福建李叶斑病病原菌种类鉴定及致病性研究
2023-11-21亓政良徐芳菲王先洪傅敏王利平洪霓王国平
亓政良 徐芳菲 王先洪 傅敏 王利平 洪霓 王国平

摘 要:【目的】鑒定明确近年在福建新发生的李叶斑病的病原菌种类。【方法】采集李叶斑病叶进行组织分离,对获得的菌株采用形态学和分子生物学相结合的方法进行种类鉴定和致病性研究。【结果】通过组织分离和纯化,并根据菌落形态特征共获得66个刺盘孢属(Colletotrichum)菌株。对这些菌株进行形态学观察和多基因(ACT、TUB2、CHS-1、GAPDH及ITS)系统发育分析的结果显示,它们分别归属于刺盘孢属的6个种,包括果生刺盘孢(C. fructicola)59个菌株、喀斯特刺盘孢(C. karstii)2个菌株、普洛柏刺盘孢(C. plurivorum)2个菌株、暹罗刺盘孢(C. siamense)1个菌株、无锡刺盘孢(C. wuxiense)1个菌株和李刺盘孢(C. pruni-salicinae)1个菌株,其中李刺盘孢(C. pruni-salicinae)为笔者鉴定出的1个新种。分离鉴定的6种刺盘孢的代表菌株,有伤接种结果显示它们均可使李叶片和果实致病,但其致病力明显不同,它们对桃、梨、柑橘和猕猴桃的致病也存在显著差异。【结论】引起福建李叶斑病的病原菌有果生刺盘孢、喀斯特刺盘孢、普洛柏刺盘孢、暹罗刺盘孢、无锡刺盘孢和李刺盘孢6种,其中果生刺盘孢(C. fructicola)为优势种,占刺分离获得的盘孢属(Colletotrichum)菌株的89.4%。不同刺盘孢菌的致病性存在明显差异。
关键词:李;叶斑病;刺盘孢菌;多基因系统发育分析;致病性
中图分类号:S662.3 文献标志码:A 文章编号:1009-9980(2023)11-2423-12
Identification and pathogenicity of pathogenic species of plum leaf spot disease in Fujian
QI Zhengliang, XU Fangfei, WANG Xianhong, FU Min, WANG Liping, HONG Ni, WANG Guoping*
(College of Plant Science and Technology, Huazhong Agricultural University/Key Lab of Plant Pathology of Hubei Province, Wuhan 430070, Hubei, China)
Abstract: 【Objective】 In recent years, a new leaf disease has occurred in plum producing areas in Fujian Province, causing yellow to brown spots on the leaves in preliminary stage, irregular gray to dark brown stripes in later period and leaf fall in severe cases. According to the symptoms, it is named plum leaf spot disease. The disease has a trend of spreading and increasing every year, which has aroused high local attention. This study investigated the occurrence of the disease and identified the pathogen species. 【Methods】 A survey of leaf spot disease was conducted in two plum orchards from 2020 to 2021 in Liancheng county, Fujian province. The leaves with the symptoms were collected from the plum trees of Furong, Younai and Huanai (Prunus sallcina) in the surveyed orchards and 4-5 mm2 diseased tissues (neighboring the asymptomatic regions) were surface-sterilized with 75% ethanol for 45 s, washed two times in sterile water and dried on sterilized filter paper, and placed onto potato dextrose agar (PDA) plates and incubated at a temperature of 28 ℃ in the dark. The single mycelium was used for purifying strains, and pure cultures were stored in 25% glycerol at -80 ℃. The pathogen genomic DNA was extracted with cetyltrimethylammonium bromide (CTAB) buffer, which was identified through partial actin (ACT), beta-tubulin (TUB2), chitin synthase (CHS-1), a 200-bp intron of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and partial regions of six loci including partial rDNA-ITS (ITS) region sequence analysis. The multiple sequences were aligned using MAFFT v.7 with default settings, manually adjusted in MEGA v.5.2.2 if necessary and phylogenetic tree was constructed using maximum-likelihood (ML) by IQ-TREE. The morphological characteristics, sporulation phenotype, conidia morphology and ascospore morphology for representative strains of the identified Colletotrichum spp. were recorded. The pathogenicity was determined on the detached leaves and fruits of plum inoculated with mycelial plugs and conidia suspension of representative strains of the identified Colletotrichum spp., the host ranges were determined on the detached leaves of peach (Prunus persica), pear (Pyrus pyrifolia), citrus (Citrus reticulate) and kiwifruit (Actinidia chinensis) inoculated with representative strains of the identified Colletotrichum spp..【Results】 Through tissue isolation and purification, 66 strains with the similar morphology to Colletotrichum spp. were isolated in total. The obtained strains were identified by multiple genes (ACT, TUB2, CHS-1, GAPDH, and ITS) and were clustered in six different branches, including 59 strains clustered in the branch of C. fruticola, 2 strains in C. karstii, 2 strains in C. pluriforum, 1 strain in C. siamense, 1 strain in C. wuxiensis and 1 strain (2FRL-3-1) close to C. tropicicola. Morphological observation showed that 2FRL-3-1 was significantly different from C. tropicicola in colony color and conidia size, which indicated that 2FRL-3-1 was a novel species of Colletotrichum spp. identified in this study, named as C. pruni-salicinae. The results of pathogenicity test showed that the representative strains of the above 6 species of Colletotrichum spp. induced lesions on the leaves and fruits of P. salicina Lindl. The strains isolated from infected sites were identical to the strains inoculated. The results showed that they were all pathogenic and responsible for plum leaf spot and fulfilled the Kochs postulates. But there were significant differences of pathogenicity in the fruits. The pathogenicity of C. siamense on the fruits was significantly stronger than the other 5 species. The pathogenicity of HN-6-1 was significantly stronger than that of 2FRL-2-3, although they all belonged to the species C. karstii. The results indicated that different species and different strains of the same species of Colletotrichum spp. had significant pathogenicity differentiation against the same host. The results of determination for the host range showed that the above six species of Colletotrichum spp. infected the leaves of the plants other than plum, C. fruticola and C. wuxiense infected the leaves of peach (P. persica), pear (P. pyrifolia), citrus (Citrus reticulata) and kiwifruit (A. chinensis), C. siamense and C. pluriforum infected the leaves of peach, pear and kiwifruit, while C. karstii and C. pruni-salicinae also infected the leaves of peach and pear. 【Conclusion】 Based on morphological characteristics and phylogenetic analysis of the multiple genes (ACT, TUB2, CHS-1, GAPDH, and ITS), the pathogens of plum leaf spot disease in Fujian were identified as 6 species, including C. fruticola, C. karstii, C. pluriforum, C. siamense, C. wuxiense and C. pruni-salicinae. Among them, C. fruticola was the dominant species, accounting for 89.4% of Colletotrichum strains. There were significant differences in pathogenicity between different species and strains of the same species on the same host. C. siamense had the strongest pathogenicity on the fruits of plum. This study is the first report on the identification of pathogens associated with plum leaf spot disease in Fujian.
Key words: Plum; Leaf spot; Colletotrichum spp.; Multi-gene phylogenetic analysis; Pathogenicity
收稿日期:2023-03-31 接受日期:2023-07-31
基金项目:国家梨产业技术体系树体病害防控岗位(CARS-28-16)
作者简介:亓政良,男,在读硕士研究生,从事果树病理学研究。Tel:18366319588,E-mail:qizhengliang000@163.com
*通信作者 Author for correspondence. E-mail:gpwang@mail.hzau.edu.cn
近年在福建连城等地的李产区发生一种新的叶部病害,根据其症状表现称之为李叶斑病。该病初期在李叶上产生黄色至浅褐色病点,之后逐渐扩展成形状不规则的灰色至深褐色病斑,发生严重时叶片干枯。该病有扩展蔓延和逐年加重的趋势,已引起当地的高度关注。
据Olawole等[1]报道,在美国的李产区发生一种细菌病害,导致李叶枯焦,潮湿时病部产生菌脓,研究证实系由叶缘焦枯病菌(Xylella fastidiosa)所致。我国四川的李产区也发生有李叶枯病,叶片边缘或尖端形成不规则的褐色斑点,继而扩展为较大的灰褐色病斑。经病原鉴定证实是由两种镰刀菌(Fusarium pernambucanum和F. sulawesiense)引起的[2]。2021年在我国广西李产区发现李黄斑病,该病田间症状表现为最初叶上形成小的黄色斑点,逐渐扩展为不规则深褐色凹陷病斑,其边缘显现黄色晕圈。研究表明该病的病原菌为埃斯钦诺梅刺盘孢(Colletotrichum aeschynomenes)[3]。不难看出,福建李叶斑病的症状表现与由细菌引起的李叶枯焦病[1]和由镰刀菌引起的李叶枯病[2]有明显的不同,而与由刺盘孢菌引起的李黄斑病[3]相似。
植物病原刺盘孢属(Colletotrichum)的寄主范围广泛,可为害许多作物造成严重的经济损失,该属种类繁多,不同的种可导致症状相同的病害,同一种刺盘孢也可引起不同症状表现的病害。据日本报道,引起李果实炭疽病的病原菌有尖孢炭疽菌(C. acutatum)、胶孢炭疽菌(C. gloeosporioides)和内乏亚炭疽菌(C. nymphaeae)[4-5]。而在韩国栽培的日本李(Prunus salicina Lindl.)上,导致果实炭疽病的病原菌为胶孢炭疽菌(C. gloeosporioides)、内乏亚炭疽菌(C. nymphaeae)、松针炭疽菌(C. foriniae)和暹罗刺盘孢(C. siamense)[5-6]。我国福建芙蓉李炭疽病的病原菌为胶孢炭疽菌(C. gloeosporioides)[7],而在广东栽培的日本李(Prunus salicina Lindl.)和广西栽培的三华李(P. salicina Lindl. cv. Sanhua)上,叶片与果实炭疽病则分别由松针炭疽菌(C. fioriniae)[8]和埃斯钦诺梅刺盘孢(C. aeschynomenes)[3]所致。
笔者在本研究中为明确福建李叶斑病的病原种类,对该病进行田间调查,采集病样进行病原菌分离、形态学观察及多基因鉴定、致病性验证。研究结果可为产区李叶斑病的防控提供理论依据,并为深入了解植物病原刺盘孢种类和致病多样性提供新的信息。
1 材料和方法
1.1 材料
2020年6月至2021年6月,在福建省连城县李叶斑病发生最严重的2个李园进行病害系统观察,并从3个李品种(芙蓉李、油柰、花柰)园,分别采用五点取样法,每点选取5株李树,每株李树上随机采集4枚显现有叶斑病典型症状的叶片,作为病叶样品用于病菌的分离。
1.2 方法
1.2.1 病原菌的分离纯化 从病叶样品病健交界处切取4~5 mm2组织块,放入75%的乙醇中消毒45 s后,使用无菌水中漂洗2次后取出,放在灭菌滤纸上晾干后,置于PDA培养基上,在28 ℃恒温培养箱中黑暗培养2~3 d,待菌落长出后,挑取小块未污染的菌丝块移至新的PDA培养基上培养5 d。可产生分生孢子的分离株的纯化方法参考《植病研究方法》概述的琼胶平板表面单孢子挑取方法[9],未观察到产孢的分离株通过单菌丝纯化方法[10]。纯化后的菌株分别置于PDA斜面上4 ℃保存和25%甘油中-80 ℃保存备用。
1.2.2 培养特征观察 挑取培养5 d的菌落边缘的菌丝块(直径5 mm)转接至PDA平板中央,置于28 ℃恒温培养箱中黑暗条件下培养,每个菌株重复3皿。每天记录菌落在培养基上的生长情况并测量菌落直径。在体视显微镜(SZX16,Japan)下观察分生孢子堆和子囊壳。在光学显微镜(Olympus BX63,Japan)下观察分生孢子、子囊、子囊孢子等并测量大小。
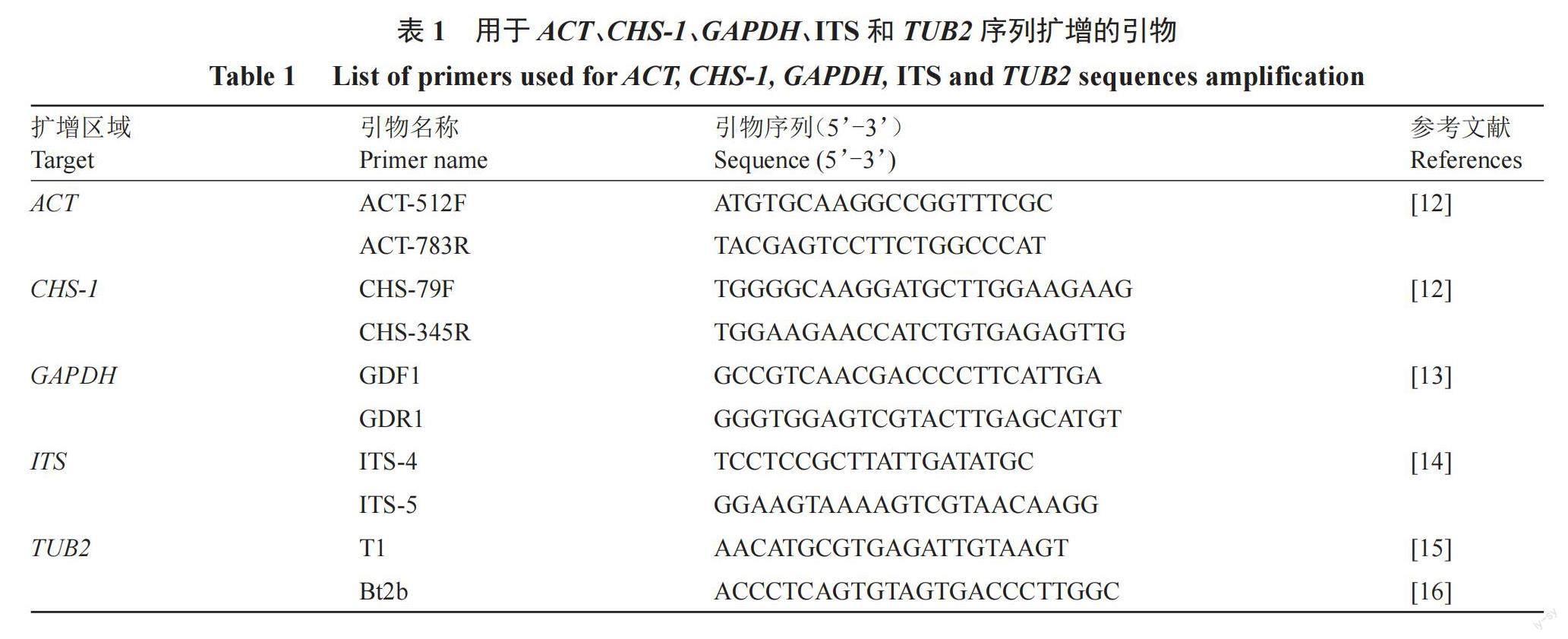
1.2.3 多基因扩增与测序 将供试菌株的菌丝块转入铺有灭菌玻璃纸的PDA平板中央,置于28 ℃智能光照培养箱中培养6 d后收集菌丝,采用CTAB(十六烷基三乙基溴化铵)法提取分离物基因组DNA[11],并通过1.2%琼脂糖凝胶电泳后检测提取的DNA质量。采用引物ITS4/ITS5、GDF1/GDR1、ACT-512F/ACT-783R、CHS-79F/CHS-345R和T1/Bt2b分别扩增菌株的ITS序列、GAPDH 200 bp的内含子以及ACT、CHS-1和TUB2基因的部分序列。具体引物信息见表1[12-16]。PCR反应体系和扩增参考Weir等[17]的方法。将PCR产物委托生工生物工程股份有限责任公司进行测序。
1.2.4 系统发育分析 在核酸序列数据库GenBank(http://www.ncbi.nlm.nih.gov)中BLAST搜索并下载与测序所得序列相似性较高的序列和刺盘孢属各模式种的序列,使用DNAMAN 9.0软件对本研究中得到的产物序列与从GenBank中下载的序列进行多重比对和分析,再使用默认设置的MAFFT v.7对序列进行多重比对,必要时用MEGA7对序列进行手工校正。将比对好的基因序列按照ACT、TUB2、CHS-1、GAPDH和ITS的顺序首尾相连进行拼接[18]。使用IQ-TREE对合并的数据集进行遗传进化分析,采用最大似然法(Maximum-likelihood)并选用GTR+G核苷酸替代模型构建系统进化树,用自展檢验法以1000次重复计算支持率。
1.2.5 致病性测定 分生孢子悬浮液有伤接种:以健康的李叶片为接种材料,用75%乙醇表面消毒后,使用无菌的昆虫针(直径0.5 mm)在叶脉两侧各针刺1次,将代表菌株的分生孢子悬浮液(浓度为106 个·mL-1)7 μL滴注在叶片的刺伤点上[19],每个菌株接种叶片8枚,3次重复,并以无菌水接种作为对照。接种后的叶片置于接种盘中(盘底铺滴有无菌水的灭菌纱布,上覆保鲜膜),再用塑料保鲜膜密封保湿,将其置于25 ℃、12/12 h光暗交替环境中培养。培养期间每天观察叶片发病情况,接种第6天测量病斑大小并拍照记录。
菌丝块有伤接种:以健康的李叶片和果实为接种材料,用75%乙醇表面消毒后,分别用3根捆绑一起的无菌昆虫针(直径0.5 mm)在果实侧面中央位置刺入约5 mm深,用单根无菌昆虫针在每枚叶片的叶脉两侧各针刺1次。用打孔器打取供试菌株的菌丝块(直径5 mm),将菌丝块有菌丝的一面接种于刺伤点,每个菌株接种叶片8枚或果实5个,3次重复,以接种空白PDA培养基为对照,用无菌的脱脂棉蘸取适量无菌水后敷在菌丝块上保湿,接种48 h后去除脱脂棉和菌丝块,培养条件同分生孢子悬浮液有伤接种,第10天测量病斑直径。
1.2.6 寄主范围测定 选取桃(Prunus persica)、梨(Pyrus pyrifolia)、柑橘(Citrus reticulata)、猕猴桃(Actinidia chinensis)的离体叶片,有伤接种6种刺盘孢的共10个代表菌株,每个菌株接种5枚叶片,3次重复。接种方法与观察同1.2.5。
2 结果与分析
2.1 李叶斑病的田间症状表现与获得的刺盘孢属(Colletotrichum)菌株
2020年6月至2021年6月在福建省连城县李叶斑病发生最严重的2个李园进行病害系统观察,结果显示,病害发生初期,叶片上形成浅褐色斑点(图1-A),之后扩展成灰色至深褐色条状病斑(图1-B),病斑的周围有黄色晕圈,发病后期叶尖和叶缘向上卷缩并焦枯(图1-C)。发病严重时,整株叶片发生干枯(图1-D)。
同期在福建省连城县,从3个李品种(芙蓉李、油柰和花柰)上采集显现李叶斑病典型症状的病叶,并通过组织分离法进行病菌分离和纯化,共从30份病叶样品中获得98个分离物。根据对其菌落形态特征观察,其中有66个分离物属于刺盘孢属(Colletotrichum)菌株,占总分离物的67.3%。
2.2 多基因系统发育分析和鉴定出的刺盘孢种类
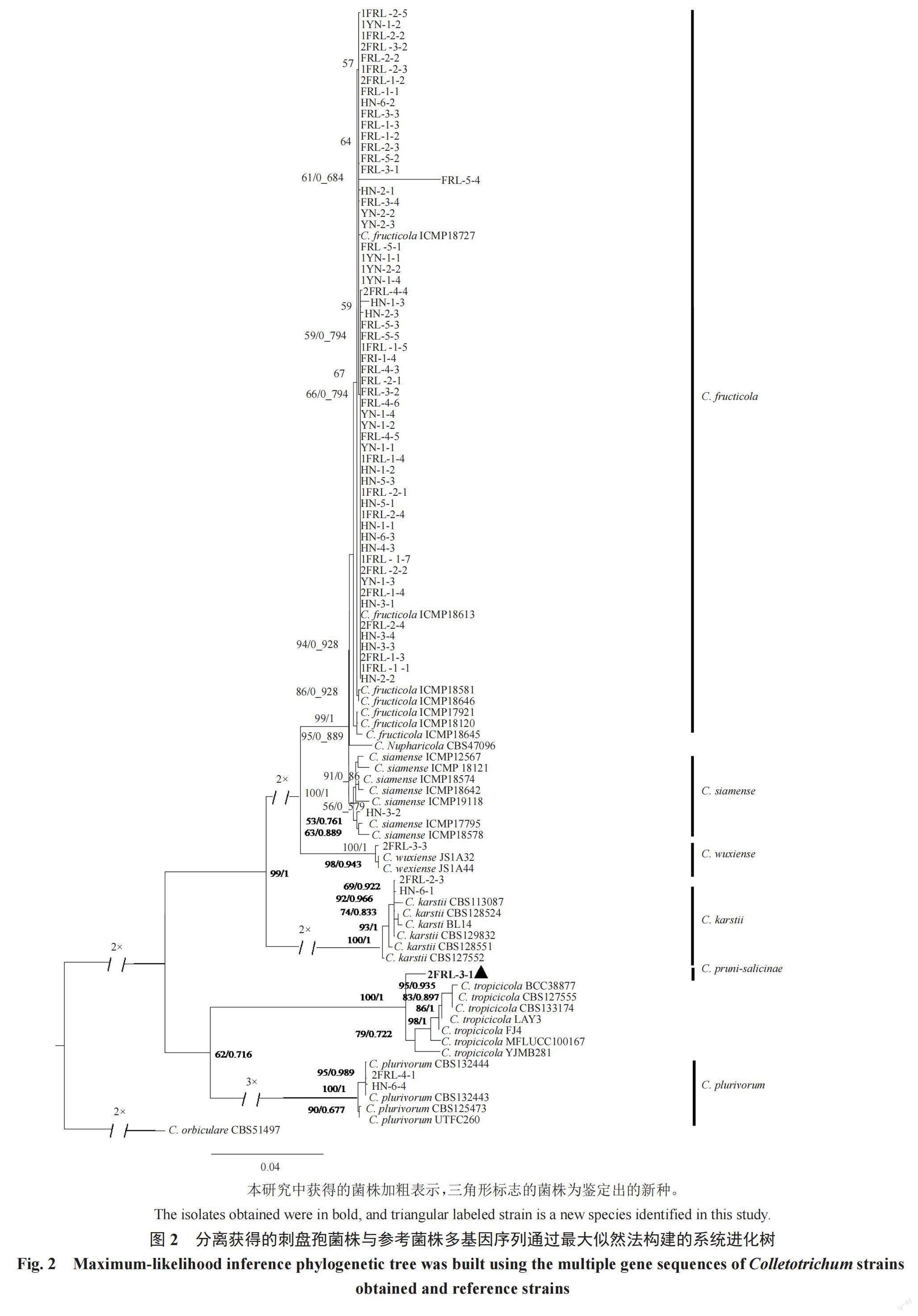
对获得的66个刺盘孢属(Colletotrichum)菌株进行DNA提取和ACT、TUB2、CHS-1、GAPDH、ITS序列扩增,分别得到大小约280、780、285、270、600 bp的特异性片段。按照ACT-TUB2-CHS-1-GAPDH-ITS顺序进行序列串联,以瓜类炭疽菌(C. orbiculare)(CBS51497)为外群构建系统发育进化树,序列分析结果(图2)显示,这66个菌株的序列聚集在6个不同的分支上,其中59个菌株与果生刺盘孢(C. fructicola)聚集在一个分支上,2个菌株(2FRL-2-3和HN-6-1)与喀斯特刺盘孢(C. karstii)的来源于美国海棠分离物(CBS 113087)亲缘关系最近,2个菌株(HN-6-4和2FRL-4-1)与普洛柏刺盘孢(C. plurivorum)的来源于巴西棉花分离物(CBS 132444)聚为一个分支,菌株HN-3-2与暹罗刺盘孢(C. siamense)的来源于泰国咖啡分离物(ICMP 18578)亲缘关系最近,菌株2FRL-3-3与无锡刺盘孢(C. wuxiense)的来源于中国茶分离物(JS1A44和JS1A32)聚为一个分支,菌株2FRL-3-1以高支持率单独形成一个分枝,与热带生刺盘孢(C. tropicicola)BCC 38877分离株的亲缘关系最近。进一步研究显示,菌株2FRL-3-1的培养特性与形态特征(结果见2.3中的描述)与热带生炭疽菌(C. tropicicola)模式菌株BCC 38877[20]存在明显差异,由此认定2FRL-3-1为刺盘孢属的一个新种,并根据其寄主命名为李刺盘孢(C. pruni-salicinae),Mycobank登陆号:MB843544,主模式标本号:HMAS 352256,中国普通微生物菌种保藏管理中心保存编号:CGMCC3.20959。
研究结果(表2)表明,从福建李叶斑病样品中分离获得的66个刺盘孢属(Colletotrichum)菌株,分属于刺盘孢属的6个种,其中果生刺盘孢(C. fructicola)59个菌株、喀斯特刺盘孢(C. karstii)2个菌株、普洛柏刺盘孢(C. plurivorum)2个菌株、暹罗刺盘孢(C. siamense)1个菌株、无锡刺盘孢(C. wuxiense)1个菌株和李刺盘孢(C. pruni-salicinae)1个菌株。结果显示果生刺盘孢(C. fructicola)为优势种,其菌株数占刺盘孢属(Colletotrichum)菌株数的89.4%(59/66),而其余5種刺盘孢的菌株数仅占10.6%(7/66)。
2.3 6种刺盘孢菌(代表菌株)的形态学特征
(1)果生刺盘孢(C. fructicola,YN-2-5):在PDA培养基上菌落灰白色,可产生黑色色素,气生菌丝发达;培养14 d后,产生橙黄色分生孢子堆(图3-a),分生孢子圆柱状(图3-i),两端钝圆,大小为(14~18.5) μm×(4.5~6.5) μm;培养20 d后可见有性态(图3-g),子囊壳内含有棍棒状的子囊,子囊内有8个呈梭状的子囊孢子(图3-h)。
(2)暹罗刺盘孢(C. siamense,HN-3-2):在PDA培养基上菌落灰白色,可产生黑色色素,气生菌丝发达;培养12 d后,产生橙色的分生孢子堆(图3-b),分生孢子圆柱状,两端钝圆,透明,无隔膜(图3-j),大小为(11~17.2) μm×(4.3~6.9) μm;培养20 d可见有性态(图3-e),子囊壳内含有棍棒状子囊,子囊内产生有梭状子囊孢子(图3-f)。
(3)无锡刺盘孢(C. wuxiense,2FRL-3-3):在PDA培养基上菌落白色,不产生色素,气生菌丝发达;未观察到孢子产生。
(4)喀斯特刺盘孢(C. karstii,HN-6-1):在PDA培养基上菌落白色,可产生淡黄色色素,气生菌丝较弱;培养14 d后,产生棕黄色分生孢子堆(图3-c),分生孢子光滑,无隔膜,短棒状(图3-k),一端稍微凸起,大小为(11~15) μm×(5~7) μm;未观察到有性态。
(5)普洛柏刺盘孢(C. plurivorum,HN-6-4):在PDA培养基上菌落墨绿色,可产生黑色色素,气生菌丝发达;未观察到孢子产生。
(6)李刺盘孢(C. pruni-salicinae,2FRL-3-1):菌落正面灰色,背面产生黄绿色色素,生长速率为1.20~1.22 cm·d-1。培养14 d后,产生浅黄色的分生孢子堆(图3-d),分生孢子透明、无隔膜,两端钝圆(图3-l),大小为(15~22) μm×(5~10) μm,`x =(17.7±1.26) μm×(7.2±0.98) μm,n = 50。
2.4 致病性测定结果
从已鉴定出的6种刺盘孢中各选取1個代表菌株,采用分生孢子悬浮液或菌丝块有伤接种到李(P. salicina Lindl.)叶片。结果显示,果生刺盘孢(C. fructicola)YN-2-5菌株、喀斯特刺盘孢(C. karstii)HN-6-1菌株、暹罗刺盘孢(C. siamense)HN-3-2菌株、李刺盘孢(C. pruni-salicinae)2FRL-3-1菌株的分生孢子悬浮液和无锡刺盘孢(C. wuxiense)2FRL-3-3菌株、普洛柏刺盘孢(C. plurivorum)HN-6-4菌株的菌丝块接种后均能使叶片发病,发病率为100%(24/24),产生的近圆形黄褐色病斑(图4)与田间症状表现相同。对发病组织进行病菌再分离和鉴定,结果显示再分离出的病菌种类与接种的菌株一致。结果表明,笔者在所鉴定出的果生刺盘孢(C. fructicola)、喀斯特刺盘孢(C. karstii)、普洛柏刺盘孢(C. plurivorum)、暹罗刺盘孢(C. siamense)、无锡刺盘孢(C. wuxiense)和李刺盘孢(C. pruni-salicinae)等6种刺盘孢均为引起福建李叶斑病的病原菌。
选取6种刺盘孢的代表菌株,采用菌丝块有伤接种李果实。结果显示,这些代表菌株均能使果实产生坏死斑症状,发病率为100%(15/15)。对接种10 d后产生的病斑进行长度观测(图5),可以看出,不同刺盘孢种的致病力有异。暹罗刺盘孢(C. siamense)的致病力最强,平均病斑直径2.23 cm;果生刺盘孢(C. fructicola)和无锡刺盘孢(C. wuxiense)致病力居中,平均病斑直径1.55 cm;普洛柏刺盘孢(C. plurivorum)和李刺盘孢(C. pruni-salicinae)的致病力较弱,平均病斑直径0.86 cm。喀斯特刺盘孢(C. karstii)的不同菌株之间的致病力也存在较大差异,菌株HN-6-1产生的平均病斑直径达1.34 cm,而菌株2FRL-2-3平均病斑直径则为0.61 cm。
对寄主范围测定的结果显示,6种刺盘孢的10个代表菌株对桃、梨、柑橘和猕猴桃的致病存在明显差异。普洛柏刺盘孢(C. plurivorum)和李刺盘孢(C. pruni-salicinae)在梨叶上的发病率分别为44%和66%,其余4种刺盘孢在桃和梨叶上的发病率均100%。果生刺盘孢(C. fructicola)和无锡刺盘孢(C. wuxiense)在柑橘叶上的发病率分别为66%和33%,在猕猴桃叶上的发病率分别为55%和33%。暹罗刺盘孢(C. siamense)在猕猴桃叶上的发病率66%。
3 讨 论
近年在福建省,特别是连城县的李产区新发生的李叶斑病危害严重,已成为李生产上的突出问题。笔者在本研究中通过对连城李产区叶斑病的发生状况和危害特点进行系统性调查和病原菌分离纯化、形态特征观察和多基因序列分析及致病性验证,首次明确了福建李叶斑病的病原菌种类,其中果生刺盘孢(C. fructicola)为优势种,占总分离株数的89.4%,研究结果为该地李叶斑病的有效防控提供了理论依据。
研究结果表明,引起福建李叶斑病的病原菌有果生刺盘孢(C. fructicola)、喀斯特刺盘孢(C. karstii)、普洛柏刺盘孢(C. plurivorum)、暹罗刺盘孢(C. siamense)、无锡刺盘孢(C. wuxiense)和李刺盘孢(C. pruni-salicinae)等6种,引起日本李果实炭疽病的病原菌有尖孢炭疽菌(C. acutatum)、胶孢炭疽菌(C. gloeosporioides)和内乏亚炭疽菌(C. nymphaeae)等3种[4-5],韩国李果实炭疽病的病原菌有胶孢炭疽菌(C. gloeosporioides)、内乏亚炭疽菌(C. nymphaeae)、松针炭疽菌(C. foriniae)和暹罗刺盘孢(C. siamense)等4种[5-6]。这类由多种刺盘孢菌复合侵染所致的病害,其病原菌组成的差异是否由李的种类或品种、环境条件等的不同导致,还需进一步研究。
植物病原刺盘孢属(Colletotrichum)有多个种和复合种,多数菌株能够表现出稳定且特定的形态特征,形态培养特性对刺盘孢属种间的鉴定具有重要的意义[21],可以作为初步判断所属复合种的依据[22]。Liu等[23]研究发现平头刺盘孢(C. truncatum)、辣椒刺盘孢(C. scovillei)、南瓜刺盘孢(C. brevisporum)和部分果生刺盘孢(C. fructicola)有独特相对稳定的菌落形态便于区分。Than等[24]认为菌落生长速率是区分胶孢刺盘孢(C. gloeosporioides)、平头刺盘孢(C. truncatum)和尖孢刺盘孢(C. acutatum)的重要指标。而仅利用形态学特征很难准确鉴定到种,目前对刺盘孢菌种的鉴定,多采用形态学观测结合分子生物学鉴定[25-26]。笔者在本研究中通过多基因(ACT、TUB2、CHS-1、GAPDH和ITS)系统进化分析,发现2FRL-3-1菌株以高支持率单独形成一个分化枝,与热带生刺盘孢(C. tropicicola)BCC 38877分离株的亲缘关系最近,进一步研究发现二者在生长速率和分生孢子大小方面存在明显不同,由此认定2FRL-3-1为刺盘孢属的一个新种,并根据其寄主命名为李刺盘孢(C. pruni-salicinae)。本研究结果为植物病原刺盘孢菌的分类鉴定提供了新的有用信息。
炭疽病可由多种刺盘孢菌复合侵染,引起墨西哥杧果炭疽病的病原有暹罗刺盘孢(C. siamense)、杧果刺盘孢(C. asianum)、热带生刺盘孢(C. tropicicola)、隐秘刺盘孢(C. alienum)和果生刺盘孢(C. fructicola),从杧果病组织中它们的分离比例存在差异,且所有分离株均对杧果果实具有致病性,不同种间存在分化现象,暹罗刺盘孢(C. siamense)和杧果刺盘孢(C. asianum)的致病力强于隐秘刺盘孢(C. alienum)和果生刺盘孢(C. fructicola)[27]。本研究结果显示,在试验条件下果生刺盘孢(C. fructicola)、喀斯特刺盘孢(C. karstii)、普洛柏刺盘孢(C. plurivorum)、暹罗刺盘孢(C. siamense)、无锡刺盘孢(C. wuxiense)和李刺盘孢(C. pruni-salicinae)等6种刺盘孢菌均可使李的叶片和果实致病,但在田间病叶中它们的分离比例存在显著差异,后5种仅占10.6%。因此它们在病害中的作用以及与果生刺盘孢(C. fructicola)之間的关系还有待深入研究。致病性测定显示,在引起福建李叶斑病的6种病原刺盘孢中,暹罗刺盘孢(C. siamense)对李果实的致病力较其余5种明显增强。而喀斯特刺盘孢(C. karstii)的HN-6-1和2FRL-2-3菌株,接种到李果实后所产生的病斑的平均直径也存在显著差异,前者为1.34 cm,后者仅0.61 cm。这些结果表明植物病原刺盘孢的不同种以及同种的不同菌株对同一种寄主均存在明显的致病力分化现象[28]。
植物病原刺盘孢的寄主范围极为广泛,同一种刺盘孢菌可侵染多种植物造成严重的病害[29-31]。刺盘孢菌在多种寄主上具有交叉侵染的潜力[32],来源于杧果和牛油果的胶孢刺盘孢(C. gloeosporioides)的分离株可以侵染辣椒、草莓、番石榴和木瓜[33]。本研究结果显示,果生刺盘孢(C. fructicola)和无锡刺盘孢(C. wuxiense)可侵染李、桃、梨、柑橘和猕猴桃叶片,喀斯特刺盘孢(C. karstii)、普洛柏刺盘孢(C. plurivorum)和李刺盘孢(C. pruni-salicinae)也可侵染李、桃和梨叶片。不同宿主上分离得到的刺盘孢菌株的致病力存在差异,分离自牛油果的分离株致病力强于杧果分离株,分离株在原寄主叶片上的致病性要比在其他叶片上的致病性强得多,这些差异可能由于病原体对不易感的宿主的适应,从而具有更强的致病性,以克服宿主的防御机制[33-34]。因此,在果园田间管理中应注意防范交互感染。
4 结 论
引起福建李叶斑病的病原菌有果生刺盘孢(C. fructicola)、喀斯特刺盘孢(C. karstii)、普洛柏刺盘孢(C. plurivorum)、暹罗刺盘孢(C. siamense)、无锡刺盘孢(C. wuxiense)和李刺盘孢(C. pruni-salicinae)等6种,其中果生刺盘孢为优势病原种,占刺盘孢属(Colletotrichum)菌株的89.4%。不同刺盘孢菌的致病性存在明显差异。
参考文献 References:
[1] OLAWOLE O I,URIBE P,RODRIGUEZ N A,GONZALEZ C F,ONG K L. First report of bacterial leaf scald of plum caused by Xylella fastidiosa in texas[J]. Plant Disease,2022,106(12):3198.
[2] LU M M,ZHANG Y J,LI Q L,HUANG S P,TANG L H,CHEN X L,GUO T X,MO J Y,MA L A. First report of leaf blight caused by Fusarium pernambucanum and Fusarium sulawesiense on plum in Sichuan,China[J]. Plant Disease,2022,106(10):2759.
[3] LU M M,MA L A,TANG L H,CHEN X L,GUO T X,MO J Y,HUANG S P,LI Q L. First report of anthracnose of Sanhua plum caused by Colletotrichum aeschynomenes in Guangxi,China[J]. Plant Disease,2022,107(4):1223.
[4] CHANG T H,HASSAN O,LEE Y S. First report of anthracnose of Japanese plum (Prunus salicina) caused by Colletotrichum nymphaeae in Korea[J]. Plant Disease,2018,102(7):1461.
[5] HASSAN O,LEE Y S,CHANG T. Colletotrichum species associated with Japanese plum (Prunus salicina) anthracnose in South Korea[J]. Scientific Reports,2019,9:12089.
[6] LEE Y S,HA D H,LEE T Y,PARK M J,CHUNG J B,JEONG B R. Isolation and characterization of Colletotrichum isolates causing anthracnose of Japanese plum fruit[J]. Korean Journal of Environmental Agriculture,2017,36(4):299-305.
[7] 林雄杰,王賢达,胡菡青,潘昌,范国成. 芙蓉李炭疽病的病原鉴定及生物学特性[J]. 中国南方果树,2016,45(4):28-34.
LIN Xiongjie,WANG Xianda,HU Hanqing,PAN Chang,FAN Guocheng. Identification of the pathogenic fungus causing anthracnose of Furong plums and its biological characteristics[J]. South China Fruits,2016,45(4):28-34.
[8] CUI Y P,PENG A T,SONG X B,CHEN X,LING J F. First report of Japanese plum anthracnose caused by Colletotrichum fioriniae in China[J]. Journal of Plant Pathology,2021,103(3):1009.
[9] CHOI Y W,HYDE K D,HO W H. Single spore isolation of fungi[J]. Fungal Diversity,1999,3:29-38.
[10] YAEGASHI H,KANEMATSU S,ITO T. Molecular characterization of a new hypovirus infecting a phytopathogenic fungus,Valsa ceratosperma[J]. Virus Research,2012,165(2):143-150.
[11] FREEMAN S,KATAN T,SHABI E. Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests[J]. Applied and Environmental Microbiology,1996,62(3):1014-1020.
[12] CARBONE I,KOHN L M. A method for designing primer sets for speciation studies in filamentous ascomycetes[J]. Mycologia,1999,91(3):553-556.
[13] GUERBER J C,LIU B,CORRELL J C,JOHNSTON P R. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns,mtDNA and intron RFLPs,and mating compatibility[J]. Mycologia,2003,95(5):872-895.
[14] WHITE T J,BRUNS T,LEE S,TAYLOR J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[J]. PCR Protocols,A Guide to Methods and Application,1990(1):315-322.
[15] ODONNELL K,CIGELNIK E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous[J]. Molecular Phylogenetics and Evolution,1997,7(1):103-116.
[16] GLASS N L,DONALDSON G C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J]. Applied and Environmental Microbiology,1995,61(4):1323-1330.
[17] WEIR B S,JOHNSTON P R,DAMM U. The Colletotrichum gloeosporioides species complex[J]. Studies in Mycology,2012,73:115-180.
[18] KATOH K,STANDLEY D M. MAFFT multiple sequence alignment software version 7:improvements in performance and usability[J]. Molecular Biology and Evolution,2013,30(4):772-780.
[19] LIN Q,KANCHANA-UDOMKARN C,JAUNET T,MONGKOLPORN O. Genetic analysis of resistance to pepper anthracnose caused by Colletotrichum capsici[J]. Thai Joural Agricultural Science,2002,35(3):259-264.
[20] NOIREUNG P,PHOULIVONG S,LIU F,CAI L,MCKENZIE E H C,CHUKEATIROTE E,JONES E B G,BAHKALI A H,HYDE K D. Novel species of Colletotrichum revealed by morphology and molecular analysis[J]. Cryptogamie Mycologie,2012,33(3):347-362.
[21] PRIHASTUTI H,CAI L,CHEN H,MCKENZIE E H C,HYDE K D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand[J]. Fungal Diversity,2009,39(1):89-109.
[22] GUARNACCIA V,GROENEWALD J Z,POLIZZI G,CROUS P W. High species diversity in Colletotrichum associated with citrus diseases in Europe[J]. Persoonia - Molecular Phylogeny and Evolution of Fungi,2017,39(1):32-50.
[23] LIU F L,TANG G T,ZHENG X J,LI Y,SUN X F,QI X B,ZHOU Y,XU J,CHEN H B,CHANG X L,ZHANG S R,GONG G S. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province,China[J]. Scientific Reports,2016,6:32761.
[24] THAN P P,JEEWON R,HYDE K D,PONGSUPASAMIT S,MONGKOLPORN O,TAYLOR P W J. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand[J]. Plant Pathology,2008,57(3):562-572.
[25] ZHANG W,DAMM U,CROUS P W,GROENEWALD J Z,NIU X L,LIN J M,LI Y T. Anthracnose disease of carpetgrass (Axonopus compressus) caused by Colletotrichum hainanense sp. nov.[J]. Plant Disease,2020,104(6):1744-1750.
[26] XUE L H,ZHANG Y W,DUAN T Y,LI M Y,WHITE J F,LIU Y,LI C J. Characterization and pathogenicity of Colletotrichum species on Philodendron tatei cv. Congo in Gansu Province,China[J]. Plant Disease,2020,104(10):2571-2584.
[27] TOVAR-PEDRAZA J M,MORA-AGUILERA J A,NAVA-D?AZ C,LIMA N B,MICHEREFF S J,SANDOVAL-ISLAS J S,C?MARA M P S,T?LIZ-ORTIZ D,LEYVA-MIR S G. Distribution and pathogenicity of Colletotrichum species associated with mango anthracnose in Mexico[J]. Plant Disease,2020,104(1):137-146.
[28] FU M,CROUS P W,BAI Q,ZHANG P F,XIANG J,GUO Y S,ZHAO F F,YANG M M,HONG N,XU W X,WANG G P. Colletotrichum species associated with anthracnose of Pyrus spp. in China[J]. Persoonia,2019,42:1-35.
[29] SHU J,NING P,GUO T X,TANG L H,HUANG S P,LI Q L,MO J Y,YU Z H,HSIANG T. First report of leaf spot caused by Colletotrichum fructicola on Callerya speciosa (Millettia speciosa) in Guangxi,China[J]. Plant Disease,2020,104(12):3256.
[30] 鐘杰,尹秀娟,钟双玉,陈锦,朱俊子,李晓刚. 富贵草叶斑病病原的鉴定[J]. 湖南农业大学学报(自然科学版),2022,48(1):60-64.
ZHONG Jie,YIN Xiujuan,ZHONG Shuangyu,CHEN Jin,ZHU Junzi,LI Xiaogang. Identification of pathogen causing Colletotrichum liriopes leaf spot on Pachysandra terminalis[J]. Journal of Hunan Agricultural University (Natural Sciences),2022,48(1):60-64.
[31] 刘梅,王彩霞,燕继晔,贾静怡,李兴红. 辽宁省北镇市葡萄叶斑病的病原鉴定[J]. 植物保护,2021,47(2):185-188.
LIU Mei,WANG Caixia,YAN Jiye,JIA Jingyi,LI Xinghong. Identification of the pathogen causing leaf spot on grape in Beizhen city of Liaoning Province[J]. Plant Protection,2021,47(2):185-188.
[32] FREEMAN S,KATAN T,SHABI E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits[J]. Plant Disease,1998,82(6):596-605.
[33] SANDERS G M,KORSTEN L. Comparison of cross inoculation potential of South African avocado and mango isolates of Colletotrichum gloeosporioides[J]. Microbiological Research,2003,158(2):143-150.
[34] ALAHAKOON P W,BROWN A E,SREENIVASAPRASAD S. Cross-infection potential of genetic groups of Colletotrichum gloeosporioides on tropical fruits[J]. Physiological and Molecular Plant Pathology,1994,44(2):93-103.
