Cigarette induced release of exo-miR-34a from 16HBE vesicles targeting CASP2 promoted the proliferation of COPD MRC-5 cell
2023-12-23LISiguangLINLingsangCHENJieZHAOJieDINGYipeng
LI Si-guang, LIN Ling-sang, CHEN Jie, ZHAO Jie, DING Yi-peng,3✉
1.Hainan Affiliated Hospital of Hainan Medical University, Haikou 570311, China
2.Department of General Practice Medical, Hainan General Hospital, Haikou 570311, China
3.Department of Respiratory and Critical Care Medicine, Hainan General Hospital, Haikou 570311, China
Keywords:
ABSTRACT Objective: To explore how cigarette smoke extract (CES) regulates the expression of exosomal miR-34a in 16 HBE bronchial epithelial cells, thus affecting the proliferation of MRC-5 lung fibroblasts.Methods: CES was prepared from commercially available cigarettes, and 16HBE cells were treated with CES.The exosomal miR-34a collected from Yipeng Ding,Chief Physician, M.D..the supernatant was used for MRC-5 cell culture.The expression level of exosomal miR-34a was detected by RT-PCR.The proliferation ability of MRC-5 cells was determined by CCK-8 cell counting kit.The expression of CASP2 was detected by Western blot, and the target binding of miR-34a and CASP2 gene was verified by dual luciferase.Results: Under the transmission electron microscope, the exosomes in the supernatant of 16 HBE were spherical, with a particle size of about 100 nm; after CES treatment, the expression of exosomal miR-34a significantly increased.Further research showed that the exosomal miR-34a induced by CES can promote the proliferation of MRC-5 cells; miR-34a and CASP2 have a target binding relationship; miR-34a mimic significantly inhibited the expression of CASP2.Conclusion: In CES-induced 16HBE cells, exosomal miR-34a plays a key role in fibroblast proliferation through target binding with the CASP2 gene.
1.Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a persistent illness that negatively impacts the respiratory system[1].It is projected that by 2030, COPD will become the third leading cause of death globally, posing a significant threat to public respiratory health[2].Smoking is considered a principal trigger of COPD,and the deleterious effects of tobacco smoke on persistent airway deterioration have been extensively studied[3].In this process, the role of pulmonary fibroblasts is crucial[4].The hyperproliferation of pulmonary fibroblasts in COPD patients may be one of the primary factors leading to pulmonary function impairment[5].
Exosomes are capable of extruding various substances, such as lipids, proteins, and nucleic acids, out of the cell via multivesicular bodies.These nano-scale extracellular vesicles influence the physiological activities of recipient cells through intercellular signal molecule transmission[6].In the absence of this vesicular transport’s organizing function, cells would be in a disorganized state.It has been reported that exosomal miRNAs (exo-miRNAs)can mediate the transportation and expression of miRNAs, thereby promoting cellular migration, apoptosis, proliferation, autophagy,and regulating the function of recipient cells[7].Numerous studies suggest that exo-miRNAs could potentially aid in the diagnosis of COPD, and might serve as effective prognostic biomarkers, or even as clinical therapeutic targets[8].The role of exo-miRNAs in the pathogenesis of COPD is garnering increasing attention, albeit their function in COPD onset remains unclear.Among these, miR-34a holds promise as a novel intervention target and may play a significant role in COPD and other oxidative stress-related senile diseases[9].
CASP2, also known as Caspase-2, is a cysteine-aspartic acid protease that functions as a tumor suppressor in various oncogenedriven processes[10].CASP2 is a protein that gets activated under external or extrinsic stimuli, initiating a cascade of cellular apoptosis[6].Simultaneously, CASP2 can exert inflammatory effects by modulating the activation of immune cells and the transmission of inflammatory signals[11].Early studies revealed that miR-34a could enhance neuronal survival and alleviate neuronal damage induced by Aβ deposition by downregulating CASP2 expression[12].In lung cancer cells, miR-494 promotes the proliferation of non-small cell lung cancer (NSCLC) cells by targeting CASP2[13].
In this study, we explored the expression of miR-34a in exosomes from bronchial epithelial cells 16 HBE treated with cigarette smoke extract (CES), as well as the impact of exosomes and miR-34a mimics on the proliferation and apoptosis of lung fibroblasts MRC-5.This further confirmed the relationship between miR-34a and CASP2.The results showed that upon cigarette smoke stimulation,miR-34a expression was significantly elevated, and miR-34a in exosomes could target the downregulation of CASP2, promoting the proliferation of MRC-5.Based on these findings, we concluded that exosomal miR-34a, released from bronchial epithelial cells induced by cigarette smoke, participates in the pathogenesis and progression of COPD through its mediation of abnormal proliferation of lung fibroblasts.
2.Materials and Methods
2.1 Materials and Reagents
Two types of cells: human bronchial epithelial cells (16 HBE)purchased from the Shanghai Institute of Cell Science of the Chinese Academy of Sciences, and embryonic lung fibroblast line (MRC-5,ATCC®CCL-171™).Culture medium: FBS, MEM and DMEM culture media were obtained from Gibco, USA.Gene primers: miR-34a primer, miR-34a mimic, and mimic negative control (NC) were synthesized by Shanghai GenePharma Co.Antibodies: CASP2 antibody (ab179520), GAPDH antibody (ab8245) were purchased from Abcam.Reagent kits: RNA extraction kit was purchased from Tiangen Biotech (Beijing) Co., Ltd., CCK-8 kit was purchased from Wuhan Huamei Biotech Co., Ltd., and Dual-Luciferase®Reporter Assay System was purchased from Promega Corporation.Instrumentation: The transmission electron microscope was of FEI brand, USA, and the CO2 incubator (3111) was purchased from Thermo Fisher Scientific.Other: Lipofectamine 2 000 reagent(L3000015) was also purchased from Thermo Fisher Scientific.
2.2 Preparation of Cigarette Smoke Extract (CSE)
Twenty commercially available Golden Leaf brand cigarettes were fully burned, and the generated smoke was dissolved in 20 mL of serum-free DMEM culture medium.This solution was then sterilized by filtration through a 0.22 μm filter (EMDMillipore, Billerica, MA)to remove suspended particles and bacteria, resulting in a DMEM stock solution containing CSE.The extract (defined as 100% CSE)was diluted to the prescribed concentration and used within 10 minutes of preparation.
2.3 Experimental Methods
2.3.1 Cell Culture and Treatment with CES
16HBE cells and MRC-5 cells were maintained in DMEM and MEM containing 10% fetal bovine serum, 100 mg/mL streptomycin,and 100 U/mL penicillin (Sigma, Poole, UK), and cultured in a 5% CO2, 37°C incubator until reaching 50%-60% cell density.The 16HBE cells were divided into groups for treatment.The experimental group was treated with 20% CES, while the control group was left untreated (blank control group).After 48 hours of incubation, the supernatant containing extracellular vesicles(including membrane-bound vesicles) was collected from the culture medium of each group.The extracellular vesicles obtained from 16HBE cells were used for treatment of MRC-5 cells.
2.3.2 Isolation of Extracellular Vesicles (EVs)
Extracellular vesicles were isolated from the culture medium of 16HBE cells treated with either normal or CES.The supernatant from the 16HBE cell culture was collected and centrifuged at room temperature at 3 000 g for 15 min using a benchtop centrifuge.After centrifugation, the supernatant was filtered through a 0.22 μm PVDF filter and transferred to a new tube.Exoquick-TC Solution (System Biosciences) was added to the filtered supernatant at a 1:5 ratio,mixed well, and then refrigerated at 4 ℃ for at least 12 h.After the incubation, the mixture was centrifuged for a second time at 1500 g for 30 min.The supernatant was discarded after centrifugation.For the third centrifugation, the remaining liquid was removed after 5 minutes of centrifugation.The pellet containing the EVs was then resuspended in nuclease-free water.ZetaView nanoparticle tracking analysis (ParticleMetrix, Germany) was used to analyze the size distribution and concentration of the EVs.
2.3.3 Transmission Electron Microscopy (TEM)
The isolated extracellular vesicles were subjected to the following pretreatment: diluted with PBS buffer to an appropriate concentration(50 μg/mL), 20 μL of the EV suspension was added to a pre-prepared solution of polyethylene glycol (PEG-8000, 2.0% v/v), mixed well,and left to settle at room temperature for 30 minutes to precipitate.The supernatant was removed, and the pellet was resuspended in 0.1 mol/L PBS buffer.A small amount of the suspension was dropped onto a carbon-coated and support-film-coated copper grid, excess liquid was gently removed, and the grid was allowed to air-dry for about 20 min in a ventilated area.
The prepared samples were examined and observed using a transmission electron microscope (JEM-1200EX, JEOL Ltd,Japan).The electron beam was set to 100 kV.The sample position was first located at low magnification, and then switched to high magnification for higher-resolution imaging.The images were exposed and converted into digital signals using a scintillation screen.Image software was used to analyze the photos and calculate the size and quantity of the extracellular vesicles.
2.3.4 Extraction of Extracellular Vesicle RNA for RT-PCR Detection
First, the total RNA from the exosomes is extracted using an extracellular vesicle RNA extraction kit (System Biosciences, USA).The extracted RNA is then quantified and assessed for integrity using a Bioanalyzer 2 100 instrument (Agilent Technologies,USA).Next, the RNA is reverse transcribed into cDNA using M-MLV reverse transcriptase (18057018; Thermo Fisher Scientific)and random primers, based on the EasyBlue kit (Laboratory Technology).GAPDH is used as a loading control for mRNA.The resulting cDNA is subjected to PCR amplification using specific primers.The PCR products are analyzed by 1.5% agarose gel electrophoresis and visualized using a 10% ethidium bromide gel system (Daihan, WGD30).Finally, RT-PCR is performed using SYBR Green PCR Master Mix (Solebao Biotechnology,Beijing, China), specific primers, and probes for miR-34a and CASP2.The relative expression levels of miR-34a and CASP2 are determined by simultaneously detecting the reference gene U6 and miR-34a and using the 2-ΔΔctmethod to compare the expression differences between the CES-treated group and the normal control group.The primer sequences for amplifying miR-34a and CASP2 are as follows: miR-34a: (upstream primer) 5'-GGCTCGAGTAGTTGCCTGGGCTGGTCTT-3' and (downstream primer) 5'-GGGCGGCCGCCCTGTGCCTTTTTCCTTCC-3';CASP2: (upstream primer) 5'-CAGTTACCTGCACACCGAGT-3'and (downstream primer) 5'-CAGTTACCTGCACACCGAGT-3'.
2.3.5 CCK-8 Assay for MRC-5 Cell Proliferation
The Cell Counting Kit-8 (CCK-8) assay (CK04, Dojindo, China)was used to assess MRC-5 cell proliferation.100 μL of cell suspension was added to each well of a 96-well plate, with three wells per group and a cell density of 1×104cells per well.The plate was then incubated overnight at 37 ℃ with 5% CO2in a cell culture incubator.At 24, 48, 72, and 96 hours of cell culture, 100 μL of 10% CCK-8 reagent was added to each well.After incubating for 2 hours, the absorbance at 450 nm was measured using a microplate reader (BioTek, USA).The x-axis represents the detection time (t),and the y-axis represents the absorbance values (OD).Relevant data was collected and a cell growth curve was plotted to demonstrate the growth and proliferation ability of MRC-5 cells.
2.3.6 Dual-Luciferase Reporter Gene Assay
The Targetscan database (http://www.targetscan.org/) was used to predict the binding sites of miR-34a and Caspase-2.To establish a model for detecting the interaction between miRNA and mRNA,wild-type (WT) and mutant (MUT) sequences of the binding sites for miR-34a and CASP2 were inserted downstream of the firefly luciferase gene, resulting in expression vectors.Next, miR-34a mimics were mixed with pmirGLO-CASP2-WT and MUT recombinant plasmids.In the control group, miRNA-NC was mixed with CASP2-WT and MUT recombinant plasmids.The mixtures were then transfected into HEK293T cells along with Lipo-2000 liposomes and incubated for 48 hours.Finally, luciferase activity was measured according to the instructions of the assay kit to determine the interaction between miR-34a and CASP2.
2.3.7 Western Blot Analysis
Cell lysates were prepared by extracting proteins from each group of cells.The total protein concentration was determined using the BCA assay kit.Denaturation of the proteins was achieved by adding 5×loading buffer and incubating at 100 ℃ in a metal bath for 15 minutes.20 μg of protein from each sample was loaded onto an SDS-PAGE gel for protein electrophoresis.The proteins were then transferred onto a PVDF membrane with a pore size of 0.22 μm.The membrane was blocked with 5% skim milk for 1 hour.Subsequently,the membrane was incubated with the primary antibody (diluted 1:1 000) overnight at 4 ℃.The membrane was washed 6 times for 5 min each to remove unbound primary antibody.After washing, the membrane was incubated with a goat anti-rabbit secondary antibody(diluted 1:5 000) for 1 h at room temperature.The membrane was washed 3 times for 5 min each after secondary antibody incubation.Finally, chemiluminescent reagents were added to the gel imaging system to visualize the protein bands, and the images were captured.GAPDH was used as an internal reference.The protein band intensities were analyzed using Image J software to calculate the relative expression levels of the proteins.
2.3.8 Statistical Analysis
Statistical analysis and graphical representation of experimental data were conducted using GraphPad Prism 9.0 software.Each experiment was independently repeated three times.Normally distributed quantitative data are presented as mean ± standard deviation (mean ± SD).Statistical significance was considered when the p-value was less than 0.05, indicating the presence of a significant statistical difference.
3.Results
3.1 Characterization of 16HBE Exosomes
Transmission electron microscopy (TEM) was performed to observe the typical characteristics of exosomes.It was found that exosomes released by 16HBE cells exhibited spherical vesicular structures with an approximate diameter of 100 nm (Figure 1).This result indicates that the exosome isolation technique utilized in this study was effective and reliable, and the obtained exosomes are suitable for subsequent processing in MRC-5 cells.

Fig 1 Observation of the structure of 16HBE exosome vesicles treated with cigarettes by transmission electron microscopy
3.2 CES Significantly Promotes Exosomal miR-34a Secretion in 16HBE cells
The relative expression abundance of exosomal miR-34a in the supernatant of cigarette smoke mimic (CSM)-treated bronchial epithelial cells was determined by RT-PCR.As shown in Figure 2, the expression level of miR-34a in the CSM-treated group(3.22±0.33) was significantly higher than that in the control group(1.04±0.07) (t=11.09, P=0.0004<0.001).
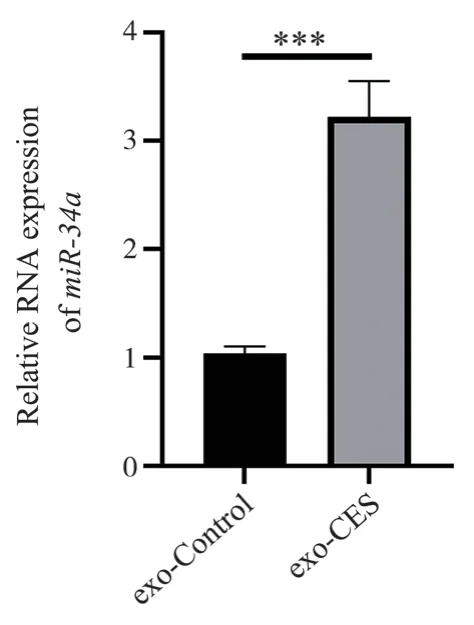
Fig 2 Relative expression level of exosome miR-34a in supernatant of CES -treated cells and control, *** means P<0.001
3.3 CES Induces Exosomal miR-34a in 16HBE cells to Promote MRC-5 Proliferation
MRC-5 cells were cultured with exosomal miR-34a isolated from the supernatant of CSE-treated 16HBE cells (exo-CES).Cell proliferation was assessed using the CCK-8 assay to compare the growth dynamics between the exo-CES group and the control group.The results revealed that the proliferation activity of MRC-5 cells in the exo-CES group (2.51±0.13) was significantly higher than that in the blank control group (1.71±0.19) (t=16.31, P<0.0001), as shown in Figure 3.
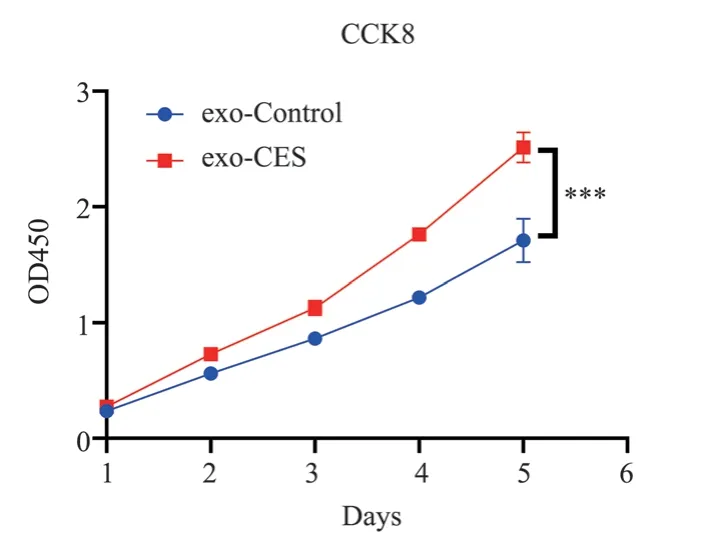
Fig 3 exo-CES promoted the proliferation of MRC-5 cells, *** means P<0.001
3.4 Regulation of CASP2 by miR-34a
To investigate the effect of miR-34a mimic on COPD fibroblasts,MRC-5 cells were cultured with miR-34a mimic, and cell proliferation was assessed using the CCK-8 assay.The results demonstrated that the proliferation activity of MRC-5 cells in the miR-34 mimic group (2.77±0.10) was significantly higher than that in the NC group (1.60±0.12), as shown in Figure 4B.The Targetscan database predicted the binding of miR-34a to CASP2, and the binding site is shown in Figure 4E.The dual-luciferase reporter assay results (Figure 4F) revealed that the fluorescence intensity of the CASP2-WT+miR-34a group (0.38±0.02) was significantly lower than that of the NC group (1.00±0.05) (t=20.61, P<0.001),while the CASP2-MUT+miR-34a group (0.94±0.02) showed no significant difference in fluorescence intensity compared to the NC group (t=2.777, P=0.05), thus confirming CASP2 as a target gene of miR-34a.Furthermore, Western blot analysis demonstrated that the expression of miR-34a in the miR-34 mimic group (2.76±0.22) was significantly higher than that in the NC group (1.04±0.08) (t=12.69,P=0.0002<0.001), as shown in Figure 4A.The expression of CASP2 in the miR-34 mimic group (0.46±0.06) was significantly lower than that in the NC group (1.07±0.12) (t=7.94, P=0.0014<0.01), as shown in Figure 4C.
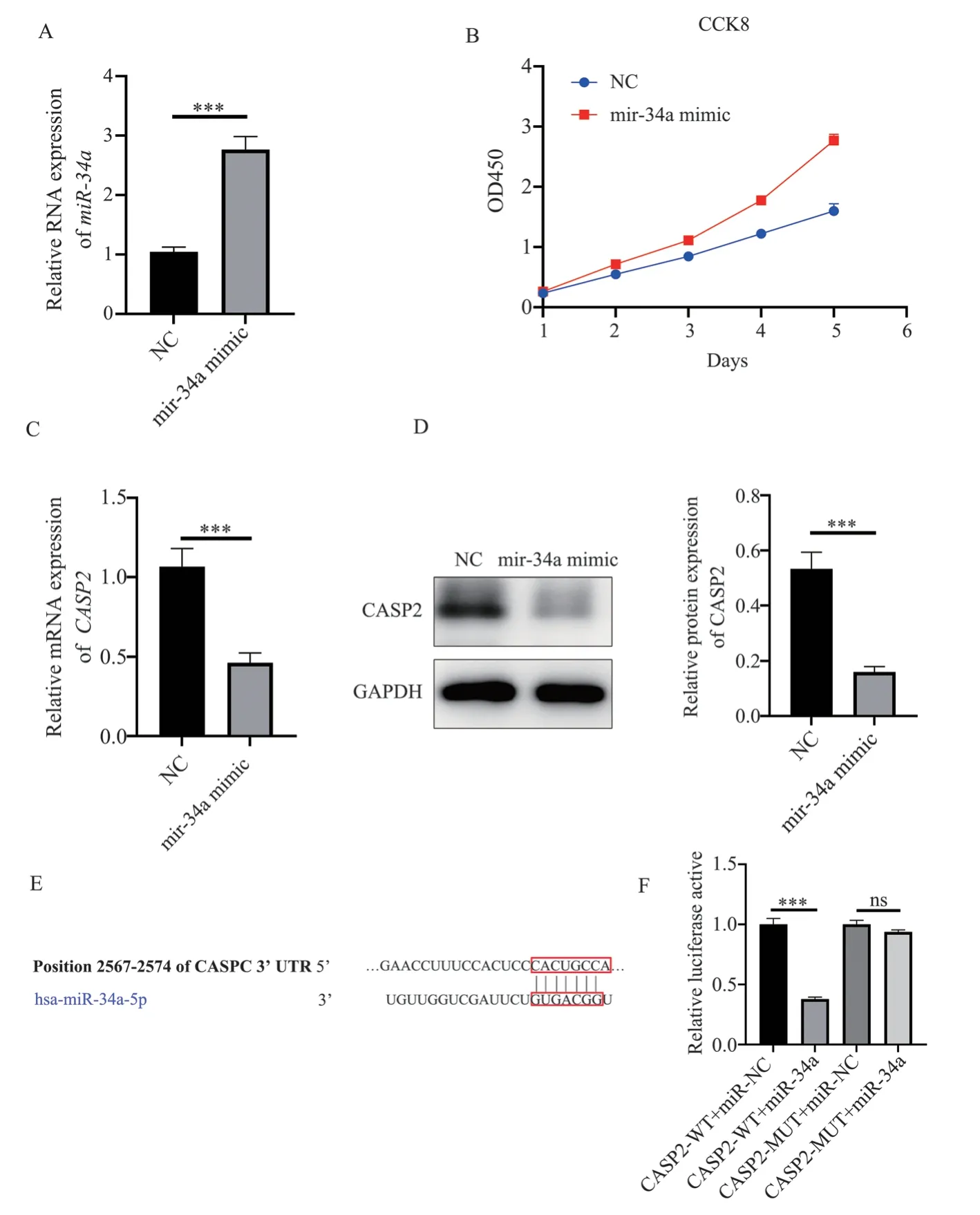
Fig 4 miR-34a transfection promotes MRC-5 lung fibroblast proliferation and inhibits the expression of CASP2
4.Discussion
As a widely prevalent chronic respiratory disease[14], chronic obstructive pulmonary disease (COPD) continues to be a primary focus of current research into respiratory diseases.Chronic obstructive lung injury is a significant clinical symptom of COPD,and smoking can cause airway epithelial injury and inflammatory response, with approximately 20% of individuals developing COPD[15].In 2017, the global incidence of COPD reached 544 million, with males and smokers being the most commonly affected groups[16,17].Studies have shown that exposure to cigarette smoke(CS) potentially impairs the defensive functions of the airways,weakening the immune functions of airway epithelial cells[18], This influence, through exacerbating inflammatory responses, oxidative stress, and accelerating cell death, may ultimately lead to the onset of COPD[19].At present, there is no definitive cure for COPD;its development can only be delayed by alleviating symptoms.Therefore, deepening the understanding of the pathogenic mechanisms of COPD to seek effective intervention measures has become a key issue and challenge in current COPD research.
In lung tissue, the main type of fibroblast is pulmonary fibroblasts[20].An obvious fibrosis can be found in the histopathology of COPD, and pulmonary fibroblasts play a crucial role[21].Numerous studies have shown that the redox state[22],oxygen free radicals mediated by inflammatory cells[23], growth factors[24] and cytokines[25] etc., all participate in the proliferation regulation of pulmonary fibroblasts.Existing evidence suggests that pulmonary fibroblasts cause bronchial epithelial inflammation by producing inflammatory mediators and cytokines[26].In addition,the abnormal proliferation of pulmonary fibroblasts may lead to cell cycle apoptosis and dysregulation, resulting in the formation of scars in lung tissue, thereby promoting the progression of fibrotic lesions[27,28].In pulmonary fibroblasts, the increased expression of transforming growth factor-β1 (TGF-β1) triggers the production of matrix components such as collagen, and the deposited collagen thickens the airway wall, resulting in airway remodeling[29].These research findings support that pulmonary fibroblasts play a vital role in the development and progression of COPD, and inhibiting the excessive proliferation of pulmonary fibroblasts may contribute to the control of COPD progression and inflammatory response[28].
Exosomes play the role of vesicular transport in the cellular environment, acting as key carriers for material and information communication between cells.They affect various functions of neighboring or distant cells, depending on the bioactive substances they carry and deliver to target cells, thereby influencing the body’s physiological and pathological functions[30].Their unique biological properties make them ideal nanoscale carriers for efficient drug delivery[31].Furthermore, miRNAs derived from exosomes have been found to be involved in the pathogenesis of many diseases and are considered potential early diagnostic and prognostic markers for these diseases[32].Many studies have shown that exosomes and miRNAs play significant roles in the pathological process of COPD[33,34].
The influence of miR-34a has been widely studied and confirmed in various tumor developments[35].For example, miR-34a might promote the apoptosis of neutrophils in patients with myelodysplastic syndrome (MDS) by regulating the Cdc42-WASP-Arp2/3 pathway,potentially promoting changes in F-actin and the production of ROS[36].On the other hand, miR-34a can inhibit the expansion and invasion of gastric cancer as a tumor suppressor by adjusting PDL1 in the immune microenvironment[37].It is noteworthy that the reduction of miR-34a might promote the occurrence of head and neck cancer, and the mechanism may involve the up-regulation of the MET oncogene and adjustment of tumor immune escape strategies[38].In alcohol-induced liver injury, miR-34a plays a key role in steatohepatitis and neovascularization by guiding the Sirt1 signaling pathway in macrophages[39].Research has also found that miR-34a can promote the occurrence of renal fibrosis by inducing the transformation of renal fibroblasts into myofibroblasts [40].In addition, miRNA-34a participates in the aging process of lung epithelial cells under high oxygen conditions by adjusting the expression of Krüppel-like factor 4[41].Whether miR-34a from bronchial exosomes has a definite effect on the pulmonary fibroblasts of COPD has not been clarified to date.
In this study, we induced bronchial epithelial cells 16HBE with cigarette smoke simulation and obtained the result of a significant increase in miR-34a expression in exosomes.When we used these exosomal miR-34a produced by 16HBE cells to culture pulmonary fibroblasts MRC-5, we observed significant changes in the proliferation of MRC-5 cells.We successfully predicted that CASP2 is a target gene of miR-34a, and then confirmed the targeting relationship between miR-34a and CASP2 through luciferase reporter gene assay.In addition, we inhibited the expression of CASP2 using miR-34a mimic, further confirming its regulatory role in the proliferation of COPD pulmonary fibroblasts.This could be one of the mechanisms by which cigarette smoke promotes the proliferation of COPD pulmonary fibroblasts, or it may provide a basic scientific basis for new therapeutic targets for COPD.
Declaration
All authors have no conflicts of interest and agree to submit this manuscript.
Authors' contributions
Siguang Li: Cell culture, manuscript writing.
Lingsang Lin: Cell treatment, cell detection.
Jie Chen: Statistical analysis, data organization.
Jie Zhao: Experimental guidance, manuscript revision.
Yipeng Ding: Research design, funding support.
杂志排行
Journal of Hainan Medical College的其它文章
- To investigate the effect of Shenqi Tiaoshen Formula on CSE induced inflammatory response of MH-S cells based on TLR4/NF-kB/NLRP3 pathway
- CircROBO1 promotes retinal Y79 cell tumor invasion by targeting KLF5
- Effect of estrogen pretreatment in GnRH antagonist protocol-Metaanalysis
- Effect of Astragalus-hawthorn on ovarian reproductive function and inflammatory mechanism of action in rats with polycystic ovary syndrome
- Inhibitory effect of thymoquinone on neuroinflammation in Parkinson's disease model by regulating NLRP3 inflammatory bodies
- Abnormal expression and significance of circ-CBLB/miR-486-5p in patients with rheumatoid arthritis of spleen deficiency and dampness excess type
