脉结构的适应机制。李晓鹏[23]研究表明,枣的叶脉密度随温度升高而增加。为了适应高温下的高蒸腾速率,叶脉系统必须增加水分运输的量与速率,因此要匹配较高的叶脉密度。在干旱胁迫下水稻叶片的叶脉密度显著增加[24],较高的叶脉密度能够在干旱造成木质部栓塞时,通过在栓塞周围运输水以保证单位面积内的水分充足和高光合效率。【本研究切入点】以往研究中对于大豆光合特性对光强的响应主要集中在光反应,但围绕光合作用中CO2扩散过程对弱光的响应相对欠缺。关于leaf和s相关关系的研究主要集中在饱和光条件下,而leaf和s在不同光强下是否协同变化尚不明确。此外,大豆叶脉性状对不同光强适应的研究也鲜见报道。【拟解决的关键问题】以大豆为研究对象,阐明光强对大豆叶片水力导度、叶片水势及光合特性的影响。探讨leaf和s在不同光强处理下的协调关系,并从影响leaf的叶脉性状出发阐明光强对光合作用的调控机理。研究大豆叶片水分运输能力对光强的响应,为提高光合能力提供理论依据。
1 材料与方法
试验于2022年在四川农业大学完成。
1.1 试验材料
根据武晓玲等[25]不同耐荫型大豆品种筛选的结果,选择强耐荫型的南豆12(四川省南充市农业科学院)和弱耐荫型的桂夏7(广西农业科学院)作为试验材料。
1.2 试验设计
试验在四川农业大学成都校区人工气候室中进行。气候室相对湿度保持在55%,白天温度25 ℃,晚上22 ℃,光周期设置为12 h光照/12 h黑暗。挑选均匀饱满的大豆种子种植于PINDSTRUP营养土﹕蛭石体积比3﹕1的基质中,每盆1株,每个处理10盆。出苗后2 d开始对大豆幼苗进行光强处理。通过使用具有不同透光性能的黑色遮阳网,参照陈吉玉等[26]的研究设置以下处理:高光强(HL)、中光强(ML)和低光强(LL),光环境数据见表1。光强处理20 d后,选择每个处理大豆的倒数第二片完全展开叶进行相关指标的测定。
1.3 项目测定与方法
1.3.2 光合参数 上午9:00—11:00,选择每个处理下长势一致的6株大豆进行测试。使用Li-Cor公司的Li-6400光合仪,在叶室内,光强设置为500 µmol·m-2·s-1,CO2浓度调节至400 µmol CO2·mol-1。测量过程中温度设置为25 ℃。达到稳定状态后,记录净光合速率(n)、s、胞间CO2浓度(i)和[18]。取3次测量的平均值作为最终测量值。
1.3.3 气孔密度 在光合参数测量完成后,从每片叶子的顶部、中部和底部分别切下1 cm2的样品,并在固定液中固定保存24 h。所有样品均在当天的同一时间采集。叶片样品分别在5种梯度乙醇溶液(50%、70%、85%、95%和100%)中各脱色30 min,然后置于5% NaOH(w/v)水溶液中2 d。叶片样品用蒸馏水清洗后在三氯乙醛水合物中固定和清除,直到叶子半透明。最后,使用1%亚甲基蓝溶液染色后置于载玻片上[28]。利用尼康公司的TI-E型倒置荧光显微镜在10倍放大倍率下拍摄每个样品3张图像。用ImageJ软件测定气孔密度。
1.3.4 叶脉密度 将一级、二级和三级叶脉归为主叶脉,四级及以上归为小叶脉[29]。使用尼康公司D5600相机拍摄完全展开的大豆叶片,以量化其面积和主叶脉长度。小叶脉用尼康公司的TI-E型倒置荧光显微镜在10倍放大倍率下观察和拍照[30]。采用ImageJ软件分别测定小叶脉密度和主叶脉密度。
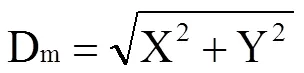
1.3.6 叶脉解剖结构 用手术刀沿大豆叶片主叶脉中部取样(0.5 cm×2 cm左右),固定液固定24 h,固定后的叶脉组织用梯度酒精进行脱水。将组织于包埋机内进行包埋,随后用石蜡切片机切片。石蜡切片用环保型脱蜡透明液脱蜡后用甲苯胺蓝染色液进行染色,切片入干净的二甲苯透明5 min,中性树胶封片。在尼康公司Eclipse E100显微镜下观察,采用NikonS-U3成像系统获取图片。使用ImageJ软件分别测量小叶脉木质部导管面积(Xminor)和主叶脉木质部导管面积(Xmajor)。
1.4 数据处理与分析
采用Microsoft Excel 2016软件整理数据并作图,SPSS 25.0软件进行方差分析和相关性分析。
2 结果
2.1 光强对大豆叶片水力导度及叶片水势的影响
随着光强降低,两个大豆品种的leaf具有相同的变化趋势。在中、低光强处理下,南豆12和桂夏7的leaf较高光强处理分别降低7.56%和21.24%(中光强和低光强,下同)、42.16%和23.71%。在相同处理下,南豆12的leaf均高于桂夏7。与高光强相比,中、低光强处理对两个大豆品种的Ψleaf影响均不显著。在高、低光强处理下两个品种间Ψleaf无显著差异,在中光强处理下桂夏7的Ψleaf显著低于南豆12(图1)。
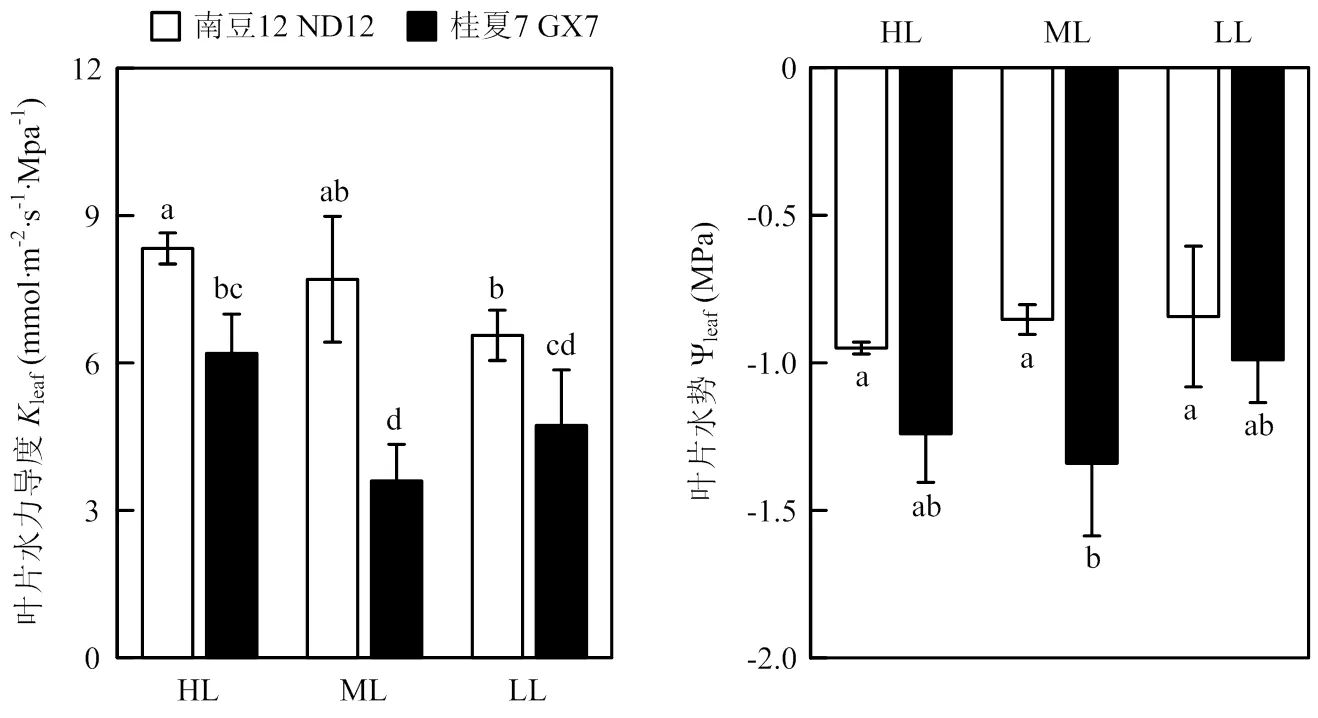
柱上不同小写字母表示各处理间差异显著(P<0.05)Different lowercases on the bars represent significantly different atp<0.05 level
2.2 光照对大豆叶片光合特性的影响
由表2可知,弱光降低了大豆叶片s、n和。与高光强相比,中、低光强处理下南豆12的s显著降低43.96%和58.89%,桂夏7显著降低54.55%和45.79%;南豆12叶片的n显著降低29.44%和46.49%,桂夏7显著下降37.03%和42.06%;南豆12的显著降低16.95%和27.56%,桂夏7显著下降30.99%和31.44%。在相同光强处理下,南豆12和桂夏7的n无显著差异,但在高、中光强处理下南豆12的s均显著高于桂夏7。南豆12和桂夏7的i在处理间无显著差异。在中、低光强处理下,南豆12的i均显著高于桂夏7,在中光强处理下,南豆12的显著高于桂夏7。随着光强的降低,两个大豆品种的leaf/s均显著增加。在中、低光强处理下,两大豆品种的leaf/s较高光强增加112.38%和93.86%、27.58%和57.70%。
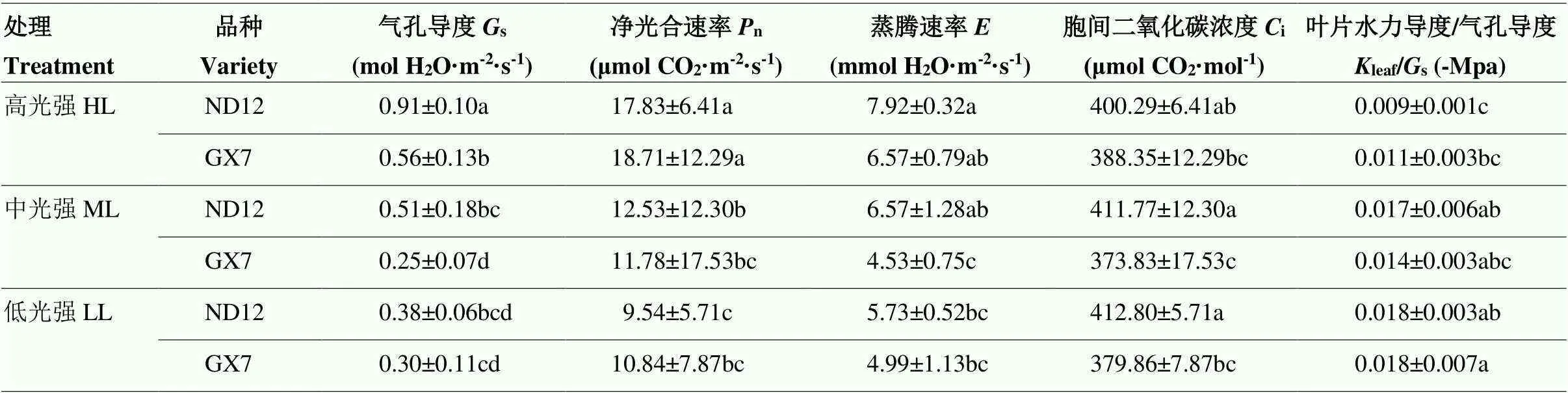
表2 光强对大豆叶片光合特性的影响
同列数据后不同小写字母表示在0.05水平差异显著。表3同
Different lowercases after the data in the same column indicate significant differences at 0.05 level. The same as Table 3
2.3 大豆叶片水力导度与气孔导度、净光合速率的相关性分析
3个光强处理下s与leaf均呈极显著正相关,且相关性较强,相关性系数分别为0.96、0.93和0.99,因此leaf的变化能够部分解释s在不同光强下发生变化的原因。在3个光强处理下,leaf与n之间均无显著的相关关系(图2-A)。Pearson相关性分析表明,在光强处理间leaf与s呈极显著正相关,与n呈显著正相关,相关性系数分别为0.83和0.44(图2-B)。
2.4 光强对大豆叶脉性状和气孔密度的影响
由表3可知,南豆12的VLAmajor在处理间无显著差异,桂夏7的VLAmajor在中、低光强处理下较高光强显著降低11.4%和15.0%。与高光强相比,中、低光强处理下大豆小叶脉变细、变少(图3-A),且随着光强的降低,VLAminor降低的幅度增加。南豆12在中、低光强下的VLAminor较高光强分别降低34.56%和38.88%,桂夏7在中、低光强下的VLAminor较高光强分别降低26.42%和37.09%(表3)。由图3-B可知,弱光下大豆气孔密度均降低,且随着光强的降低变化幅度增加。与高光强相比,中、低光强处理下南豆12的气孔密度分别降低38.29%和49.11%,桂夏7分别显著降低33.35%和50.49%。在3个光强处理下,南豆12的气孔密度均高于桂夏7,且在高、低光强处理下达到显著水平(表3)。由图3-C可知,弱光处理增长了Dm。与高光强相比,南豆12在中、低光强处理下Dm分别增长21.33%和60.01%,桂夏7的Dm分别增长31.50%和53.59%。光强降低减少了大豆叶片木质部导管大小(图3-D)。南豆12在中、低光强处理下的Xmajor、Xminor较高光强分别显著降低18.33%和32.29%、14.12%和27.09%。桂夏7在中、低光强处理下的Xmajor、Xminor较高光强分别显著降低19.36%和34.67%、25.77%和42.79%。
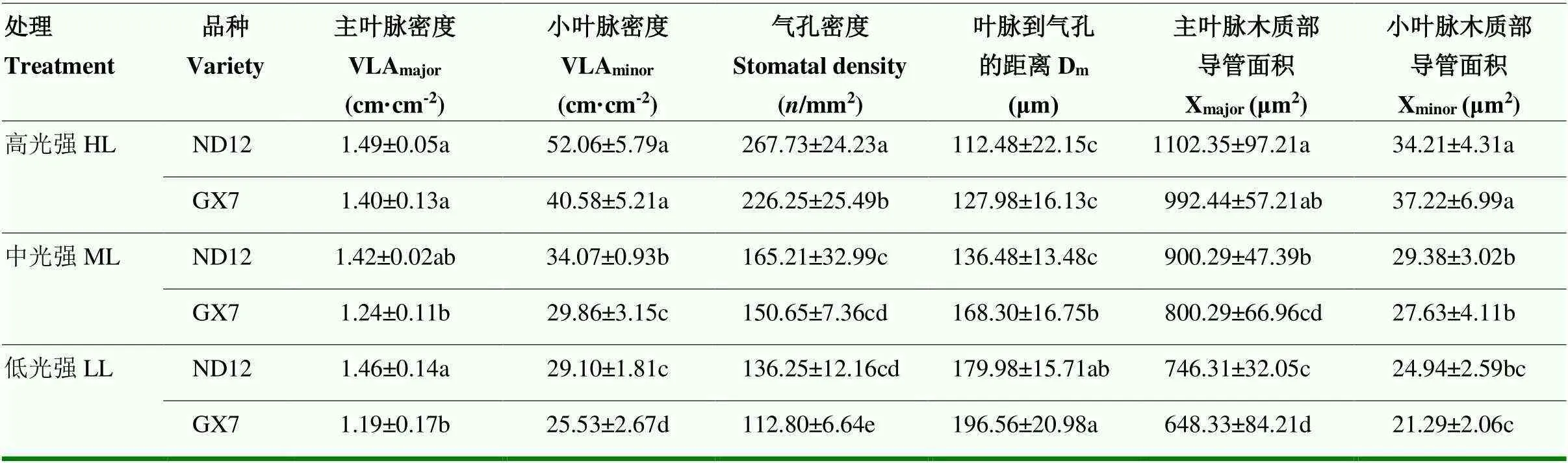
表3 光强对大豆叶脉结构和气孔密度的影响
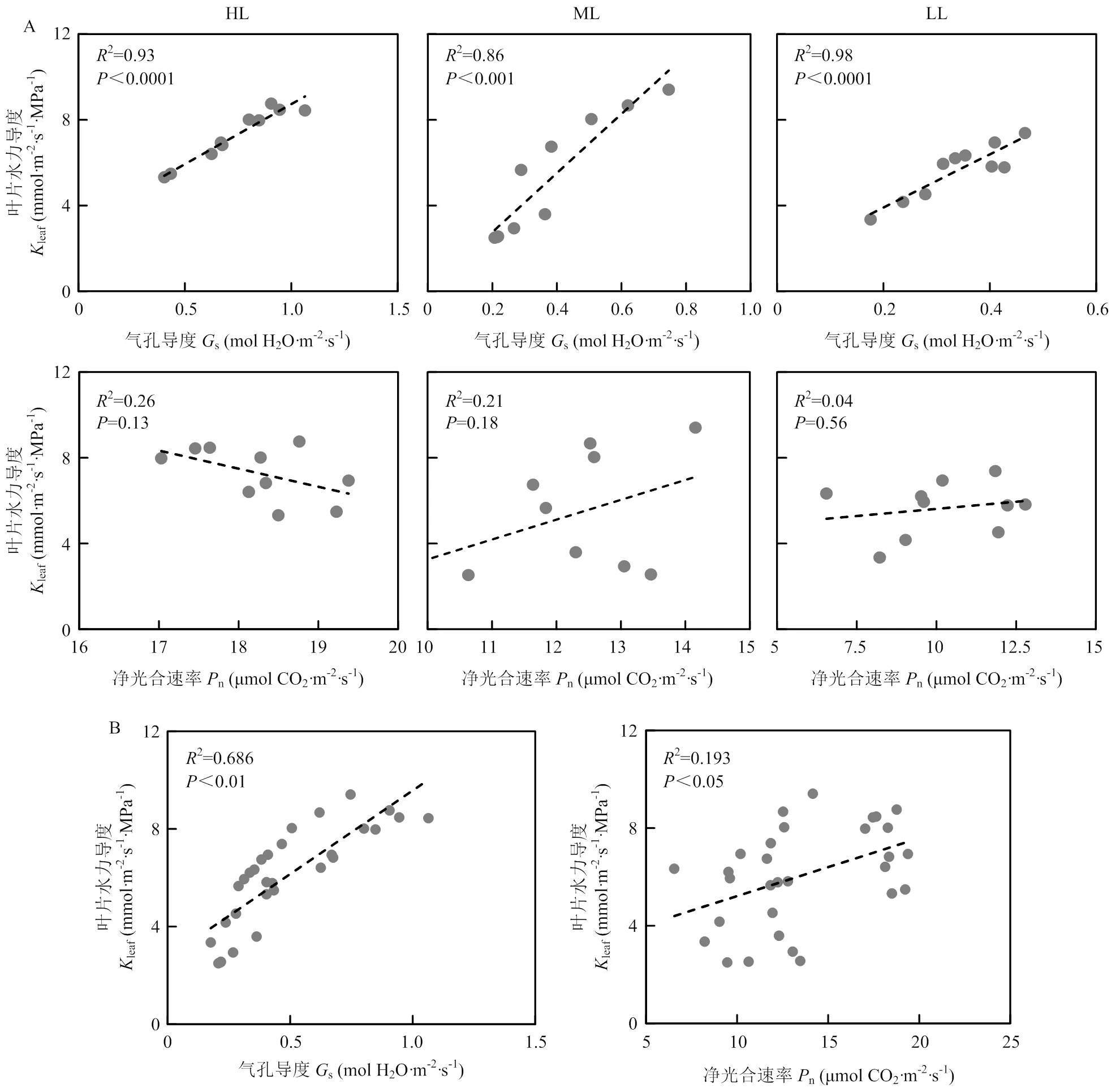
A:不同光强处理下叶片水力导度和光合参数相关性分析the correlation analysis of leaf hydraulic conductivity and photosynthetic parameters under different light intensity treatments;B:处理间叶片水力导度和光合参数的相关性分析The correlation analysis of leaf hydraulic conductivity and photosynthetic parameters among treatments
2.5 大豆叶片水力导度和叶脉性状的相关性分析
leaf与VLAminor呈显著正相关,与Dm极显著负相关,且相关性较强,相关性系数分别为0.73和0.80。leaf与Xmajor、Xminor呈显著正相关,相关性系数分别为0.68和0.69。这表明随着光强的降低,Xmajor、Xminor和VLAminor的变化均会显著影响leaf。VLAminor与Dm呈极显著负相关,相关性系数达到了0.91。大豆气孔密度与leaf呈显著正相关。此外,大豆气孔密度与VLAminor极显著正相关,与Dm极显著负相关,相关性系数分别为0.97和0.93(表4)。

A:叶脉密度Leaf vein density;B:气孔密度Stomatal density;C:小叶脉解剖结构,V、sto和Dm分别代表叶脉、气孔、叶脉到气孔的距离Minor leaf vein anatomy, V, sto and Dm represent leaf veins, stomata and distance from leaf veins to stomata, respectively;D:主叶脉解剖结构,Xy代表木质部导管Major leaf vein anatomy, Xy represents xylem conduit
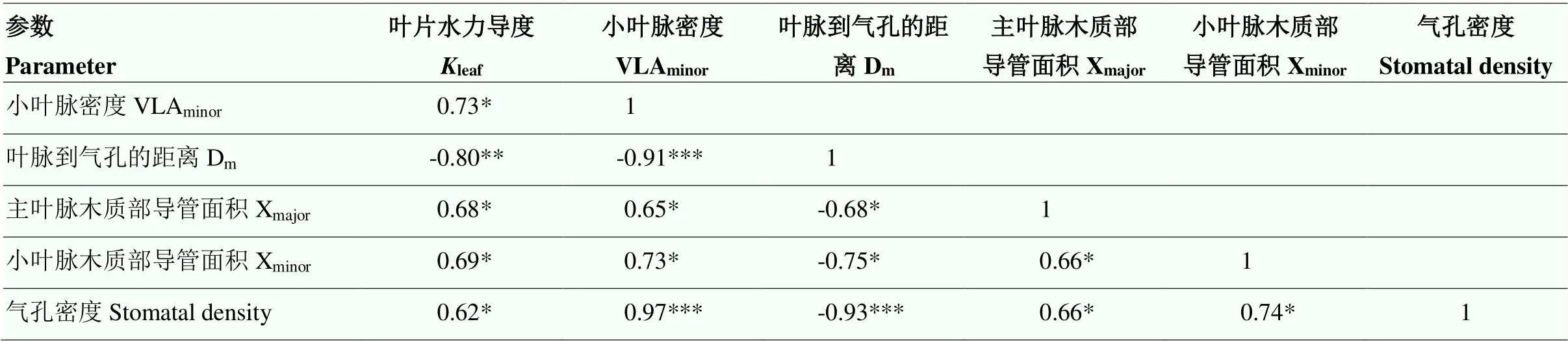
表4 大豆叶片水力导度与叶脉性状的相关性分析
*:在0.05水平上差异显著significant difference at 0.05 level;**:在0.01水平上差异显著significant difference at 0.01 level;***:在0.001水平上差异显著significant difference at 0.001 level
3 讨论
3.1 光强对大豆叶片水力导度的影响
叶片是植物水力系统的瓶颈,大约贡献了整个植株中30%—98%的水分运输阻力[31]。与根系和茎秆相比,叶片更容易受到环境的影响,因此是限制气体交换和干物质形成的根本因素[32]。与高光强相比,低光强处理显著降低了两个大豆品种的leaf,但中光强处理下南豆12的leaf变化不显著(图1)。Raimondo等[33]指出橄榄树的leaf不受冠层光强影响,leaf对光强缺乏差异可能是由于阴生叶冠层的光强仍较高(约1 000 µmol·m-2·s-1)。因此,只有在较低光强下才可能引起leaf的显著变化。与高光强相比,中光强下南豆12和桂夏7的leaf分别减少7.56%和42.16%(图1),可见不同大豆品种的leaf对光强的响应具有不同的可塑性。南豆12的leaf在3个光强下均显著高于桂夏7,因此南豆12在苗期具有更高的耐旱性,桂夏7的光合作用则更容易受到叶片干燥的影响。这种差异可能与两个品种的种植区域相关,南豆12适宜在四川夏季套作种植,正逢夏季干旱[34],而桂夏7适宜在广西作夏大豆种植,种植区域气候湿热,雨水丰沛[35]。
3.2 大豆叶片水力导度和气孔导度协调变化以适应不同的生长光强
弱光对大豆光合特性有很大影响[9,36]。与前人的研究结果相同,低光强处理显著降低了大豆叶片的n、和s(表2)。leaf和s之间的联系是近年来的研究热点。在较高的生长光强下,夏威夷半边莲的leaf显著增加,而s无显著变化[37]。而在白桦树冠层上部的阳生叶到冠层下部的阴生叶中,leaf和s协同变化[38]。本研究结果与Sellin等[38]的结果一致,leaf和s在不同光强处理下均呈极显著相关,而且随着光强的降低,leaf和s紧密协调(图2),因此leaf是决定大豆CO2扩散的重要限制因子。而本研究结果与Scoffoni等[37]结果的差异可能与物种分布有关。夏威夷半边莲来自干旱地区,相比于s,leaf具有更高的可塑性让植物能够在不关闭气孔的情况下耐受短暂的土壤干旱[15]。leaf/s代表了叶片水分供应与需求的比值。与高光强相比,中、低光强处理下两个大豆的leaf/s显著增加,表明弱光下大豆叶片对水分需求的减少与水分供应的减少相匹配。气孔是leaf和s的共同影响因素,气孔保持开放的能力取决于叶片中稳定的水分状态,当Ψleaf降至一定的阈值后,就会引起气孔关闭,防止水分进一步散失[39]。Sellin等[40]报道阴生叶的Ψleaf低于阳生叶。在本研究中,Ψleaf在不同生长光强下无显著差异(图1),表明弱光并没有通过影响大豆叶片的水分供应情况来降低叶片水势进而影响叶片气孔的张开程度。
3.3 大豆叶脉性状对不同光强的适应机制
leaf对光强的长期适应主要由于叶片结构变化[29]。对双子叶植物大豆而言,水分从叶柄进入叶片后,首先通过主脉依次向下一级叶脉运输,并通过维管束鞘向叶肉细胞扩散[41]。由于木质部导管的存在,叶脉被直观地认为是leaf的决定因素[21]。一个高效、流畅的木质部导管对叶片水分运输非常重要[42]。同一物种的阳生叶比阴生叶具有更大的木质部导管[15],这与本研究结果一致(表3)。此外,相关性分析表明木质部导管大小与leaf显著正相关,leaf随着Xminor、Xmajor的减少而减少(表4)。North等通过Hagen-Poiseuille方程模拟计算叶脉内水力导度,在该方程中,叶脉内水力导度与木质部导管直径的四次方成正比[43]。因此木质部导管的微小减少会被放大,并影响叶片整体水分输送。
叶脉结构的等级特征导致不同级别的叶脉承担不同功能。主脉支撑和最大化铺开叶片,小叶脉以其显著的高密度为水转移至叶肉提供更大的接触面积[44]。在低光强处理下,大豆叶片维管组织发育不良,小叶脉变少、变细(图3-A),这与李盛蓝[30]、韩玲等[45]的研究结果一致。本研究中光强对叶脉密度的影响主要归因于小叶脉的变化,随着光强的降低南豆12和桂夏7的VLAminor显著减少,而南豆12的VLAmajor无显著变化(表3)。叶脉和气孔是叶片水分供给和散失的基础结构,二者的数量和形态深刻影响叶片水分利用率和碳同化[46]。本研究结果表明随着光强的降低大豆叶脉密度和气孔密度呈极显著正相关(表4)。叶脉密度和气孔密度的协同变化体现了气孔蒸腾对水分需求和叶脉结构对水分供给的相互匹配[47]。因此大豆叶脉密度与气孔密度随着光环境的不同协同变化,以适应不同的光强环境。
在喜荫和喜阳树种中,leaf与叶脉密度正相关[15]。本研究结果与之一致,VLAminor与leaf呈显著正相关(表4)。Xiong等[48]研究发现,在11个栽培和野生水稻品种中,leaf与叶脉密度不相关;North等[43]报道在低辐照度下生长的凤梨叶脉密度降低,但leaf与叶脉密度不相关。本研究结果与这些报道矛盾的原因可能是水稻和凤梨均属于单子叶植物,单子叶植物气孔总是聚集在平行脉两侧,这意味着叶脉密度的降低对叶片水分运输,尤其是叶脉外水分运输的影响可以忽略不计。与叶脉木质部的水力阻力相比,叶肉组织对水流的阻力非常大[15],Brodribb等[21]采集了43种植物(包括苔藓植物和被子植物),证明Dm与叶片水分运输阻力之间的强相关性,该水力阻力主要由叶脉密度所驱动。因此,水分从叶脉末端流经叶肉组织至气孔蒸发这段距离,深刻影响着leaf。这与本研究中Dm与leaf呈显著负相关吻合,两者的相关性由VLAminor驱动(表4)。因此,弱光下大豆VLAminor的降低不仅减少了水分转移至叶肉的接触面积,而且增加了水分从叶脉到表皮蒸发部位的输送距离,从而增加了叶片水分运输的阻力,导致leaf降低。前人对弱光下不同大豆品种光合能力评价的结果表明南豆12具有较强的耐荫性,属于强光合能力品种[25,49]。在本研究中,中、低光强处理下南豆12叶脉密度显著高于桂夏7,而且南豆12的leaf、s和i均显著高于桂夏7,较高的叶脉密度能够保证较好的叶片水分供应能力,有利于CO2的扩散和维持较高的i,从而提高光合速率。因此增加叶脉密度,缩短叶脉到气孔的水分运输距离是耐荫型大豆适应弱光环境的又一策略。
4 结论
随着光强降低,大豆leaf显著减少,而Ψleaf在不同光强处理下无显著差异。leaf和s在不同光强处理下呈极显著正相关。弱光引起大豆叶脉性状的变化,叶脉密度显著降低,叶脉到气孔的距离显著增加,木质部导管面积减小,从而导致leaf降低。因此,大豆叶脉性状会调整以适应不同光强环境下的叶片水分需求,弱光下对水分供应的减少与水分需求的减少相匹配。
[1] 蒋慕东. 二十世纪中国大豆改良、生产与利用研究[D]. 南京: 南京农业大学, 2006.
Jiang M D. Study on improvement, production and utilization of soybean in China during the 20th century[D]. Nanjing: Nanjing Agricultural University, 2006. (in Chinese)
[2] Iqbal N, Hussain S, Ahmed Z, YANG F, WANG X C, LIU W G, YONG T W, DU J B, SHU K, YANG W Y, LIU J. Comparative analysis of maize-soybean strip intercropping systems: a review. Plant production science, 2019, 22(2): 131-142.
[3] Long S P, Zhu X, Naidu S L, ORT D R. Can improvement in photosynthesis increase crop yields? Plant, cell & environment, 2006, 29(3): 315-330.
[4] Sagardoy R, Vázquez S, Florez-Sarasa I D, ALBACETEA, RIBAS-CARBÓ M, FLEXAS J, ABADÍA J, MORALES F. Stomatal and mesophyll conductances to CO2are the main limitations to photosynthesis in sugar beet () plants grown with excess zinc. The New phytologist, 2010, 187(1): 145-158.
[5] Limousin J, Misson L, Lavoir A, MARTIN N K, RAMBAL S. Do photosynthetic limitations of evergreenleaves change with long-term increased drought severity? Plant, cell & environment, 2010, 33(5): 863-875.
[6] Flexas J, Barón M, Bota J, DUCRUET J M, GALLÉ A, GALMÉS J, JIMÉNEZ M, POU A, RIBAS-CARBÓ M, SAJNANI C, TOMÀS M, MEDRANO H. Photosynthesis limitations during water stress acclimation and recovery in the drought-adaptedhybrid Richter-110 (×). Journal of experimental botany, 2009, 60(8): 2361-2377.
[7] GLEASON S M, WIGGANS D R, BLISS C A, COMAS L H, COOPER M, DEJONGE K C, YOUNG J S, ZHANG H H. Coordinated decline in photosynthesis and hydraulic conductance during drought stress in. Flora, 2017, 227: 1-9.
[8] 张强强. 水稻气孔导度和光合作用在不同光环境下的响应差异与机理[D]. 武汉: 华中农业大学, 2020.
Zhang Q Q. Studies on the mechanisms of the responses of stomatal conductance and photosynthesis to different light environments in[D]. Wuhan: Huazhong Agricultural University, 2020. (in Chinese)
[9] Fan Y F, Chen J X, Wang Z L, TAN T T, LI S L, LI J F, WANG B B, ZHANG J W, CHENG Y J, WU X L, YANG W Y, YANG F. Soybean (L. Merr.) seedlings response to shading: leaf structure, photosynthesis and proteomic analysis. BMC Plant Biology, 2019, 19(1): 34.
[10] 范元芳, 杨峰, 刘沁林, 谌俊旭, 王锐, 罗式伶, 杨文钰. 套作荫蔽对苗期大豆叶片结构和光合荧光特性的影响. 作物学报, 2017, 43(2): 277-285.
Fan Y F, Yang F, Liu Q L, Chen J X, Wang R, Luo S L, Yang W Y. Effects of shading on leaf structure and photosynthetic fluorescence characteristics of soybean seedlings in maize-soybean relay intercropping system. Acta Agronomica Sinica, 2017, 43(2): 277-285. (in Chinese)
[11] COCHARD H, VENISSE J, BARIGAH T S, BRUNEL N, HERBETTE S, GUILLIOT A, TYREE M, SAKR S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant physiology, 2007, 143(1): 122-133.
[12] NARDINI A, SALLEO S, ANDRI S. Circadian regulation of leaf hydraulic conductance in sunflower (L. cv Margot). Plant, Cell & Environment, 2005, 28(6): 750-759.
[13] Xiong D L, Douthe C, Flexas J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant, cell & environment, 2018, 41(2): 436-450.
[14] Rockwell F E, Holbrook N M, Zwieniecki M A. Hydraulic conductivity of red oak (L.) leaf tissue does not respond to light. Plant, cell & environment, 2011, 34(4): 565-579.
[15] Brodribb T J, Jordan G J. Water supply and demand remain balanced during leaf acclimation oftrees. The New phytologist, 2011, 192(2): 437-448.
[16] Nardini A, Gortan E, Salleo S. Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. Functional plant biology, 2005, 32(10): 953-961.
[17] Sack L, Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology, 2006, 87(2): 483-491.
[18] Lu Z F, Xie K L, Pan Y H, REN T, LU J W, WANG M, SHEN Q R, GUO S W. Potassium mediates coordination of leaf photosynthesis and hydraulic conductance by modifications of leaf anatomy. Plant, cell & environment, 2019, 42(7): 2231-2244.
[19] Huang G J, Zhang Q Q, Wei X H, PENG S B, LI Y. Nitrogen can alleviate the inhibition of photosynthesis caused by high temperature stress under both steady-state and flecked irradiance. Frontiers in Plant Science, 2017, 8: 945.
[20] Théroux-Rancourt G, Earles J M, Gilbert M E, ZWIENIECKI M A, BOYCE C K, MCELRONE A J, BRODERSEN C R. The bias of a two-dimensional view: comparing two- dimensional and three-dimensional mesophyll surface area estimates using noninvasive imaging. The New Phytologist, 2017, 215(4): 1609-1622.
[21] Brodribb T J, Feild T S, Jordan G J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant physiology, 2007, 144(4): 1890-1898.
[22] Xiong D L, Nadal M. Linking water relations and hydraulics with photosynthesis. The Plant Journal, 2020, 101(4): 800-815.
[23] 李晓鹏. 枣叶脉序及其功能性状研究[D]. 杨凌: 西北农林科技大学, 2016.
Li X P. Leaf venation characteristics and functional traits of Chinese jujube[D]. Yangling: Northwest A & F University, 2016. (in Chinese)
[24] Ye M, Wu M, Zhang H, ZHANG Z L, ZHANG Z J. High leaf vein density promotes leaf gas exchange by enhancing leaf hydraulic conductance inL. plants. Frontiers in Plant Science, 2021, 12: 693815.
[25] 武晓玲, 梁海媛, 杨峰, 刘卫国, 佘跃辉, 杨文钰. 大豆苗期耐荫性综合评价及其鉴定指标的筛选. 中国农业科学, 2015, 48(13): 2497-2507. doi:10.3864/j.issn.0578-1752.2015.13.002.
Wu X L, Liang H Y, Yang F, Liu W G, She Y H, Yang W Y. Comprehensive evaluation and screening identification indexes of shade tolerance at seedling in soybean. Scientia Agricultura Sinica, 2015, 48(13): 2497-2507. doi:10.3864/j.issn.0578-1752.2015.13.002. (in Chinese)
[26] 陈吉玉, 冯铃洋, 高静, 时健祎, 周雨晨, 涂发涛, 陈元凯, 杨文钰, 杨峰. 光照强度对苗期大豆叶片气孔特性及光合特性的影响. 中国农业科学, 2019, 52(21): 3773-3781. doi:10.3864/j.issn.0578-1752. 2019.21.006.
Chen J Y, Feng L Y, Gao J, Shi J Y, Zhou Y C, Tu F T, Chen Y K, Yang W Y, Yang F. Influence of light intensity on stoma and photosynthetic characteristics of soybean leaves. Scientia Agricultura Sinica, 2019, 52(21): 3773-3781. doi:10.3864/j.issn.0578-1752.2019. 21.006. (in Chinese)
[27] Sack L, Scoffoni C. Measurement of leaf hydraulic conductance and stomatal conductance and their responses to irradiance and dehydration using the evaporative flux method (EFM). Journal of Visualized Experiments, 2012, 70: 4179.
[28] Mamun M, Tang C, Sun Y, ISLAM M, LIU P, WANG X J, KANG Z S. Wheat genecontributes to stripe rust resistance. International Journal of Molecular Sciences, 2018, 19(6): 1666.
[29] Sack L, Scoffoni C. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. The New phytologist, 2013, 198(4): 983-1000.
[30] 李盛蓝, 谭婷婷, 范元芳, 杨文钰, 杨峰. 玉米荫蔽对大豆光合特性与叶脉、气孔特征的影响. 中国农业科学, 2019, 52(21): 3782-3793. doi:10.3864/j.issn.0578-1752.2019.21.007.
Li S L, Tan T T, Fan Y F, Yang W Y, Yang F. Effects of maize shading on photosynthetic characteristics, vein and stomatal characteristics of soybean. Scientia Agricultura Sinica, 2019, 52(21): 3782-3793. doi:10.3864/j.issn.0578-1752.2019.21.007. (in Chinese)
[31] 熊栋梁. 水稻叶片结构对水力导度与光合作用的影响及其机理[D]. 武汉: 华中农业大学, 2016.
Xiong D L. Coordination of leaf morpho-anatomical traits, photosynthesis and leaf hydraulic conductance in[D]. Wuhan: Huazhong Agricultural University, 2016. (in Chinese)
[32] Sack L, Holbrook N M. Leaf hydraulics. Annual Review of Plant Biology, 2006, 57: 361-381.
[33] Raimondo F, Trifilò P, Lo Gullo M A, Buffa R, Nardini A, Salleo S. Effects of reduced irradiance on hydraulic architecture and water relations of two olive clones with different growth potentials. Environmental and Experimental Botany, 2009, 66(2): 249-256.
[34] 吴海英, 张明荣. 不同播期对夏大豆南豆12产量和品质的影响. 大豆科技, 2009(6): 30-32.
Wu H Y, Zhang M R. Effects of different planting dates on yield and quality in summer soybean Nandou 12. Soybean Science and Technology, 2009(6): 30-32. (in Chinese)
[35] 韦清源, 陈渊, 汤复跃, 陈文杰, 郭小红, 梁江. 高产大豆新品种桂夏7号的选育及栽培技术要点. 种子, 2020, 39(2): 122-124, 127.
Wei Q Y, Chen Y, Tang F Y, Chen W J, Guo X H, Liang J. Breeding and cultivation techniques of a new high-yielding soybean variety Guixia 7. Seed, 2020, 39(2): 122-124, 127. (in Chinese)
[36] Yang F, Liu Q L, Cheng Y J, FENG L Y, WU X L, FAN Y F, RAZA M A, WANG X C, YONG T W, LIU W G, LIU J, DU J B, SHU K, YANG W Y. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biology, 2020, 20(1): 148.
[37] Scoffoni C, Kunkle J, Pasquet-Kok J, VUONG C, PATEL A J, MONTGOMERY R A, GIVNISH T J, SACK L. Light-induced plasticity in leaf hydraulics, venation, anatomy, and gas exchange in ecologically diverse Hawaiian lobeliads. The New Phytologist, 2015, 207(1): 43-58.
[38] Sellin A, Kupper P. Effects of light availability versus hydraulic constraints on stomatal responses within a crown of silver birch. Oecologia, 2005, 142(3): 388-397.
[39] Brodribb T J, Holbrook N M. Forced depression of leaf hydraulic conductance in situ: effects on the leaf gas exchange of forest trees. Functional Ecology, 2007, 21(4): 705-712.
[40] Sellin A, Ounapuu E, Kupper P. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (). Physiologia plantarum, 2008, 134(3): 412-420.
[41] Tholen D, Zhu X G. The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2diffusion. Plant physiology, 2011, 156(1): 90-105.
[42] Huang G J, Shu Y, Peng S B, LI Y. Leaf photosynthesis is positively correlated with xylem and phloem areas in leaf veins in rice () plants. Annals of Botany, 2022, 129(5): 619-631.
[43] North G B, Lynch F H, Maharaj F D R, PHILLIPS C A, WOODSIDE W T. Leaf hydraulic conductance for a tank bromeliad: axial and radial pathways for moving and conserving water. Frontiers in plant science, 2013, 4: 78.
[44] 龚容, 高琼. 叶片结构的水力学特性对植物生理功能影响的研究进展. 植物生态学报, 2015, 39(3): 300-308.
Gong R, Gao Q. Research progress in the effects of leaf hydraulic characteristics on plant physiological functions. Chinese Journal of Plant Ecology, 2015, 39(3): 300-308. (in Chinese)
[45] 韩玲, 赵成章, 冯威, 徐婷, 郑慧玲, 段贝贝. 张掖湿地芨芨草叶脉密度和叶脉直径的权衡关系对3种生境的响应. 植物生态学报, 2017, 41(8): 872-881.
Han L, Zhao C Z, Feng W, Xu T, Zheng H L, Duan B B. Trade-off relationship between vein density and vein diameter ofin response to habitat changes in Zhangye wetland. Chinese Journal of Plant Ecology, 2017, 41(8): 872-881. (in Chinese)
[46] Carins MURPHY M R, Jordan G J, Brodribb T J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant, cell & environment, 2012, 35(8): 1407-1418.
[47] 潘莹萍, 陈亚鹏. 叶片水力性状研究进展. 生态学杂志, 2014, 33(10): 2834-2841.
Pan Y P, Chen Y P. Recent advances in leaf hydraulic traits. Chinese Journal of Ecology, 2014, 33(10): 2834-2841. (in Chinese)
[48] Xiong D L, Yu T, Zhang T, LI Y, PENG S B, HUANG J L. Leaf hydraulic conductance is coordinated with leaf morpho-anatomical traits and nitrogen status in the genus. Journal of experimental botany, 2015, 66(3): 741-748.
[49] 王贝贝, 何乾瑞, 张佳伟, 王仲林, 范元芳, 杨文钰, 杨峰. 弱光下不同大豆品种光合能力综合评价. 大豆科学, 2021, 40(1): 45-58.
Wang B B, He Q R, Zhang J W, Wang Z L, Fan Y F, Yang W Y, Yang F. Comprehensive evaluation of photosynthetic capacity of different soybean varieties under low light. Soybean Science, 2021, 40(1): 45-58. (in Chinese)
Effect of Light Intensity on Leaf Hydraulic Conductivity and Vein Traits of Soybean at Seedling Stage
GAO Jing, CHEN JiYu, TAN XianMing, WU YuShan, YANG WenYu, YANG Feng
College of Agronomy, Sichuan Agricultural University/Key Laboratory of Crop Ecophysiology and Farming System in Southwest China, Ministry of Agriculture and Rural Affairs/Sichuan Engineering Research Center for Crop Strip Intercropping System, Chengdu 611130
【Objective】The objective of this study is to explore the effects of light intensity on leaf hydraulic conductivity, photosynthetic traits, and water potential in soybean seedlings, analyze the adaptive mechanisms of leaf vein traits in response to varying light intensities, and to provide theoretical support for enhancing future light energy utilization in soybean.【Method】Two soybean varieties, Nandou 12 (shade-tolerant) and Guixia 7 (shade-sensitive), were cultivated and placed in growth chambers. The plants were exposed to varying light conditions, including high light intensity (HL) at (424.47±12.32) µmol·m-²·s-¹, medium light intensity (ML) at (162.52±20.31) µmol·m-²·s-¹, and low light intensity (LL) at (93.93±9.87) µmol·m-²·s-¹. After a 20-day treatment period, the impacts of different light intensities on hydraulic conductivity, photosynthetic parameters, leaf water potential, and leaf vein traits in the seedling leaves of soybean were examined.【Result】Compared with HL treatment, the leaf hydraulic conductivity of Nandou 12 and Guixia 7 under LL treatment was significantly decreased, and the leaf hydraulic conductivity of Nandou 12 under the three treatments was significantly higher than that of Guixia 7 under the three treatments. Compared with HL treatment, the leaf hydraulic conductivity of Nandu 12 under ML and LL treatments decreased by 7.56% and 21.24%, stomatal conductance decreased by 43.96% and 58.89%, and net photosynthetic rate decreased by 29.44% and 46.49%, respectively. Similarly, the leaf hydraulic conductivity of Guixia 7 under the ML and LL treatments decreased by 42.16% and 23.71%, stomatal conductance decreased by 54.55% and 45.79%, and net photosynthetic rate decreased by 37.03% and 42.06%, respectively. Additionally, no statistically significant differences were observed in the leaf water potential of both soybean varieties across the various treatments. Notably, leaf hydraulic conductivity and stomatal conductance of soybean exhibited a highly significant positive correlation (<0.01) under the three light intensity treatments. As the light intensity decreased, a positive correlation was observed between leaf hydraulic conductivity and net photosynthetic rate (<0.05) as well as stomatal conductance (<0.01). Conversely, there was a noticeable decrease in the minor leaf vein density and the area of xylem conduits in major and minor veins under the ML and LL treatments for both soybean varieties. In the case of the minor leaf vein density and the area of xylem conduits in major veins, Nandou 12 exhibited significantly higher values than Guixia 7 under the ML and LL treatments. The major leaf vein density of Nandou 12 remained relatively stable across treatments, while that of Guixia 7 experienced a significant reduction of 11.4% and 15.0% under the ML and LL treatments compared to the HL treatment. Furthermore, a decrease in light intensity had a notable effect on increasing the distance between leaf veins and stomata. Specifically, under the ML and LL treatments, the distance from veins to stomata increased by 21.33% and 60.01% for Nandou 12 and by 31.50% and 53.59% for Guixia 7 in comparison to the HL treatment. The correlation analyses revealed significant positive correlations (<0.05) between the hydraulic conductivity of soybean leaves and the density of minor leaf veins, the area of xylem conduits in major and minor veins. Conversely, a significant negative correlation (<0.01) was observed between hydraulic conductivity and the distance from veins to stomata.【Conclusion】Light intensity exerts an influence on the leaf hydraulic conductivity by modulating the leaf vein structure of soybean. Under low light conditions, there is a reduction in leaf hydraulic conductivity in soybean; however, the coordination between leaf hydraulic conductivity and stomatal conductance is maintained to establish equilibrium between leaf water supply and demand as light intensity diminishes. The presence of a higher vein density under low light serves to abbreviate the distance required for water transport, thereby enhancing leaf water supply capacity. Consequently, this facilitates CO2diffusion and photosynthesis, representing an additional strategy employed by shade-tolerant soybean to acclimate to low-light environments.
soybean; leaf hydraulic conductivity; stomatal conductance; light intensity; leaf vein

10.3864/j.issn.0578-1752.2023.22.005
2023-03-27;
2023-05-04
国家重点研发计划(2022YFD2300902)、国家自然科学基金(32071963)
高静,E-mail:1787913440@qq.com。通信作者杨峰,E-mail:f.yang@sicau.edu.cn
(责任编辑 岳梅)
