PPR蛋白响应植物非生物胁迫的研究进展
2023-12-28李程路凯王才林张亚东
李程,路凯,王才林,张亚东
PPR蛋白响应植物非生物胁迫的研究进展
李程,路凯,王才林,张亚东
江苏省农业科学院粮食作物研究所/国家耐盐碱水稻技术创新中心华东中心/江苏省优质水稻工程技术研究中心/国家水稻改良中心南京分中心/江苏省农业生物学重点实验室,南京 210014
非生物胁迫是造成全球粮食减产的主要因素之一。研究植物逆境相关蛋白的功能及应答机制,对于提高作物抗逆性具有重要意义。三角状五肽重复(PPR)蛋白属于高等植物中最大的核编码蛋白家族,因其包含高度特异性的PPR基序而得名。依据基序类型及其排列,PPR蛋白可分为P和PLS两类,PLS类蛋白又可以根据其羧基末端的结构域进一步分为PLS、E、E+、DYW等亚类。PPR蛋白广泛分布于陆生植物中,主要定位于叶绿体和线粒体,亦有少数定位于细胞核中。作为序列特异性RNA结合蛋白,PPR蛋白参与植物RNA加工的多个方面,包括RNA编辑、RNA剪接、RNA稳定和RNA翻译。PPR蛋白在植物的整个生命进程中发挥多种重要作用,但对其在植物抗逆性中的作用机制还不清楚。本文在总结已有报道的非生物胁迫相关PPR蛋白定位和功能的基础上,重点综述了PPR蛋白参与调控植物非生物胁迫的作用机制(包括转录后调控和逆行信号),并对其进行讨论。转录后调控与PPR蛋白参与RNA转录后的修饰作用有关,其一般被认为通过结合RNA并调节细胞器RNA代谢来调控逆境相关基因的表达,从而影响植物抗逆性。逆行信号方面,PPR蛋白的损伤导致线粒体或叶绿体功能受损,然后产生各类逆行信号(如ROS),进而调控相关基因表达,抵御逆境。然而,由于质体中的逆行信号会受到许多环境因素的影响,这些因素部分还未明确,导致PPR蛋白在逆行信号中的作用机制仍有很多问题有待阐明。此外,PPR蛋白存在一因多效性,部分蛋白在作用于抗逆性的同时,还会对植物的生长和生殖产生重要影响。最后,本文阐述了利用PPR蛋白作为RNA编辑工具的研究现状,探讨了目前PPR蛋白响应植物非生物胁迫方面尚待解决的问题及研究前景,提出了未来研究仍需关注的重点和难点,为深入研究PPR蛋白的功能和作物非生物胁迫抗性育种提供参考。
PPR蛋白;植物;非生物胁迫
随着全球人口的不断增长,粮食安全问题日益突出[1]。由于无法移动,气候变化所带来的干旱、高温、土地盐碱化和紫外线辐射等非生物逆境对农作物的生长、发育和结实造成了严重的不良影响,现已成为全球农业减产的重要因素[2]。因此,解析植物抗逆性机制,提高作物的非生物胁迫抗性对农业生产至关重要。
植物的抗逆反应是一个复杂的调控过程,包括从胁迫信号感知到调节基因表达,再到产生功能性蛋白质,最后到形态和生理生化代谢上的一系列调整[3]。胁迫应答蛋白质决定了植物对非生物胁迫的抗性[3],近年来,大量与植物非生物胁迫响应相关的蛋白已被报道。由于细胞扩张驱动的生长与细胞壁的不断重塑有关,当逆境发生时,植物体内的类受体激酶(receptor-like kinases,RLKs)先通过感应细胞壁的变化调节逆境胁迫下的细胞生长[4]。胁迫一旦被感应,刺激信号就会立即被第二信使(钙离子、一氧化氮及不同类型的蛋白激酶等)传递和放大,以启动复杂的特异性信号级联反应。比如,钙结合蛋白可以检测到应激引起的细胞质钙离子浓度变化,并将信号传递给相互作用的蛋白激酶或直接与之融合的激酶,如钙依赖性蛋白激酶(calcium-dependent protein kinases,CDPKs),以进一步激活下游的应答过程[5]。值得一提的是,SNF1相关蛋白激酶(SnRKs)广泛介导高等植物在各种胁迫下的胁迫信号,是细胞能量稳态的主要调节因子[6]。胁迫信号经过感应和转导后会引起植物对胁迫的应答。其中,bHLH、ERF/AP2、MYB和WRKY等转录因子在非生物胁迫下可以快速反应,促进胁迫信号的转导并调节相关基因的表达[7]。如,的转录水平在非生物胁迫下显著提高,且能够通过直接结合的启动子来激活其表达,正向调节水稻的耐盐性[8]。产生非生物胁迫后,末端作用蛋白可以在胁迫条件下保护蛋白质生物活性,进而恢复细胞稳态,减轻非生物胁迫对植物的损害,例如胚胎发育晚期丰富蛋白(late embryogenesis abundant proteins,LEA)、热激蛋白(heat shock proteins,Hsps)和组成型光形态建成1(constitutively photomorphogenic 1,COP1)等均属于此类[9-11]。随着植物基因组学研究的不断深入,越来越多新的蛋白不断被报道和研究[12-15]。
三角状五肽重复(pentatricopeptide repeat,PPR)蛋白因包含一种三角状五肽重复结构域而得名,最早在拟南芥基因组测序分析中被发现[16]。PPR蛋白家族成员主要由细胞核基因编码,并以多个氨基酸螺旋重复序列简并串联为特征,这些重复序列堆叠在一起形成可识别RNA的延伸表面[17]。对PPR蛋白功能的单独或系统研究表明,PPR蛋白主要功能是与细胞器基因组转录产物特异RNA相结合,参与转录后修饰,以获得成熟的转录本[18-22]。PPR家族对RNA的修饰主要分为以下4种类型,包括RNA编辑:PPR蛋白特异识别编辑位点上游的顺式作用元件,并招募相关编辑因子共同起作用,将胞嘧啶(C)转化为尿嘧啶(U)或者将U转化为C,从而改变密码子,进而影响氨基酸序列的构成[23-25];RNA剪接:DNA转录后的前体RNA含有丰富的内含子,PPR蛋白从最初转录产物中去除顺式和反式内含子,并将外显子拼接成为成熟的RNA[26-28];RNA稳定:PPR蛋白可以修饰mRNA前体的末端,阻碍RNA外切酶的活性,使其形成稳定的单顺反子进行表达,以及结合在多顺反子转录物中开放阅读框(open reading frame,ORF)之间,处理产生的末端[29-31];RNA翻译:PPR蛋白能够结合ORF的5′端特定非翻译区(untranslated region,UTR),激活mRNA的翻译调控[28, 32-33]。
本文收集了近年来与植物非生物胁迫响应相关PPR蛋白的信息,综述了其对植物抗逆性的影响和作用机制,并就未来PPR蛋白在作物非生物胁迫抗性育种上的研究应用进行了展望。
1 PPR蛋白的结构和种类
PPR蛋白的基本结构特征是在其氨基末端区域存在一个由35个氨基酸组成的重复序列基序——PPR基序[16]。因PPR基序具有高度特异性,根据基序类型及其排列,PPR蛋白可分为P和PLS两类,P类蛋白仅含有35个氨基酸的典型P基序,而PLS类蛋白是由P-、L-和S-基序组成串联重复的PLS三联体[34]。PLS类蛋白大多具有高度保守的E、E+或DYW结构域的羧基末端延伸,因此,PLS类蛋白可以根据其羧基末端的结构域进一步分为PLS、E、E+和DYW亚类[35]。此外,有人根据第一个螺旋的差异将P基序分为P1和P2;根据第二个螺旋的不同将L基序分为L1(35个氨基酸)和L2(36个氨基酸);同样,S基序也可以分为S1(31个氨基酸)和S2(32个氨基酸);还有一种SS基序,其同时存在与S1和P1基序重叠的序列[18]。目前,也有一些报道称少数P类PPR蛋白的C末端包含额外的特征序列,如小MutS相关(small MutS-related,SMR)结构域,LAGLIDADG(His-Cys box and GIY-YIG,H-N-H)结构域和RNA识别结构域(RNA recognition motif,RRM)等(图1)[36-39]。晶体结构解析发现,PPR蛋白的每个重复基序通常会形成一对稳定反向平行的α螺旋结构,多个串联基序还可进一步螺旋化形成右手超螺旋结构,从而与相关蛋白发生互作[40-41]。
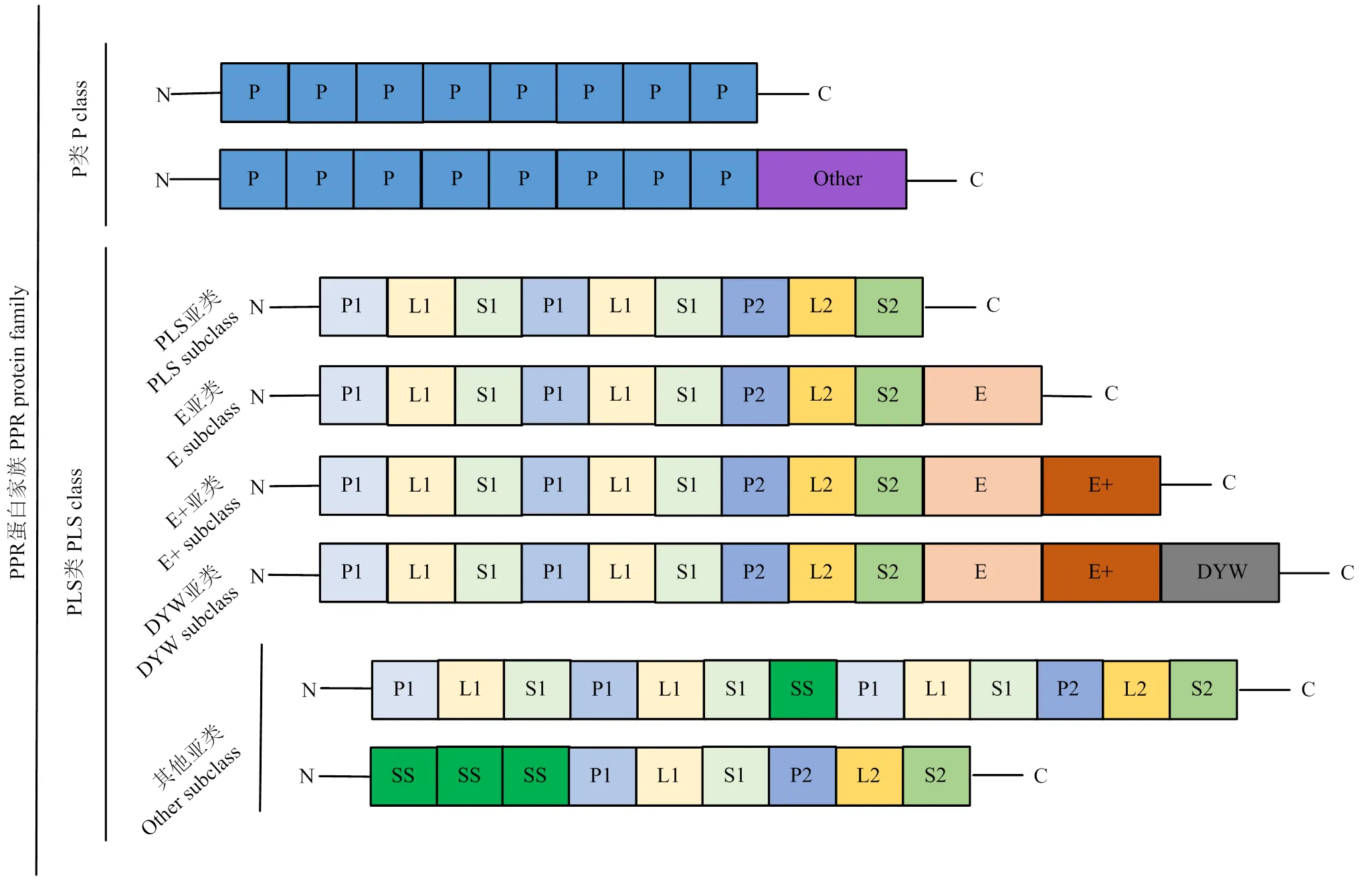
图1 PPR蛋白家族主要分类
2 PPR蛋白分布和定位
PPR蛋白主要存在于真核生物中,且在陆生植物中数量最多,原核生物中的数量很少[35]。有研究表明,PPR蛋白在陆地植物中的多数量与其调控细胞器mRNA从C到U的转录后编辑功能有关[42]。然而,同样是陆生植物,低等的苔藓中仅有103个PPR基因,而在高等的被子植物(如在拟南芥和水稻)中,PPR基因却多达400个以上,因此,推测PPR基因的数量随着植物从低等到高等进化的过程中不断增加和分化[43]。
大多数PPR蛋白的N末端具有定位信号序列[44]。在高等植物中,PPR蛋白多位于线粒体或叶绿体,也有极少数在细胞核中行使功能[45-46]。如,拟南芥MTL1蛋白定位于线粒体中,影响线粒体NADH脱氢酶亚基7(nad7)mRNA的翻译;AtECB2定位于叶绿体的类囊体膜上,影响拟南芥叶绿体中多个基因转录本的编辑;细胞核定位的OsNPPR1参与了线粒体发育且影响水稻胚乳发育[47]。此外,还有少数PPR蛋白存在双定位模式,如水稻OsPGL1蛋白(pale green leaf 1)同时定位于叶绿体和线粒体,其功能缺失会影响叶绿体和线粒体RNA编辑缺陷[48],而拟南芥PNM1蛋白则同时定位于细胞核和线粒体,可能在线粒体和细胞核之间的基因表达调节中发挥作用[49]。因此,明确PPR蛋白的亚细胞定位将有助于揭示PPR蛋白的功能。
3 PPR蛋白对植物抗逆性的影响
叶绿体和线粒体是植物细胞中半自主性的细胞器,能够感受逆境信号,在植物响应内外界环境变化的逆向信号传导过程中发挥着重要的功能,因此,定位于叶绿体或线粒体中的PPR蛋白很可能与植物非生物胁迫有关。在拟南芥中,已有10个以上的PPR蛋白被证明对非生物胁迫有反应。如,在叶绿体中,PPR蛋白RARE1负责-C794位点的编辑,其与植物耐热性有关,人工提高C794编辑的表达能够增强拟南芥的耐热性[50];核编码叶绿体PPR蛋白SVR7参与拟南芥抗氧化胁迫反应,其突变体积累更多活性氧(reactive oxygen species,ROS),并表现出较低的光氧化应激耐受性[51];GUN1亦是一种叶绿体定位的PPR蛋白,突变体对蔗糖和脱落酸(ABA)高度敏感,同时,也表现出对去黄化过程中引起的光氧化应激更敏感的表型[52-55],最新研究表明,GUN1是氧化细胞环境所必需的,通过依赖氧化还原的质体-核通讯参与了植物基础耐热性的获得[56-57]。此外,拟南芥中还存在相当数量线粒体定位的PPR蛋白,如PPR40[58]、ABO5[59]、ABO8[60]、PGN[61]、AHG11[62]、SLG1[63]、SLO2[64]、PPR96[65]、POCO1[66]和LOI1/ MEF11[67]均参与多种非生物胁迫的响应(表1)。在细胞质-核双定位的PPR蛋白中,SOAR1被证实是植物对非生物胁迫反应的一个正调节因子,与ABA信号传递和拟南芥对干旱、盐和冷胁迫的耐受性有关[68]。此外,PPR蛋白GEND1和PPR2都与拟南芥的耐热性有关,其突变体植株在高温下表现出高度敏感表型,但这些蛋白的准确定位还未见报道[69-70]。
近年来,除了模式植物,在以水稻为代表的大田作物中,关于PPR蛋白参与非生物胁迫的研究也越来越多。Chen等[71]通过全基因组分析在水稻中共发现491个PPR基因,表达谱分析表明,大量PPR基因在非生物胁迫下被诱导,其中,盐胁迫和干旱胁迫下分别有75和73个PPR基因表达上调,暗示这些PPR蛋白可能在水稻对非生物胁迫的反应中发挥作用。低温胁迫下,2种定位于叶绿体的PPR蛋白OsV4和TCD10是水稻幼苗早期叶绿体发育所必需的[72-73]。同样是定位于叶绿体的PPR蛋白WSL,其突变体在发育早期对ABA、盐和糖的敏感性增强,H2O2积累量提高[74]。此外,2种定位于线粒体的PPR蛋白PPS1和OsNBL3也被证实与水稻非生物胁迫有关,其中,的抑制导致水稻对高盐度和ABA的敏感性显著增加[75],而的抑制却导致水稻对盐胁迫的耐受性增加[76]。最近,Luo等[77]验证了2个定位于线粒体的PPR蛋白PPR035和PPR406在耐旱中的功能,和突变体均对干旱和盐胁迫表现出较强的耐受性,在提高水稻抗旱性方面具有很大的应用前景;LU等[78]在水稻中分别过表达和其同源基因,转基因植株在幼苗生长阶段的耐盐性得到增强,表明SOAR1同源PPR蛋白可通过转基因操作用于盐胁迫条件下水稻的作物改良。Su等[79]在大豆基因组中鉴定出179个DYW亚群PPR基因,并发现在盐胁迫和干旱胁迫下被诱导表达,其过表达转基因植株对干旱胁迫的耐受性增强。
4 PPR蛋白参与植物非生物胁迫调控的作用机制
4.1 转录后途径
PPR家族蛋白作为一类反式作用因子,主要参与RNA转录后的修饰,通过结合RNA并调节细胞器RNA代谢来调控基因的表达[42, 80-81]。一些与植物非生物胁迫响应相关的PPR蛋白已被证明在细胞器RNA转录后调控中发挥作用。拟南芥的突变会导致多个RNA编辑缺陷,在突变体中,ABA信号通路相关基因表达下调,进一步影响了许多与胁迫相关,特别是与干旱相关的基因表达,这与突变体的干旱敏感性增加一致[66]。另有研究表明,线粒体RNA编辑因子SLO2影响植物对ABA和非生物胁迫的敏感性,突变体中核编码的非生物胁迫响应基因和线粒体复合物Ⅰ基因()及替代呼吸途径相关基因的表达增加,进一步支持了线粒体RNA编辑事件和应激反应两者之间的联系[64]。水稻的突变会导致水稻叶绿体转录本剪接的重大缺陷,突变体中,异常转录本积累及其产物减少,质体编码聚合酶依赖的质体基因表达明显下调,质体rRNAs和翻译产物积累到非常低的水平,这表明翻译效率的降低可能会影响突变体对非生物胁迫的反应[74]。最新研究发现,主要参与线粒体基因内含子4的剪接,其突变会导致线粒体的破坏和交替呼吸途径的增加,从而产生类病变表型,增强水稻对盐的抗性和耐受性[76]。Xiong等[82]研究表明水稻细胞质雄性不育系与其保持系之间的RNA编辑的差异是导致它们在环境胁迫下表现不同的原因之一,并证实了PPR基因介导的RNA编辑与水稻非生物胁迫耐受性的潜在关系。

表1 参与调节植物非生物胁迫反应的部分PPR蛋白
4.2 逆行信号
一般来说,细胞器的发育和基因表达受核基因组调控,但来自叶绿体和线粒体的信号亦可“逆行”调控核基因的表达,这样的调控信号被称为“逆行信号”[83]。根据其功能含义,质体逆行信号被分为与质体发育相关的信号和与响应环境或代谢波动的操作微调有关的信号[84]。研究表明,PPR蛋白的损伤能够导致线粒体或叶绿体功能受损,产生各类逆行信号(如ROS),从而调控抗逆相关基因表达[85]。最典型的例子是拟南芥PPR蛋白GENOMES UNCOUPLED 1(GUN1),被鉴定为叶绿体到细胞核逆行信号通路的中心整合因子[86]。GUN1的失活会在一定条件下抑制与光合作用相关的核基因(photosynthesis associated nuclear genes,)的表达,从而促进逆行信号传导。突变体植株更容易受到叶绿体干扰因素的影响,包括光、质体翻译抑制剂林可霉素(Linc)和类胡萝卜素生物合成抑制剂去氟拉松(NF)处理[52-53]。一般来说,GUN1可能通过3条经典的逆行信号通路调控的表达:四吡咯生物合成途径(tetrapyrrole biosynthesis pathway,TPB)、氧化还原反应和质体基因表达(plastid gene expression,PGE)[87]。最近,Wu等[88]提出一个新模型:当叶绿体发育过程中遭遇逆境时,GUN1通过与cpHSC70-1互作来增强质体的蛋白输入,未及时转运进叶绿体的前体蛋白在细胞质中过度积累,诱导细胞质中HSP90蛋白表达上调,进而维持表达。
除了cpHSC70-1伴侣外,还有许多其他假定的GUN1相互作用蛋白被陆续提出,包括叶绿体核糖体蛋白S1(plastid ribosomal protein S1,PRPS1)[89]、多细胞器RNA编辑因子(multiple organellar rna editing factor,MORF)[90]、核编码RNA聚合酶(nuclear-encoded rna polymerase,NEP)[91]和各种四吡咯[92]等。研究表明,GUN1能够在蛋白水平上控制叶绿体核糖体蛋白PRPS1的积累,并和参与叶绿体蛋白稳态的蛋白质相互作用,而PRPS1的功能是质体mRNA翻译和耐热性所必需的[89]。MORF蛋白家族是线粒体和叶绿体中RNA编辑体系的重要组成部分,几乎所有位点的完全编辑都需要MORF蛋白[93]。最新研究表明,GUN1通过与MORF2发生物理相互作用,直接影响叶绿体RNA中多个位点的编辑效率,并调节核编码的叶绿体RNA聚合酶的活性,特别是在逆行信号传递过程中[91, 94]。然而,过表达系只有在使用NF处理时才能看到表型,而使用Linc处理时却无法看到表型,因此,突变对质体RNA编辑影响的潜在机制基础和功能意义仍有待阐明[90]。这些相互作用支持GUN1可能作为一种支架蛋白,在各种生物环境中促进蛋白质复合物的形成的假设[95]。但是,由于叶绿体信号受到许多环境因素的影响,其中一些因素大多是未知的,难以控制的,导致不同实验室对同一突变体得到的结果不同,因此,GUN1作为逆行通讯和细胞核信号通路的中心调节器的功能仍然有很多未解决的问题[86, 96]。部分观点认为,GUN1能够通过靶向细胞核中的多种转录因子,包括ABSCISIC ACID INSENSITIVE4(ABI4)、GOLDEN2-LIKE 1/2(GLK1/2)和ELONGATED HYPOCOTYL 5(HY5),将信息传递到细胞核[97-100],这些核转录调控因子被认为是GUN1所涉及逆境信号中的一个重要的下游成分,它们的突变体在的解偶联表达方面与突变体表现相似[101]。Veciana等[102-103]研究发现,BBX16作为GLK1的一个直接靶点,其在叶绿体损伤后通过GUN1/GLK1模块被抑制,调节暴露在破坏性强光下的区域。总之,这些研究表明GUN1对于叶绿体RNA代谢和叶绿体-核逆行信号非常重要[104]。
近年来,亦有研究表明,其他PPR蛋白通过逆行信号调控植物抗逆性。如,拟南芥线粒体PPR蛋白LOI1参与呼吸链相关基因、和的RNA编辑,并调节类异戊二烯的生物合成,突变体对2种类异戊二烯合成抑制剂(真菌植物毒素洛伐他汀和除草剂氯马松)的敏感性降低,从而引起胞质甲戊酸(cytosolic mevalonate,MVA)途径和质体非甲戊酸(plastidal non-mevalonate,MEP)途径的改变,已知此类途径会影响防御基因的表达,以应对损伤,揭示了从线粒体到细胞质逆行信号的间接作用[105-106];PPR蛋白PGN的失活会引起拟南芥线粒体编码转录物差异表达,最显著的是和表达水平的升高,它们在线粒体功能改变或抑制诱导的逆行信号传导中发挥作用,突变体幼苗内源ABA和盐胁迫下的ROS积累增加,因此,PGN可能通过转录编辑协调影响整体线粒体基因表达,从而有助于植物防御和维持细胞氧化还原平衡[61];PPR蛋白AHG11和SLG1分别参与线粒体和的编辑,和是线粒体中电子传递链复合体Ⅰ的2个亚基,和的突变致使线粒体功能的部分损伤诱导氧化还原失衡,突变体植株表现出对干旱等非生物胁迫敏感性增强[62-63];另有研究发现,ABA缺失抑制位点HAS2编码参与线粒体RNA编辑的PPR蛋白LOI1/ MEF11,证明了ABA在线粒体逆行信号调节中起着重要作用[67]。
5 PPR蛋白的多效性
目前,鉴定到的很多PPR基因都具有一因多效性,通常在影响植物适应非生物胁迫的同时,对植物的生长和生殖也会产生重要影响。例如,水稻突变体在三叶期表现为白化表型和叶绿体异常,这种现象与叶绿素含量和叶绿体发育变化有关,且受温度影响[72];同样,与低温相关的PPR蛋白基因突变体亦表现出白化和叶绿体畸形[73];拟南芥突变体不仅表现出光氧化应激耐受性降低,而且其幼期叶绿素含量也降低,呈现淡绿色表型[51],这些PPR蛋白在叶绿体早期发育过程中起重要的作用,其功能的缺失会影响叶绿体的发育,从而影响叶片生长。此外,影响线粒体功能的PPR蛋白亦被证明具有一因多效性。例如,ABO8不仅和拟南芥对ABA的敏感性有关,还能够通过ABA介导的线粒体ROS调控拟南芥根的分生组织活性[60];突变体植株不仅根系变短,分生组织大小和细胞数量减少,还表现出对高温的敏感性增强,功能分析表明,GEND1能够结合并编辑线粒体mRNA,其突变会导致拟南芥细胞色素c水平降低[69];的突变会使植株产生类病变表型(自发的细胞死亡反应和H2O2积累,对真菌和细菌病原体稻瘟病菌和水稻黄单胞菌的抗性增强),同时增强了水稻对盐胁迫的耐受性[76]。POCO1被证明能够影响拟南芥的开花时间,在长、短日照条件下,突变体均表现出早花表型,后续研究表明,POCO1还参与了拟南芥的抗旱,但二者之间的联系还未见报道[107]。
6 展望
以往研究综述大多针对PPR蛋白的起源、分类、定位,以及在植物生长发育中的功能。本文综述了近年来PPR蛋白在植物非生物胁迫中的功能研究进展,并总结分析了PPR蛋白参与植物非生物胁迫调控的分子机制,以期为作物非生物胁迫分子育种提供参考。尽管已经鉴定出多种植物PPR蛋白,但与数目众多的PPR成员相比,还有很多发挥重要功能的PPR蛋白未被研究,它们是否和植物非生物胁迫抗性有关?此外,由于PPR蛋白基因普遍存在的一因多效性,当利用PPR蛋白改良作物抗逆性时,需要注意其对作物其他生理功能的影响,这也是应用PPR蛋白育种时的重点和难点之一。
因为PPR蛋白在植物线粒体和叶绿体中负责C-to-U和U-to-C的RNA编辑,已有研究将其作为RNA编辑工具来利用[25]。如,Oldenkott等[108]通过表达来自肾叶白头翁()的含有单个DYW结构域的PPR蛋白,在大肠杆菌中构建了C-to-U RNA编辑;Ichinose等[25]成功开发了一种基于DYW:KP蛋白的U-to-C RNA编辑因子,该因子在细菌和人类细胞中起作用。在临床治疗中,相比于现在流行的以CRISPR-Cas9为代表的DNA编辑而言,RNA编辑更灵活、更安全,因为RNA编辑其通常只在特定的细胞类型中或在特定的时间表达,预期脱靶导致的编辑副作用更少,而且由于基因组序列不受影响,错误的RNA编辑也不会影响胎儿发育,停止治疗后,突变的RNA会迅速降解[109]。这些研究为未来的基因治疗和作物改良提供了参考。
近年来,虽然PPR蛋白的研究取得了重要的进展,但关于PPR蛋白参与植物非生物胁迫响应中的分子机制还不十分清楚,需要进一步深入探究。首先,PPR蛋白在调节植物非生物胁迫过程中是否存在时空性和组织特异性?其次,PPR蛋白之间如何相互作用或与其他蛋白相互作用以实现其最终功能,这些相互作用又是如何在细胞器RNA转录后加工中影响PPR活性的?第三,PPR蛋白是如何特异识别并与RNA结合的?最后,PPR蛋白在陆生植物核-细胞质相互作用的逆行信号调控网络中的具体角色是什么?也许大量的进化分析和更多其他PPR蛋白的功能鉴定,以及对共表达PPR基因的进一步分析将揭示这些问题的答案。然而,为了更详细地阐明PPR蛋白在植物非生物胁迫抗性中的具体机制,有必要对PPR蛋白的RNA靶点进行鉴定,并对蛋白质-RNA复合物的晶体结构进行分析,同时,还应加强对MORF等直接影响PPR蛋白作用的相互作用蛋白给予关注。这些研究将有助于更好地阐明PPR蛋白在植物非生物胁迫下的调控网络和特异性,有助于了解植物细胞器RNA加工的细节,从而为作物育种改良提供支撑。值得期待的是,人工PPR蛋白可以被定制并在体内结合特定的内源性RNA,这为开发用于分子设计育种的RNA结合蛋白(RNA binding protein,RBPs)提供了广阔的研究前景[110]。
[1] Gong Z Z, Xiong L M, Shi H Z, Yang S H, Herrera- Estrella L R, Xu G H, Chao D Y, Li J R, Wang P Y, Qin F,LI J, DING Y L, SHI Y T, WANG Y, YANG Y Q, GUO Y, ZHU J K. Plant abiotic stress response and nutrient use efficiency, Science China Life Sciences, 2020, 63(5): 635-674.
[2] Chang Y N, Zhu C, Jiang J, Zhang H M, Zhu J K, Duan C G. Epigenetic regulation in plant abiotic stress responses. Journal of integrative plant biology, 2020, 62(5): 563-580.
[3] 陈柯岐, 邓星光, 林宏辉, 植物响应非生物胁迫的分子机制, 生物学杂志, 2021, 38(6): 1-8.
Chen K Q, Deng X G, Lin H H. Molecular mechanisms of plant in response to abiotic stress, Journal of Biology, 2021, 38(6): 1-8. (in Chinese)
[4] Zhang H, Zhao Y, Zhu J K. Thriving under stress: how plants balance growth and the stress response, Developmental Cell, 2020, 55(5): 529-543.
[5] YUAN F, YANG H M, XUE Y, KONG D D, YE R, LI C J, ZHANG J Y, THEPRUNGSIRIKUL L, SHRIFT T, KRICHILSKY B, JOHNSON D M, SWIFT G B, HE Y K, SIEDOW J N, PEI Z M. OSCA1 mediates osmotic-stress-evoked Ca2+increases vital for osmosensing in. Nature, 2014, 514(7552): 367-371.
[6] JAMSHEER K M, JINDAL S, LAXMI A. Evolution of TOR-SnRK dynamics in green plants and its integration with phytohormone signaling networks. Journal of Experimental Botany, 2019, 70(8): 2239-2259.
[7] YOON Y, SEO D H, SHIN H, KIM H J, KIM C M, JANG G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy, 2020, 10(6): 788.
[8] ZHANG M X, ZHAO R R, WANG H T, REN S L, SHI L Y, HUANG S Z, WEI Z Q, GUO B Y, JIN J Y, ZHONG Y, CHEN M J, JIANG W Z, WU T, DU X L. OsWRKY28 positively regulates salinity tolerance by directly activating OsDREB1B expression in rice. Plant Cell Reports, 2023, 42(2): 223-234.
[9] Haq S u, Khan A, Ali M, Khattak A M, Gai W X, Zhang H X, Wei A M, Gong Z H. Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. International journal of molecular sciences, 2019, 20(21): 5321.
[10] Kim J Y, Jang I C, Seo H S. COP1 controls abiotic stress responses by modulating AtSIZ1 function through its E3 ubiquitin ligase activity. Frontiers in plant science, 2016, 7: 1182.
[11] LIANG Y, KANG K, GAN L, NING S B, XIONG J Y, SONG S Y, XI L Z, LAI S Y, YIN Y T, GU J W, XIANG J, LI S S, WANG B S, LI M T. Drought‐responsive genes, late embryogenesis abundant group3 (LEA 3) and vicinal oxygen chelate, function in lipid accumulation inandmainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnology Journal, 2019, 17(11): 2123-2142.
[12] YE Y Y, DING Y F, JIANG Q, WANG F J, SUN J W, ZHU C. The role of receptor-like protein kinases (RLKs) in abiotic stress response in plants. Plant cell reports, 2017, 36(2): 235-242.
[13] HAN G L, LU C X, GUO J R, QIAO Z Q, SUI N, QIU N W, WANG B S. C2H2zinc finger proteins: master regulators of abiotic stress responses in plants. Frontiers in plant science, 2020, 11: 115.
[14] Yu Z Y, Wang X, Zhang L S. Structural and functional dynamics of dehydrins: a plant protector protein under abiotic stress. International Journal of Molecular Sciences, 2018, 19(11): 3420.
[15] Robles P, Quesada V. Unveiling the functions of plastid ribosomal proteins in plant development and abiotic stress tolerance. Plant Physiology and Biochemistry, 2022, 189: 35-45.
[16] Small I D, Peeters N. The PPR motif-a TPR-related motif prevalent in plant organellar proteins. Trends in biochemical sciences, 2000, 25(2): 45-47.
[17] Rovira A G, Smith A G. PPR proteins-orchestrators of organelle RNA metabolism. Physiologia plantarum, 2019, 166(1): 451-459.
[18] CHENG S F, GUTMANN B, ZHONG X A, YE Y T, FISHER M F, BAI F Q, CASTLEDEN I, SONG Y E, SONG B, HUANG J Y, LIU X, XU X, LIM B L, BOND C S, YIU S M, SMALL I. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. The Plant Journal, 2016, 85(4): 532-547.
[19] Khrouchtchova A, Monde R A, Barkan A. A short PPR protein required for the splicing of specific group Ⅱ introns in angiosperm chloroplasts. Rna, 2012, 18(6): 1197-1209.
[20] Meierhoff K, Felder S, Nakamura T, Bechtold N, Schuster G. HCF152, anRNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. The Plant Cell, 2003, 15(6): 1480-1495.
[21] Yamazaki H, Tasaka M, Shikanai T. PPR motifs of the nucleus-encoded factor, PGR3, function in the selective and distinct steps of chloroplast gene expression in. The Plant Journal,2004, 38(1): 152-163.
[22] Li X L, Sun M D, Liu S J, Teng Q A, Li S H, Jiang Y S. Functions of PPR proteins in plant growth and development. International Journal of Molecular Sciences, 2021, 22(20): 11274.
[23] ANDRÉS-COLÁS N, ZHU Q A, TAKENAKA M, DE RYBEL B, WEIJERS D, VAN DER STRAETEN D. Multiple PPR protein interactions are involved in the RNA editing system inmitochondria and plastids. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(33): 8883-8888.
[24] GUILLAUMOT D, LOPEZ-OBANDO M, BAUDRY K, AVON A, RIGAILL G, FALCON DE LONGEVIALLE A, BROCHE B, TAKENAKA M, BERTHOMÉ R, DE JAEGER G, DELANNOY E, LURIN C. Two interacting PPR proteins are majorediting factors in plastid and mitochondria. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(33): 8877-8882.
[25] ICHINOSE M, KAWABATA M, AKAIWA Y, SHIMAJIRI Y, NAKAMURA I, TAMAI T, NAKAMURA T, YAGI Y, GUTMANN B. U-to-C RNA editing by synthetic PPR-DYW proteins in bacteria and human culture cells. Communications Biology, 2022, 5(1): 968.
[26] CHEN X Z, FENG F, QI W W, XU L M, YAO D S, WANG Q SONG R T. Dek35 encodes a PPR protein that affects cis-splicing of mitochondrial nad4 intron 1 and seed development in maize. Molecular Plant, 2017, 10(3): 427-441.
[27] SUN F, ZHANG X Y, SHEN Y, WANG H C, LIU R, WANG X M, GAO D H, YANG Y Z, LIU Y W, TAN B C. The pentatricopeptide repeat protein EMPTY PERICARP8 is required for the splicing of three mitochondrial introns and seed development in maize. The Plant Journal, 2018, 95(5): 919-932.
[28] LEGEN J, RUF S, KROOP X, WANG G W, BARKAN A, BOCK R, SCHMITZ-LINNEWEBER C. Stabilization and translation of synthetic operon-derived mRNA s in chloroplasts by sequences representing PPR protein-binding sites. The Plant Journal, 2018, 94(1): 8-21.
[29] ZHANG Y F, SUZUKI M, SUN F, TAN B C. The mitochondrion- targeted PENTATRICOPEPTIDE REPEAT78 protein is required for nad5 mature mRNA stability and seed development in maize. Molecular Plant, 2017, 10(10): 1321-1333.
[30] WANG C D, AUBÉ F, PLANCHARD N, QUADRADO M, DARGEL- GRAFFIN C, NOGUÉ F, MIREAU H. The pentatricopeptide repeat protein MTSF2 stabilizes a nad1 precursor transcript and defines the 3΄ end of its 5΄-half intron. Nucleic Acids Research, 2017, 45(10): 6119-6134.
[31] LEE K, HAN J H, PARK Y I, COLAS DES FRANCS-SMALL C, SMALL I, KANG H. The mitochondrial pentatricopeptide repeat protein PPR19 is involved in the stabilization of NADH dehydrogenase 1 transcripts and is crucial for mitochondrial function anddevelopment. New Phytologist, 2017, 215(1): 202-216.
[32] HAÏLI N, PLANCHARD N, ARNAL N, QUADRADO M, VRIELYNCK N, DAHAN J, FRANCS-SMALL C C D, MIREAU H. The MTL1 pentatricopeptide repeat protein is required for both translation and splicing of the mitochondrial NADH DEHYDROGENASE SUBUNIT7 mRNA in. Plant Physiology, 2016, 170(1): 354-366.
[33] ZOSCHKE R, WATKINS K P, MIRANDA R G, BARKAN A. The PPR-SMR protein PPR53 enhances the stability and translation of specific chloroplast RNA s in maize. The Plant Journal, 2016, 85(5): 594-606.
[34] LURIN C, ANDREÉS C, AUBOURG S, BELLAOUI M, BITTON F, BRUYÈRE C, CABOCHE M, DEBAST C, GUALBERTO J, HOFFMANN B. Genome-wide analysis ofpentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. The Plant Cell, 2004, 16(8): 2089-2103.
[35] 李景芳, 王宝祥, 刘艳, 刘金波, 陈庭木, 孙志广, 杨波, 邢运高, 迟铭, 徐波. PPR蛋白在水稻生长发育中的功能研究进展. 植物遗传资源学报, 2022, 23(2): 358-367.
LI J F, WANG B X, LIU Y, LIU J B, CHEN T M, SUN Z G, YANG B, XING Y G, CHI M, XU B. Progress of research in functions of PPR proteins in growth and development of rice. Journal of Plant Genetic Resources, 2022, 23(2): 358-367. (in Chinese)
[36] ZHANG Y, LU C. The enigmatic roles of PPR-SMR proteins in plants. Advanced Science, 2019, 6(13): 1900361.
[37] ZHANG J H, GUO Y P, FANG Q, ZHU Y L, ZHANG Y, LIU X J, LIN Y J, BARKAN A, ZHOU F. The PPR-SMR protein ATP4 is required for editing the chloroplastmRNA in rice and maize. Plant Physiology, 2020, 184(4): 2011-2021.
[38] LONGEVIALLE A F D, HENDRICKSON L, TAYLOR N L, DELANNOY E, LURIN C, BADGER M, MILLAR A H, SMALL I. The pentatricopeptide repeat genewith two LAGLIDADG motifs is required for the cis-splicing of plastidintron 2 in. The Plant Journal, 2008,56(1): 157-168.
[39] SCHMITZ-LINNEWEBER C, WILLIAMS-CARRIER R E, WILLIAMS- VOELKER P M, KROEGER T S, VICHAS A, BARKAN A. A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplastpre-mRNA. The Plant Cell, 2006, 18(10): 2650-2663.
[40] YIN P, LI Q X, YAN C Y, LIU Y, LIU J J, YU F, WANG Z, LONG J F, HE J H, WANG H W, WANG J W, ZHU J K, SHI Y G, YAN N. Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature, 2013, 504(7478): 168-171.
[41] YAN J, ZHANG Q, YIN P. RNA editing machinery in plant organelles. Science China Life Sciences, 2018, 61(2): 162-169.
[42] BARKAN A, SMALL I. Pentatricopeptide repeat proteins in plants. Annual Review of Plant Biology, 2014, 65: 415-442.
[43] FUJII S, SMALL I. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytologist, 2011, 191(1): 37-47.
[44] 王婉珍, 任育军, 缪颖. PPR蛋白在植物生长发育中的作用. 热带亚热带植物学报, 2019, 27(2): 225-234.
WANG W Z, REN Y J, MIAO Y. Roles of PPR proteins in plant growth and development. Journal of Tropical and Subtropical Botany, 2019, 27(2): 225-234. (in Chinese)
[45] COLCOMBET J, LOPEZ-OBANDO M, HEURTEVIN L, BERNARD C, MARTIN K, BERTHOMÉ R, LURIN C. Systematic study of subcellular localization ofPPR proteins confirms a massive targeting to organelles. RNA Biology, 2013, 10(9): 1557-1575.
[46] HAMMANI K, TAKENAKA M, MIRANDA R A. Barkan, A PPR protein in the PLS subfamily stabilizes the 5′-end of processed rpl16 mRNAs in maize chloroplasts. Nucleic Acids Research, 2016, 44(9): 4278-4288.
[47] HAO Y Y, WANG Y L, WU M M, ZHU X P, TENG X, SUN Y L, ZHU J P, ZHANG Y Y, JING R N, LEI J, LI J F, BAO X H, WANG C M, WANG Y H, WAN J M. The nuclear-localized PPR protein OsNPPR1 is important for mitochondrial function and endosperm development in rice. Journal of Experimental Botany, 2019, 70(18): 4705-4720.
[48] XIAO H J, XU Y H, NI C Z, ZHANG Q N, ZHONG F Y, HUANG J S, ZHU Y G, HU J. A rice dual-localized pentatricopeptide repeat protein is involved in organellar RNA editing together with OsMORFs. Journal of Experimental Botany, 2018, 69(12): 2923-2936.
[49] HAMMANI K, GOBERT A, HLEIBIEH K, CHOULIER L, SMALL I, GIEGÉ P. Andual-localized pentatricopeptide repeat protein interacts with nuclear proteins involved in gene expression regulation. The Plant Cell, 2011, 23(2): 730-740.
[50] HUANG C, LIU D, LI Z A, MOLLOY D P, LUO Z F, SU Y, LI H O, LIU Q, WANG R Z, XIAO L T. The PPR protein RARE1-mediated editing of chloroplast accD transcripts is required for fatty acid biosynthesis and heat tolerance in. Plant Communications, 2023, 4(1): 100461.
[51] LV H X, HUANG C, GUO G Q, YANG Z N. Roles of the nuclear-encoded chloroplast SMR domain-containing PPR protein SVR7 in photosynthesis and oxidative stress tolerance in. Journal of Plant Biology, 2014, 57(5): 291-301.
[52] MOCHIZUKI N, SUSEK R, CHORY J. An intracellular signal transduction pathway between the chloroplast and nucleus is involved in de-etiolation. Plant Physiology, 1996, 112(4): 1465-1469.
[53] WU G Z, CHALVIN C, HOELSCHER M, MEYER E H, WU X N, BOCK R. Control of retrograde signaling by rapid turnover of GENOMES UNCOUPLED1, Plant Physiology, 2018, 176(3): 2472-2495.
[54] COTTAGE A, MOTT E K, KEMPSTER J A, GRAY J C. Theplastid-signalling mutant() shows altered sensitivity to sucrose and abscisic acid and alterations in early seedling development. Journal of Experimental Botany, 2010, 61(13): 3773-3786.
[55] GUO J G, ZHOU Y P, LI J A, SUN Y J, SHANGGUAN Y, ZHU Z N, HU Y J, LI T, HU Y H, ROCHAIX J D, MIAO Y C, SUN X W. COE 1 and GUN1 regulate the adaptation of plants to high light stress. Biochemical and biophysical research communications, 2020, 521(1): 184-189.
[56] LASORELLA C, FORTUNATO S, DIPIERRO N, JERAN N, TADINI L, VITA F, PESARESI P, PINTO M C D E. Chloroplast-localized GUN1 contributes to the acquisition of basal thermotolerance in. Frontiers in Plant Science, 2022, 13: 1058831.
[57] FORTUNATO S, LASORELLA C, TADINI L, JERAN N, VITA F, PESARESI P, PINTO M C D E. GUN1 involvement in the redox changes occurring during biogenic retrograde signaling. Plant Science, 2022, 320: 111265.
[58] ZSIGMOND L, RIGÓG, SZARKA A, SZEKELY G, OTVOS K, DARULA Z, MEDZIHRADSZKY K F, KONCZ C, KONCZ Z, SZABADOS L.PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiology, 2008, 146(4): 1721-1737.
[59] LIU Y, HE J N, CHEN Z Z, REN X Z, HONG X H, GONG Z Z., encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrialintron 3, is involved in the abscisic acid response in. The Plant Journal, 2010, 63(5): 749-765.
[60] YANG L, ZHANG J, HE J N, QIN Y Y, HUA D P, DUAN Y, CHEN Z Z, GONG Z Z. ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in. PLoS Genetics, 2014, 10(12): e1004791.
[61] LALUK K, ABUQAMAR S, MENGISTE T. Themitochondria-localized pentatricopeptide repeat protein PGN functions in defense against necrotrophic fungi and abiotic stress tolerance. Plant Physiology, 2011, 156(4): 2053-2068.
[62] MURAYAMA M, HAYASHI S, NISHIMURA N, ISHIDE M, KOBAYASHI K, YAGI Y, ASAMI T, NAKAMURA T, SHINOZAKI K, HIRAYAMA T. Isolation of, a weak ABA hypersensitive mutant defective in nad4 RNA editing. Journal of Experimental Botany, 2012, 63(14): 5301-5310.
[63] YUAN H, LIU D. Functional disruption of the pentatricopeptide protein SLG1 affects mitochondrial RNA editing, plant development, and responses to abiotic stresses in. The Plant Journal, 2012, 70(3): 432-444.
[64] ZHU Q, DUGARDEYN J, ZHANG C Y, MÜHLENBOCK P, EASTMOND P J, VALCKE R, CONINCK B DE, ÖDEN S, KARAMPELIAS M, CAMMUE B P. TheRNA editing factor SLO2, which affects the mitochondrial electron transport chain, participates in multiple stress and hormone responses. Molecular Plant, 2014, 7(2): 290-310.
[65] LIU J M, ZHAO J Y, LU P P, CHEN M, GUO C H, XU Z S, MA Y Z. The E-subgroup pentatricopeptide repeat protein family inand confirmation of the responsiveness PPR96 to abiotic stresses. Frontiers in Plant Science, 2016, 7: 1825.
[66] H. EMAMI, A. KUMAR, F. KEMPKEN. Transcriptomic analysis of poco1, a mitochondrial pentatricopeptide repeat protein mutant in. BMC Plant Biology, 2020, 20(1): 1-21.
[67] SECHET J, ROUX C, PLESSIS A, EFFROY D, FREY A, PERREAU F, BINIEK C, KRIEGER-LISZKAY A, MACHEREL D, NORTH H M. The ABA-deficiency suppressor locus HAS2 encodes the PPR protein LOI1/MEF11 involved in mitochondrial RNA editing. Molecular Plant, 2015, 8(4): 644-656.
[68] JIANG S C, MEI C, LIANG S, YU Y T, LU K, WU Z, WANG X F, ZHANG D P. Crucial roles of the pentatricopeptide repeat protein SOAR1 inresponse to drought, salt and cold stresses. Plant Molecular Biology, 2015, 88(4): 369-385.
[69] GUO Z F, WANG X Y, HU Z B, WU C Y, SHEN Z G. The pentatricopeptide repeat protein GEND1 is required for root development and high temperature tolerance in. Biochemical and Biophysical Research Communications, 2021, 578: 63-69.
[70] PARK Y J, LEE H J, KWAK K J, LEE K, HONG S W, KANG H. MicroRNA400-guided cleavage of pentatricopeptide repeat protein mRNAs rendersmore susceptible to pathogenic bacteria and fungi. Plant and Cell Physiology, 2014, 55(9): 1660-1668.
[71] CHEN G L, ZOU Y, HU J H, DING Y. Genome-wide analysis of the rice PPR gene family and their expression profiles under different stress treatments. BMC Genomics, 2018, 19(1): 720.
[72] GONG X D, SU Q Q, LIN D Z, JIANG Q, XU J L, ZHANG J H, TENG S, DONG Y J. The riceencoding a novel pentatricopeptide repeat protein is required for chloroplast development during the early leaf stage under cold stress. Journal of Integrative Plant Biology, 2014, 56(4): 400-410.
[73] WU L L, WU J, LIU Y X, GONG X D, XU J L, LIN D Z, DONG Y J. The rice pentatricopeptide repeat geneis needed for chloroplast development under cold stress. Rice, 2016, 9(1): 67.
[74] TAN J J, TAN Z H, WU F Q, SHENG P K, HENG Y Q, WANG X H, REN Y L, WANG J L, GUO X P, ZHANG X, CHENG Z J, JIANG L, LIU X M, WANG H Y, WAN J M. A novel chloroplast-localized pentatricopeptide repeat protein involved in splicing affects chloroplast development and abiotic stress response in rice. Molecular Plant, 2014, 7(8): 1329-1349.
[75] XIAO H J, LIU Z J, ZOU X, XU Y H, PENG L L, HU J, LIN H H. Silencing of rice PPR geneexhibited enhanced sensibility to abiotic stress and remarkable accumulation of ROS. Journal of Plant Physiology, 2021, 258-259: 153361.
[76] QIU T C, ZHAO X S, FENG H J, QI L L, YANG J, PENG Y L, ZHAO W S. OsNBL3, a mitochondrion-localized pentatricopeptide repeat protein, is involved in splicingintron 4 and its disruption causes lesion mimic phenotype with enhanced resistance to biotic and abiotic stresses. Plant Biotechnology Journal, 2021, 19(11): 2277-2290.
[77] LUO Z, XIONG J, XIA H, WANG L, HOU G H, LI Z Y, LI J, ZHOU H L, LI T F, LUO L J. Pentatricopeptide repeat gene-mediated mitochondrial rna editing impacts on rice drought tolerance. Frontiers in Plant Science, 2022, 13: 926285.
[78] LU K, LI C, GUAN J, LIANG W H, CHEN T, ZHAO Q Y, ZHU Z, YAO S, HE L, WEI X D, ZHAO L, ZHOU L H, ZHAO C F, WANG C L, ZHANG Y D. The PPR-Domain Protein SOAR1 Regulates Salt Tolerance in Rice. Rice, 2022, 15: 1-16.
[79] SU H G, LI B, SONG X Y, MA J, CHEN J, ZHOU Y B, CHEN M, MIN D H, XU Z S, MA Y Z. Genome-wide analysis of the DYW subgroup PPR gene family and identification ofresponses to drought stress. International Journal of Molecular Sciences, 2019, 20(22): 5667.
[80] SCHMITZ-LINNEWEBER C, SMALL I. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends in plant science, 2008, 13(12): 663-670.
[81] PRIKRYL J, ROJAS M, SCHUSTER G, BARKAN A. Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein. Proceedings of the National Academy of Sciences of the United State of America, 2011, 108(1): 415-420.
[82] XIONG J, TAO T, LUO Z, YAN S G, LIU Y, YU X Q, LIU G L, XIA H, LUO L J. RNA editing responses to oxidative stress between a wild abortive type male-sterile line and its maintainer line. Frontiers in Plant Science, 2017, 8: 2023.
[83] RICHTER A S, NÄGELE T, GRIMM B, KAUFMANN K, SCHRODA M, LEISTER D, KLEINE T. Retrograde signaling in plants: A critical review focusing on the GUN pathway and beyond. Plant Communications, 2023, 4(1): 20.
[84] POGSON B J, WOO N S, FÖRSTER B, SMALL I D. Plastid signalling to the nucleus and beyond, Trends in Plant Science, 2008, 13(11): 602-609.
[85] LEE K, KANG H. Roles of organellar RNA-binding proteins in plant growth, development, and abiotic stress responses. International Journal of Molecular Sciences, 2020, 21(12): 4548.
[86] PESARESI P, KIM C. Current understanding of GUN1: a key mediator involved in biogenic retrograde signaling. Plant Cell Reports, 2019, 38(7): 819-823.
[87] HERNÁNDEZ-VERDEJA T, STRAND Å. Retrograde signals navigate the path to chloroplast development. Plant Physiology, 2018, 176(2): 967-976.
[88] WU G Z, MEYER E H, RICHTER A S, SCHUSTER M, LING Q, SCHÖTTLER M A, WALTHER D, ZOSCHKE R, GRIMM B, JARVIS R P. Control of retrograde signalling by protein import and cytosolic folding stress. Nature Plants, 2019, 5(5): 525-538.
[89] TADINI L, PESARESI P, KLEINE T, ROSSI F, GULJAMOW A, SOMMER F, MÜHLHAUS T, SCHRODA M, MASIERO S, PRIBIL M. GUN1 controls accumulation of the plastid ribosomal protein S1 at the protein level and interacts with proteins involved in plastid protein homeostasis. Plant Physiology, 2016, 170(3): 1817-1830.
[90] ZHAO X B, HUANG J Y, CHORY J. GUN1 interacts with MORF2 to regulate plastid RNA editing during retrograde signaling. Proceedings of the National Academy of Sciences of the United State of America, 2019, 116(20): 10162-10167.
[91] TADINI L, PERACCHIO C, TROTTA A, COLOMBO M, MANCINI I, JERAN N, COSTA A, FAORO F, MARSONI M, VANNINI C. GUN1 influences the accumulation of NEP-dependent transcripts and chloroplast protein import incotyledons upon perturbation of chloroplast protein homeostasis. The Plant Journal, 2020, 101(5): 1198-1220.
[92] SHIMIZU T, KACPRZAK S M, MOCHIZUKI N, NAGATANI A, WATANABE S, SHIMADA T, TANAKA K, HAYASHI Y, ARAI M, LEISTER D. The retrograde signaling protein GUN1 regulates tetrapyrrole biosynthesis. Proceedings of the National Academy of Sciences of the United State of America, 2019, 116(49): 24900-24906.
[93] TAKENAKA M, ZEHRMANN A, VERBITSKIY D, KUGELMANN M, HÄRTEL B, BRENNICKE A. Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proceedings of the National Academy of Sciences of the United State of America, 2012, 109(13): 5104-5109.
[94] JIA Y B, TIAN H Y, ZHANG S, DING Z J, MA C L. GUN1- interacting proteins open the door for retrograde signaling. Trends in Plant Science, 2019, 24(10): 884-887.
[95] COLOMBO M, TADINI L, PERACCHIO C, FERRARI R, PESARESI P. GUN1, a jack-of-all-trades in chloroplast protein homeostasis and signaling. Frontiers in Plant Science, 2016, 7(257): 1427.
[96] BRUNKARD J O, BURCH-SMITH T M. Ties that bind: the integration of plastid signalling pathways in plant cell metabolism. Essays in Biochemistry, 2018, 62(1): 95-107.
[97] SUN X W, FENG P Q, XU X M, GUO H L, MA J F, CHI W, LIN R C, LU C M, ZHANG L X. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nature Communications, 2011, 2: 477.
[98] PAGE M T, KACPRZAK S M, MOCHIZUKI N, OKAMOTO H, SMITH A G, TERRY M J. Seedlings lacking the PTM protein do not show amutant phenotype. Plant Physiology, 2017, 174(1): 21-26.
[99] KINDGREN P, NOREN L, LOPEZ J D D B, SHAIKHALI J, STRAND Å. Interplay between Heat Shock Protein 90 and HY5 controlsexpression in response to the GUN5 plastid signal. Molecular Plant, 2012, 5(4): 901-913.
[100] KAKIZAKI T, MATSUMURA H, NAKAYAMA K, CHE F S, TERAUCHI R, INABA T. Coordination of plastid protein import and nuclear gene expression by plastid-to-nucleus retrograde signaling. Plant Physiology, 2009, 151(2): 1339-1353.
[101] KOUSSEVITZKY S, NOTT A, MOCKLER T C, HONG F X, SACHETTO-MARTINS G, SURPIN M, LIM J, MITTLER R, CHORY J. Signals from chloroplasts converge to regulate nuclear gene expression. Science, 2007, 316(5825): 715-719.
[102] MARTIN G, LEIVAR P, LUDEVID D, TEPPERMAN J M, QUAIL P H, MONTE E. Phytochrome and retrograde signalling pathways converge to antagonistically regulate a light-induced transcriptional network. Nature Communications, 2016, 7: 11431.
[103] VECIANA N, MARTÍN G, LEIVAR P, MONTE E. BBX16 mediates the repression of seedling photomorphogenesis downstream of the GUN1/GLK1 module during retrograde signalling. New Phytologist, 2022, 234(1): 93-106.
[104] WU G Z, BOCK R. GUN control in retrograde signaling: how GENOMES UNCOUPLED proteins adjust nuclear gene expression to plastid biogenesis. The Plant Cell, 2021, 33(3): 457-474.
[105] KOBAYASHI K, SUZUKI M, TANG J, NAGATA N, OHYAMA K, SEKI H, KIUCHI R, KANEKO Y, NAKAZAWA M, MATSUI M. Lovastatin insensitive 1, a novel pentatricopeptide repeat protein, is a potential regulatory factor of isoprenoid biosynthesis in. Plant and Cell Physiology, 2007, 48(2): 322-331.
[106] TANG J W, KOBAYASHI K, SUZUKI M, MATSUMOTO S, MURANAKA T. The mitochondrial PPR protein LOVASTATIN INSENSITIVE 1 plays regulatory roles in cytosolic and plastidial isoprenoid biosynthesis through RNA editing. The Plant Journal, 2010, 61(3): 456-466.
[107] EMAMI H, KEMPKEN F. PRECOCIOUS1 (POCO1), a mitochondrial pentatricopeptide repeat protein affects flowering time in. The Plant Journal, 2019, 100(2): 265-278.
[108] OLDENKOTT B, YANG Y, LESCH E, KNOOP V, SCHALLENBERG- RÜDINGER M. Plant-type pentatricopeptide repeat proteins with a DYW domain drive C-to-U RNA editing in.Communications Biology, 2019, 2: 85.
[109] NAKAMURA T. Understanding RNA editing and its use in gene editing. Gene and Genome Editing, 2022(3/4): 100021.
[110] MCDERMOTT J J, WATKINS K P, WILLIAMS-CARRIER R, BARKAN A. Ribonucleoprotein capture by in vivo expression of a designer pentatricopeptide repeat protein in. The Plant Cell, 2019, 31(8): 1723-1733.
Research progress of PPR protein in plant abiotic stress response
LI Cheng, LU Kai, WANG CaiLin, ZHANG YaDong
Institute of Food Crops, Jiangsu Academy of Agricultural Sciences/East China Branch of National Center of Technology Innovation for Saline-Alkali Tolerant Rice/Jiangsu High Quality Rice R&D Center/Nanjing Branch of China National Center for Rice Improvement/Key laboratory of Jiangsu Province for Agrobiology, Nanjing 210014
Abiotic stress is one of the main factors causing global grain yield reduction. It is of great significance to study the function and response mechanisms of plant stress-related proteins to improve crop stress resistance. Pentatricopeptide repeat (PPR) proteins, belong to the largest family of nuclear coding proteins in higher plants and are named because they contain highly specific PPR motifs. Depending on motif type and arrangement, PPR proteins can be classified as P and PLS, and PLS proteins can be further classified as PLS, E, E+, DYW, and other subclasses based on their carboxyl-terminal domains. PPR proteins are widely distributed in terrestrial plants, mainly in chloroplasts and mitochondria, and a few in the nucleus. As sequence-specific RNA binding proteins, PPR proteins are involved in multiple aspects of plant RNA processing, including RNA editing, splicing, stabilization, and translation. PPR protein plays a variety of important roles in the whole life process of plants, but the mechanism of its action in plant stress resistance is not well understood. Based on the localization and function of PPR proteins related to abiotic stress reported, the mechanism of PPR proteins involved in regulation of abiotic stress, including post-transcriptional regulation and retrograde signaling, was reviewed and discussed in this paper. Post-transcriptional regulation is related to the role of PPR proteins in the modification of RNA after transcription. It is generally believed that PPR affects stress resistance in plants by regulating the expression of stress-related genes via binding RNA and by regulating the metabolism of organelle RNA. In terms of retrograde signaling, damage to PPR proteins can lead to impaired mitochondrial or chloroplast function, and then produce various retrograde signals (such as ROS), thereby regulating the expression of related genes and resisting adversity. However, since plastid signaling is affected by many environmental factors, some of which are still unclear, the mechanism of the PPR protein in retrograde signaling remains to be clarified. In addition, PPR proteins are pleiotropic and some have important effects on plant growth and reproduction while acting on stress resistance. Finally, this paper further analyzed the current research status of PPR protein as an RNA editing tool, discussed the remaining problems and research prospects of PPR protein in the direction of abiotic stress, and pointed out the key points and difficulties that need to be paid attention to in future research, to provide references for further research on PPR protein and crop abiotic stress resistance breeding.
PPR protein; plant; abiotic stress

10.3864/j.issn.0578-1752.2023.24.001
2023-05-25;
2023-07-24
江苏省种业振兴揭榜挂帅项目(JBGS[2021]001)
李程,E-mail:cli1024shine@163.com。通信作者张亚东,E-mail:zhangyd@jaas.ac.cn
(责任编辑 李莉)
