Network pharmacology and preliminary cell screening studies on the anti-liver cancer activity of Nauclea Officinalis
2023-11-20CHENWeijiaZHOUMingyanHUJichengZHUZeXUJian
CHEN Wei-jia, ZHOU Ming-yan, HU Ji-cheng, ZHU Ze, XU Jian✉
1.Department of Organ Transplantation, the Second Affiliated Hospital of Hainan Medical University, Haikou 570105, China
2.Institute of Clinical Medicine, the Second Affiliated Hospital of Hainan Medical University, Haikou 570105, China
3.Hainan Institute of Organ Transplantation, Haikou 570105, China
Keywords:
ABSTRACT Objective: To explore the mechanism of Nauclea Officinalis of anti-liver cancer effect based on network pharmacology, and to preliminarily verify anti-liver cancer activity of Nauclea Officinalis through cell screening.Methods: Network pharmacology was used to screen for common targets of Nauclea Officinalis and liver cancer, protein-protein interaction (PPI)network was constructed, and enrichment analysis and mechanism prediction were conductd.Molecular docking of main active ingredients of Nauclea Officinalis with core targets was made.Preliminary verification was performed by in vitro cell experiments such as CCK8, cell apoptosis, and PCR.Results: After the screening, 14 active ingredients of Nauclea Officinalis were obtained, with 587 related targets.After mapping with liver cancer targets, there were 288 common targets, mainly including TP53, SRC, STAT3, and other core targets.Among them, compounds such as strictosamide, pumiloside and vincosamide may be potential active ingredients of Nauclea Officinalis of anti-liver cancer effect.They may participate in protein phosphorylation and negative regulation of the apoptosis process by mediating cancer pathways, PI3K/Akt and EGFR tyrosine kinase inhibitors resistance signaling pathways to play an anti-liver cancer role; molecular docking results showd that active ingredients of Nauclea Officinalis had a stable binding with liver cancer core targets; in vitro cell experiments showd that main ingredient strictosamide of Nauclea Officinalis had cytotoxicity against liver cancer cells, inhibited liver cancer cell proliferation (P<0.001), down-regulated gene expression of liver cancer HepG2 cells SRC, STAT3, MAPK3 (P<0.05), and induced liver cancer cell apoptosis (P<0.001).Conclusion: This study preliminarily explores the potential mechanism of active ingredients of Nauclea Officinalis against liver cancer and its preliminary pharmacological effects, providing a theoretical basis for the study of Nauclea Officinalis of anti-liver cancer mechanism.
1.Introduction
Liver cancer is the 5th most common malignant tumor and the and cause of tumor death in China, which greatly affects the life,health and property safety of our people[1].In early stage liver cancer without distant metastasis, surgical resection of tumor is the main treatment.However, most patients, once diagnosed, are already in the advanced stage of liver cancer and miss the best time for surgical treatment, so they often can only be treated by drugs.Currently, first-line therapeutic drugs such as sorafenib are mainly used clinically against hepatocellular carcinoma, but patients are prone to tolerance and adverse effects such as diarrhea, hand-foot syndrome and hypertension with long-term use[2].Therefore, it is urgent to research effective drugs for liver cancer prevention and treatment.Chinese medicine, as a kui po in China, has been proven in many clinical studies to be effective in improving the symptoms and improving the quality of life of patients with advanced liver cancer, reducing tumor recurrence, controlling disease progression and drug tolerance[3,4].
Nauclea Officinalis, also known as medicinal otan, is the only native species of otan plant in China in the Rubiaceae family[5],mainly produced in Hainan Qiongzhong, Baisha and other Li regions, with a bitter taste and cold nature, and its main active ingredient is alkaloids[6].Alkaloids have the characteristics of low toxicity, high potency and high specificity.Their anti-liver cancer mechanism does not directly kill liver cancer cells, but exerts antiliver cancer effects by changing the morphology of liver cancer cells,inhibiting cancer cell metastasis, promoting apoptosis and causing cell cycle blockage[7].It has been found that the alkaloids such as Pumiloside, 3-epi- Pumiloside and Vincosamide glycosides in Nauclea Officinalis have anti-tumor effects[8,9].
The action and mechanism of Nauclea Officinalis against hepatocellular carcinoma is not well defined, therefore, this study was conducted to predict and initially validate the action and mechanism of Nauclea Officinalis against hepatocellular carcinoma.Network pharmacology is a new tool in modern Chinese medicine research, which exploits the targets of compound-disease interactions to explore the mechanism of drug action[10,11].While traditional Chinese medicine emphasizes the holistic effect of drugs on the body network, network pharmacology matches with the holistic view of the action of traditional Chinese medicine, so network pharmacology has unique advantages for the study of traditional Chinese medicine[12].In this study, we systematically predicted the pharmacodynamic components, potential targets and pathway mechanisms of the antihepatocellular carcinoma effect of Nauclea Officinalis using a network pharmacology approach, and initially verified the antihepatocellular carcinoma pharmacological activity of Strictosamide, the main component of Nauclea Officinalis, through in vitro cellular assays, providing a theoretical basis for subsequent in-depth investigation and clinical application.
2.Materials and Methods
2.1 Nauclea Officinalis chemical composition screening and predicted target acquisition
The main compound components of Nauclea Officinalis were obtained by searching PubMed and CNKI, the molecular structure and CanonicalSMILES number of Nauclea Officinalis chemical components were obtained by searching PubChem database(http://pubchem.ncbi.nlm.nih.gov), and the target sites of Nauclea Officinalis chemical components were predicted by SwissTargetPrediction(http://swisstargetprediction.ch/) website to predict the targets of the chemical components of Nauclea Officinalis, and after correction with UniProtKnowledgebase(http://www.uniprot.org/), we used SwissTargetPredictionProb 0.10,TargetNetProb 0.60 or PharmMapperFit0.60 were used to screen the predicted targets.
2.2 Liver cancer target acquisition
Using “liver cancer” and “hepatocarcinoma” as keywords and“Homo sapiens” as the species limit, search PharmGKB(https://www.pharmgkb.org), DisGeNET(http://www.disgenet.org),OMIM(http://www.omim.org), GeneCards(https://www.genecards.org)/) databases, using GeneCards scores 15 and DisGeNET0.10 as screening criteria, and removing duplicate targets after merging to obtain targets for hepatocellular carcinoma.
2.3 Acquisition of the target of action of Nauclea Officinalis against hepatocellular carcinoma
The target of Nauclea Officinalis was intersected with the target of liver cancer using Venny2.1 (http://bioinformatics.psb.ugent.be/webtools/Venn/) to obtain the target of anti-liver cancer action of Nauclea Officinalis.
2.4 PPI network construction and analysis
The above targets were uploaded to STRING11.5 database with the species “Homo Sapiens” and confidence level of 0.9, and the free targets were hidden, and the TSV format files were downloaded and imported into Cytoscape3.9.1 software to create PPI network maps.Network Analyze” was used to analyze the gene node degree and mediator values in the network and to screen the core targets.
2.5 Biological function and pathway enrichment analysis of the anti-hepatocellular carcinoma targets of Nauclea Officinalis
The target of Nauclea Officinalis against hepatocellular carcinoma was imported into the David database (https://david.ncifcrf.gov/),and the species was set as “Homo Sapiens” with a threshold of P<0.05.Biological function and pathway enrichment analyses were performed, and the results were visualized by OMishare Tools(https://www.omicshare.com/tools/) to visualize the results.
2.6 Construction of “drug-component-target-pathwaydisease” interaction network of Nauclea Officinalis against hepatocellular carcinoma
The anti-hepatocellular carcinoma targets and components of Nauclea Officinalis and the first 14 enriched signaling pathways were imported into Cytoscape 3.9.1, and the “drug-component-targetpathway-disease” network diagram was drawn.The nodes represent drug, component, target, pathway and disease, and the active ingredients of Nauclea Officinalis against hepatocellular carcinoma were screened by the degree value.
2.7 Molecular docking of key components and core targets of Nauclea Officinalis against hepatocellular carcinoma
For the active ingredient as ligand, the 3D structure of the key ingredient was downloaded from PubChem; for the core target as receptor, the 3D molecular structure of the core target was downloaded from ProteinDataBank database (https://pubchem.ncbi.nlm.nih.gov/).The active ingredient and the core target were imported into Discovery Studio2019 Client software, and then the molecular docking was performed by DOCKLigand after dehydration and hydrogenation, and the relationship between the active ingredient and the core target was obtained.
2.8 In vitro cellular assays
2.8.1 Materials
LX-2 human normal hepatocytes, HepG2 hepatocellular carcinoma cells, DMEM and RPMI-1640 medium, 10% fetal bovine serum,1% penicillin/streptomycin, 0.25% trypsin, PBS buffer, CCK-8 kit(APExBIO), Strictosamide (purchased from MedBio, purity 98%),Annexin V-APC/PI double-stained apoptosis detection kit (KGI),GAPDH, SRC, STAT3, MAPK3 primers (Biotech Bioengineering).
2.8.2 Instruments and Reagents
Adjustable-range pipette, 0.22 μm microfilter membrane, 96-well plates, 24-well plates, constant temperature CO2cell incubator and water bath, precision electronic balance, ultra-clean bench,flow analyzer Beckman model cytoflex, real-time fluorescence quantitative PCR instrument (ABI, USA).
Sample solution preparation: Strictosamide was prepared with culture medium at a concentration of 100 mM of mother liquor and stored in the refrigerator at 4 ℃ for back up.
2.8.3 Cell culture and grouping
Cell culture: LX2 and HepG2 cells were inoculated into new 25 cm2culture flasks, and about 4 mL of RPMI-1640, penicillin or DMEM containing 10% fetal bovine serum and 1% streptomycin were added and cultured at 37 ℃ and 5% CO2, and the culture medium was changed every two days.The cells were incubated at 37 ℃ with 5% CO2, and the culture medium was changed every two days until the cell wall adherence rate was about 80%-90% and the cells were in good condition for subsequent experiments.
Cell grouping: blank group (without Strictosamide), administration group (Strictosamide at different concentrations group).
2.8.4 CCK8 measurement
The cells were inoculated in 96-well plates at a density of 10 000 cells/well, and 5 replicate wells were set up in each group, and the edges were filled with sterile PBS.The cells were incubated at 37 ℃and 5% CO2, and the intervention was administered the next day.12 h later, the liquid in the 96-well plates was aspirated and discarded,and 100μL of medium containing CCK-8 was added to each well and incubated at 37 ℃ and 5% CO2for 1 h.CO2for 1 h.After incubation, the absorbance was measured at 450 nm on an enzyme marker.2.8.5 Apoptosis detection by flow cytometry
Human hepatocellular carcinoma HepG2 cells were taken and inoculated in 6-well plates at 2×105per well, treated with 0, 40, 80,120 and 160 μM concentration of isovorin lactam solution for 12 h, rinsed by pre-chilled phosphate buffered salt solution (PBS), and resuspended with 500 μL of Binding Buffer.Annex-in V-FITC was added and gently blown, then 5 μL of PI was added and mixed, and the reaction was performed at room temperature and protected from light for 15 min, and apoptosis was detected by flow cytometry.
2.8.6 Real-time PCR assay
Human hepatocellular carcinoma HepG2 cells were taken and inoculated in 6-well plates at 2×105per well, treated with 0, 40, 80,120, 160 μM concentration of isovorin lactam solution, and after 12 h RNA was extracted from the samples and then reverse transcribed using the reverse transcription kit, according to the instructions,at 37 ℃, 15 min; 85 ℃, 5 s.PCR amplification was performed according to the instrument instructions: 95 ℃ pre denaturation for 5 min; denaturation at 95 ℃ for 10 s, annealing at 57.5 ℃ for 20 s,extension for 20 s, cyclic reactions for 40 times, 95 ℃, 15 s, lysis at 60 ℃ for 60 s.lysis curves were used, and finally Ct values were counted, and the results were compared with GAPDH as a reference,using the 2-ΔΔCt relative quantification method for each group of target mRNA expression differences, and the primer sequences are shown in the table below.
2.9 Statistical processing
Data were organized and analyzed with the statistical software GraphPad Prism 9.0, and experimental data were expressed as mean ± standard deviation (±s).Differences between groups were compared by t-test, non-parametric test was used for variance difference, and one-way ANOVA was used for comparison between multiple groups, and differences were considered statistically significant if P<0.05.
3.Results
3.1 Screening of Nauclea Officinalis components and acquisition of target sites
The chemical composition of Nauclea Officinalis was collected using specialized chemical databases as well as by searching the literature[13-15], and then 14 compounds containing the molecular structure were obtained by searching the PubChem database, mainly:Strictosamide (DM1), Vincosamide(DM2), Pumiloside(DM3),NaucleamideG (DM4), Kaempferol-3-rutinoside(DM5),NaucleamideG(DM14), Rutin (DM6), Antiarol(DM7),Dihydroactinidiolide(DM8), METHYL2,4-DIHYDROXY-3,6-DIMETHYLBENZOATE (DM9), Syringaldehyde (DM10),VinmajineI(DM11), Indole-3-carboxaldehyde(DM12),Angustine(DM13), Ethyl3-(4-hydroxy-3-methoxyphenyl)acrylate(DM14).The predicted Nauclea Officinalis targets from each database were combined according to the screening conditions,and 587 Nauclea Officinalis targets were obtained.Among them,Strictosamide is the main active component in urchinwood of Ri,which belongs to the indole alkaloids[6,16].
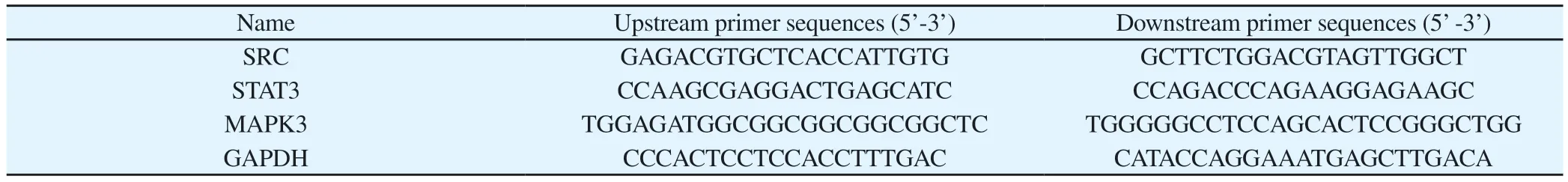
Tab 1 Primer sequence
3.2 Liver cancer target acquisition
According to the screening conditions, the obtained liver cancer targets were 1 981 in GeneCards database, 507 in OMIM database,1 396 in DisGeNET database and 284 in PharmGKB database, and 3 319 targets for liver cancer were obtained after taking the merged set, as shown in Figure 1.

Fig 1 Venn diagram of targets of HCC
3.3 Acquisition of anti-hepatocellular carcinoma targets by Nauclea Officinalis
The 587 Nauclea Officinalis targets and 3 319 liver cancer targets were introduced into Venny2.1, and 288 common targets were obtained as shown in Figure 2.
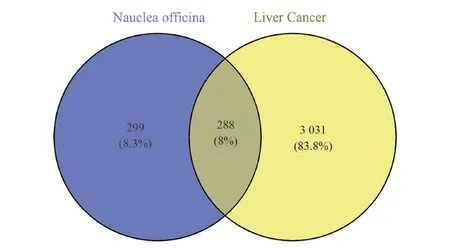
Fig 2 Venn diagram of Nauclea Officinalis and corresponding targets of HCC
3.4 PPI network construction and analysis
After importing the common targets into the STRING database,the PPI network graph was constructed in Cytoscape 3.9.1 software,including 98 nodes and 794 edges, see Figure 3.and network topology analysis was performed in the software, and the results showed that TP53, SRC, STAT3, HSP90AA1, PIK3R1, MAPK3 and other neighboring nodes were the most numerous, and in Table 2 The Nauclea Officinalis antihepatocellular carcinoma PPI network targets were ranked with the highest degree of freedom values and might be the core targets.

Fig 3 PPI diagram of anti-HCC targets of Nauclea Officinalis

Tab 2 Ranking of the degree of PPI network targets of Nauclea Officinalis against HCC
3.5 Biological processes and pathway enrichment analysis of the anti-hepatocellular carcinoma targets of Nauclea Officinalis
The Nauclea Officinalis anti-hepatocellular carcinoma targets were analyzed for GO and KEGG enrichment analysis (P<0.05).There were 994 entries related to biological process (BP) in GO functional enrichment analysis, mainly involving protein phosphorylation,protein autophosphorylation, negative regulation of apoptotic process, etc.; 125 entries related to cellular components (CC),regarding cytosol, receptor complex, plasma membrane, polymer complex, nuclear plasma, etc.; 238 entries related to molecular function (MF), mainly involving kinase activity, enzyme binding,identical protein binding, etc.The results of the visual analysis of the biological processes of the top 10 BP, CC and MF with high number of enriched genes are shown in Figure 4.KEGG pathway enrichment analysis, Nauclea Officinalis anti-hepatocellular carcinoma targets mainly act on cancer pathway, hepatitis B, EGFR tyrosine kinase inhibitor resistance, PI3K-Akt and other signaling pathways, the results of the visual analysis of the top 20 KEGG pathways See Figure 5.
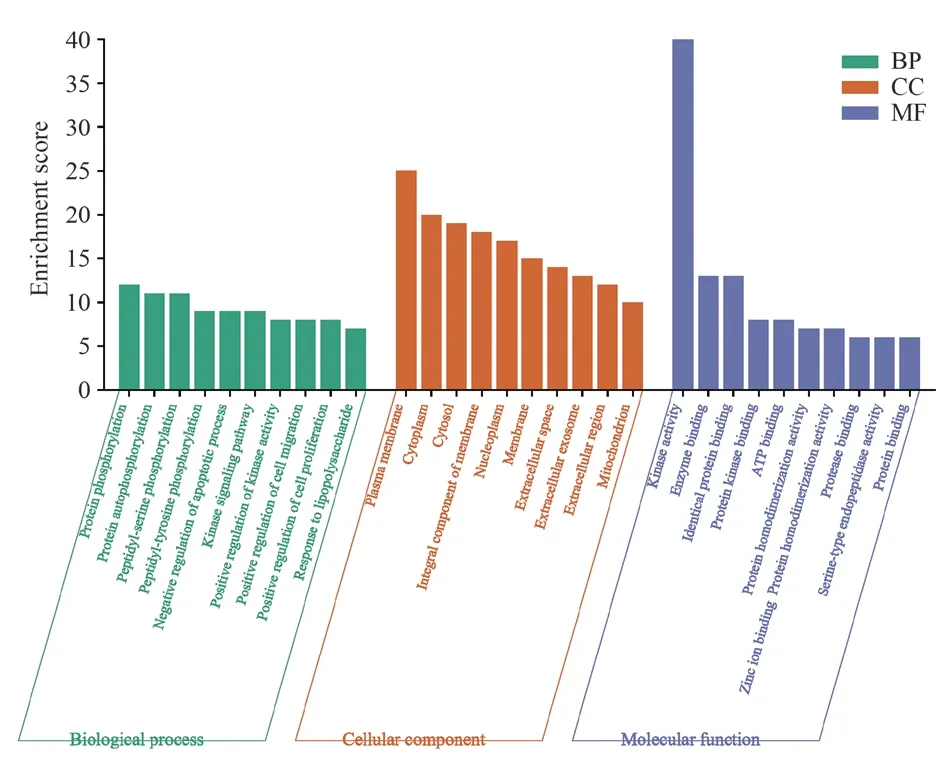
Fig 4 GO function enrichment analysis of anti-HCC targets of Nauclea Officinalis

Fig 5 KEGG pathway enrichment diagram of anti-HCC targets of Nauclea Officinalis
3.6 Construction of “drug-component-target-pathwaydisease” interaction network of Nauclea Officinalis against hepatocellular carcinoma
The network consists of 99 nodes and 406 edges, see Figure 6, and after analysis, the active ingredients are ranked according to their degrees of freedom values, see Table 3.The network can act on multiple targets and modulate multiple signaling pathways to exert anti-liver cancer effects.
3.7 Molecular docking of active components of Nauclea Officinalis to core targets
The active ingredients and core targets were imported into DiscoveryStudio2019Client software, and then molecular docking verification was performed after adding water and dehydrogenation,and when the LibDockScore was greater than 105, the ingredients were considered to have good binding activity with the targets[17],and the top-ranked active ingredients were taken to be validated with the core targets TP53, SRC, STAT3, HSP90AA1, PIK3R1, MAPK3 were docked and validated, and it was found that TP53, HSP90AA1,PIK3R1 had poor binding ability to Strictosamide, Vincosamide,and Pumiloside.The LibDockScore of the core targets SRC, STAT3,MAPK3 and the top-ranked active compounds were all higher than 105, indicating that the key components in Nauclea Officinalis have good affinity to interact with the core targets SRC, STAT3, MAPK3.The results of higher docking scores of active components with core targets are shown in Table 4, and the molecular docking visualization is shown in Figures 7-9.
3.8 Results of in vitro cellular CCK-8 experiments
Combined with the network pharmacology results, Strictosamide,the main active ingredient in Nauclea Officinalis, was selected for validation.As shown in Figure 10, Strictosamide at concentrations of 40-160 μM had no inhibitory effect on LX-2 normal hepatocyte growth (P>0.05).However, the results in Figure 11 showed that Strictosamide exhibited significant cell growth inhibitory activity for HepG2 cells after 12 h of action at concentrations of 80-160 μM, respectively (P<0.001).Therefore, Strictosamide was cytotoxic to hepatocellular carcinoma HepG2 and inhibited the proliferation and growth of hepatocellular carcinoma cells, but had no significant effect on normal LX-2 hepatocytes.
3.9 Effect of drugs on apoptosis of tumor cells
Human hepatocellular carcinoma HepG2 cells were treated with different concentrations of isovorin lactam (0, 40, 80, 120, 160 μM)for 12 h.The effect on apoptosis was detected by flow cytometry.Figure 12 The lower left quadrant represents normal cells and the upper right quadrant represents advanced apoptotic cells.Compared with the control group, apoptotic cells were significantly increased and normal cells were significantly decreased in the 0, 40, 80, 120 and 160 μM groups.The apoptotic rates were 15.99%, 24.49%,29.16%, 36.12%, and 49.11%, respectively (Table 5).Compared with the control group, the apoptosis rate of human hepatocellular carcinoma HepG2 cells increased significantly with increasing concentration (P<0.001).

Fig 6 Network diagram of Nauclea Officinalis-ingredient-pathway-HCC-target
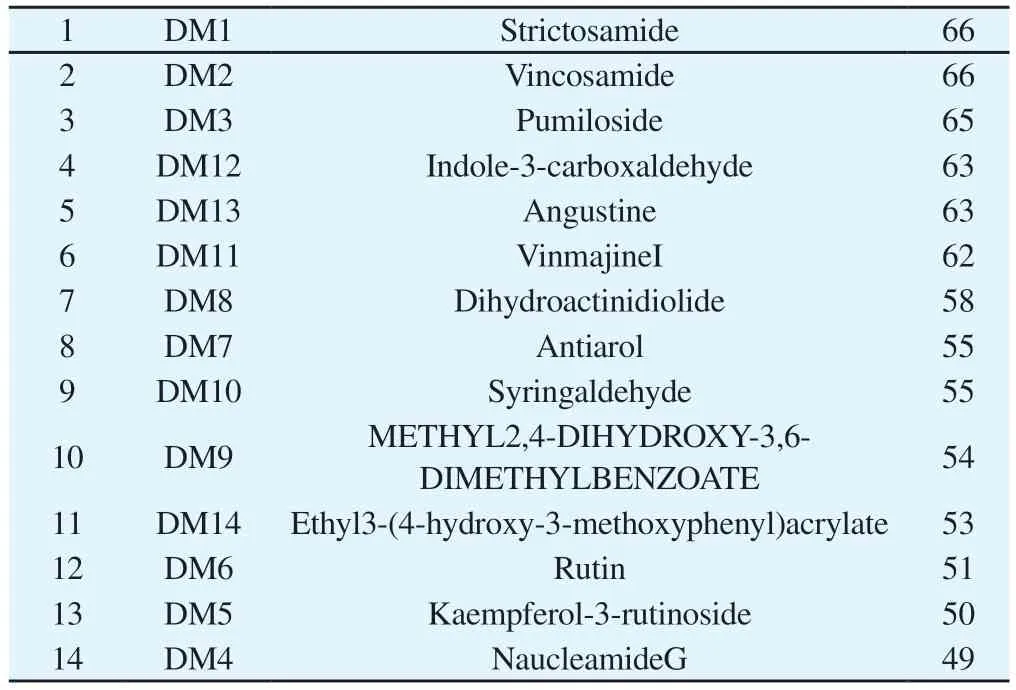
Tab 3 Ranking of degree value of drug-active ingredient-anti-HCC target network of Nauclea Officinalis Ranking Substitute Compound Dgree

Tab 4 The results of molecular docking.
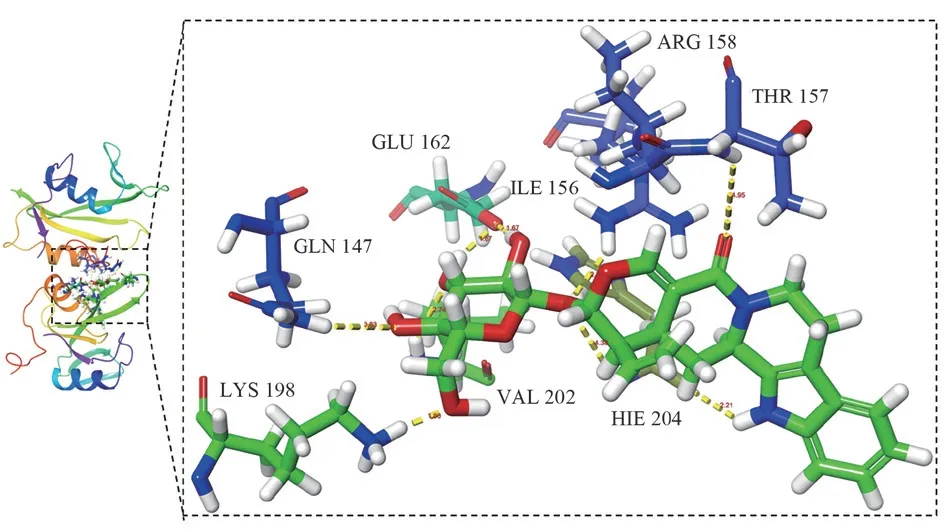
Fig 7 Molecular docking diagram of vincosamide and SRC target

Fig 8 Molecular docking diagram of pumiloside and MAPK3 target

Fig 9 Molecular docking diagram of strictosamide and MAPK3 target
Tab 5 Effect of different concentrations of strictosamide on cell apoptosis rate(n=3, ±s)

Tab 5 Effect of different concentrations of strictosamide on cell apoptosis rate(n=3, ±s)
Note: Compared with the blank group, ***P<0.001.
Group(μmol/L) Apoptosis rate(%)0 15.99±1.49 40 24.49±2.18***80 29.61±2.13***120 36.12±0.11***160 49.11±1.05***
Tab 6 Effect of strictosamide on core protein mRNA expression in hepatocellular carcinoma HepG2 cells(n=3, ±s)
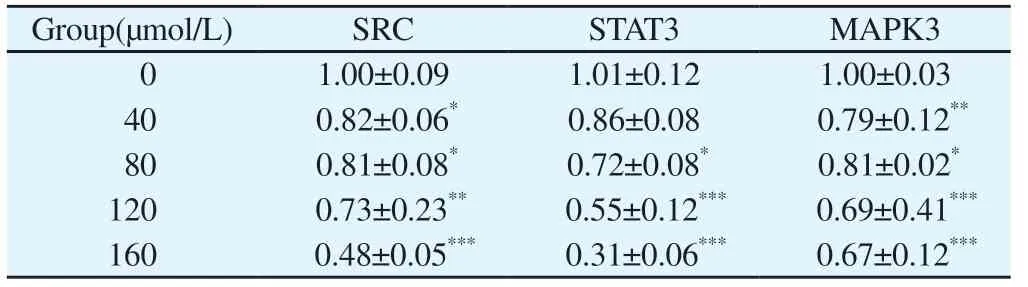
Tab 6 Effect of strictosamide on core protein mRNA expression in hepatocellular carcinoma HepG2 cells(n=3, ±s)
Note: Compared with the blank group, *P<0.05, **P<0.01, ***P<0.001.
Group(μmol/L) SRC STAT3 MAPK3 0 1.00±0.09 1.01±0.12 1.00±0.03 40 0.82±0.06* 0.86±0.08 0.79±0.12**80 0.81±0.08* 0.72±0.08* 0.81±0.02*120 0.73±0.23** 0.55±0.12*** 0.69±0.41***160 0.48±0.05*** 0.31±0.06*** 0.67±0.12***

Fig 10 Effect of strictosamide lactam on cytotoxicity of LX-2 cells

Fig 11 Effect of strictosamide lactam on cytotoxicity of HepG2 cells
3.10 Drug inhibits the expression of core target mRNAs
The expression of TP53, SRC, STAT3, MAPK3 mRNA was detected by qRT-PCR after the action of different concentrations of Strictosamide (0, 40, 80, 120 μM) on HepG2 cells for 12h.As shown in the results in Table 6, the expression of SRC, STAT3, and MAPK3 mRNA in HepG2 cells decreased with the elevation of high Strictosamide endocannabinoid (P<0.05, P<0.05, P<0.05).These data suggest that Strictosamide can down-regulate the expression levels of SRC, STAT3, and MAPK3 mRNA in HepG2 cells.

Fig 12 Effect of different concentrations of strictosamide solution on cell apoptosis
4.Discussion
The prevention and treatment of hepatocellular carcinoma, a malignant tumor occurring in the liver, remains a major clinical challenge.For advanced unresectable hepatocellular carcinoma,pharmacotherapy is the main treatment strategy.Alkaloids are the main chemical constituents of Nauclea Officinalis and are one of the current clinical antitumor research concerns.In this study, we predicted the possible active components and mechanism of action of Nauclea Officinalis against hepatocellular carcinoma by network pharmacology, and verified its antihepatocellular carcinoma activity by in vitro cellular assay.
Among the 14 active ingredients of Nauclea Officinalis, the potential active ingredients isoparaffin lactam, hippopanax glucoside and Pumiloside were predicted to have the most targets against hepatocellular carcinoma, and ranked the top three in the “drugcomponent-target-pathway-disease” network in terms of degrees of freedom.These three chemical components were molecularly docked with TP53, SRC, STAT3 and MAPK3, the key targets of choline against hepatocellular carcinoma, among which the docking scores of SRC, STAT3 and MAPK3 were all greater than 105,suggesting good binding activity.Validation using in vitro cellular assays showed that Strictosamide, the main component in Nauclea Officinalis, significantly inhibited the proliferation of hepatocellular carcinoma HepG2 cells (P<0.001).Related studies have found that alkaloids can exert anti-hepatocellular carcinogenic effects by inhibiting telomerase activity, reducing hepatocellular carcinoma proliferation, inducing hepatocellular carcinoma cell differentiation and apoptosis, and inhibiting hepatocellular carcinoma cell cycle and proliferation[18-20].
The PPI network shows that the core targets of the antihepatocellular carcinoma effect of Riopharm Nauclea Officinalis are TP53, SRC, STAT3, MAPK3, etc.TP53 is a tumor suppressor gene, and patients with hepatocellular carcinoma who inhibit its TP53 gene mutation and expression have a better prognosis[21,22].And TP53 can regulate cancer cell senescence and apoptosis[23].SRC, the first proto-oncogene identified, affects the processes of cell proliferation, migration, invasion and angiogenesis, and when SRC expression is downregulated, liver cancer disease progression is inhibited[24-26].Seo[27] et al.showed that cafestrol induces apoptosis in hepatocellular carcinoma cells by inhibiting SRC phosphorylation, as well as by inhibiting p-mTOR and p-STAT3.In the tumor ecosystem, STAT3 is widely over-activated in cancer and non-cancer cells and plays a key role in the regulation of antitumor immune responses, and when STAT3 phosphorylation is increased it promotes liver tumorigenesis[28, 29].It has been shown that when MAPK3 is overexpressed, the proliferation rate and migration ability of hepatocellular carcinoma cells are subsequently accelerated[30].In contrast, downregulation of MAPK3 resulted in a significant increase in intracellular apoptosis-related gene expression[31].Therefore,inhibition of SRC, STAT3, and MAPK3 expression may promote apoptosis in hepatocellular carcinoma cells.In the present study, we found that isoproterenolide lactam downregulated the expression of SRC, STAT3, and MAPK3 mRNA in hepatocellular carcinoma HepG2 cells (P<0.05) and induced apoptosis in hepatocellular carcinoma cells (P<0.001).
GO enrichment showed enrichment of shared targets in processes such as protein phosphorylation and negative regulation of apoptotic processes.It has been shown that herbal medicines can exert antitumor effects by regulating protein phosphorylation levels to promote apoptosis, etc[32].The core target MAPK3 is also a protein kinase that phosphorylates and activates STAT3, promoting liver tumorigenesis[29, 33].Therefore, affecting the protein phosphorylation process involved in apoptosis may be closely related to the inhibition of MAPK3 expression by cholwood to exert antihepatocarcinogenic effects.KEGG pathway showed a high enrichment of targets in the cancer pathway and EGFR receptor tyrosine kinase inhibitor resistance and PI3K-Akt signaling pathway in total, suggesting that it may be a potential pathway for cholwood against hepatocellular carcinoma.Cancer pathways, which are brought together by multiple pathways such as NF-κB, MAPK, JAK-STAT, etc., where the core target MAPK3 is in the MAPK signaling pathway, and changes in the phosphorylation levels of related proteins in this pathway are closely related to the development of hepatocellular carcinoma[30].The core targets STAT3, SRC and other genes are involved in regulating the EGFR receptor tyrosine kinase inhibitor resistance pathway.Hepatocellular carcinoma cells acquire resistance to the first-line anticancer drug lenvatinib by activating this pathway pathway and promoting the expression of EGFR, STAT3, and SRC targets on this axis[34,35].In addition, it was shown that PI3K-Akt signaling pathway, when activated, promotes proliferation, migration and invasion of hepatocellular carcinoma cells and accelerates the disease process[36].Phycocyanin can limit hepatocellular carcinoma progression by inhibiting the PI3K/Akt signaling pathway and thus transducing PI3K/Akt expression[37].Combined with the RT-PCR results, Strictosamide in Nauclea Officinalis can inhibit the expression of SRC, STAT3, and MAPK3 genes, which initially showed that Nauclea Officinalis can regulate multiple pathways by acting on SRC, STAT3, and MAPK3 targets to exert antihepatocellular carcinoma effects.
In conclusion, the possible anti-hepatocellular carcinoma mechanism of Nauclea Officinalis is that the active ingredients of Nauclea Officinalis, such as Strictosamide , Pumiloside and Vincosamide, act on hepatocellular carcinoma through SRC, STAT3 and MAPK3 targets, and finally interfere with the development of hepatocellular carcinoma by regulating multiple signaling pathways such as cancer pathway, PI3K/Akt and EGFR tyrosine kinase inhibitor resistance.In vitro cell experiments further demonstrated that Nauclea Officinalis had significant anti-proliferative effects on hepatocellular carcinoma, and could down-regulate SRC, STAT3 and MAPK3 gene expression and promote apoptosis of cancer cells.The present study provides a direction and idea for the study of the anti-hepatocellular carcinoma mechanism of Nauclea Officinalis, but given the limitations of the network pharmacology and preliminary cellular experiments, the anti-hepatocellular carcinoma efficacy of Nauclea Officinalis and its mechanism of action still need further study.
Author’s contribution
Chen Wei-jia: Cell experiments, data collection and paper writing;Xu Jian: Responsible for article conception and review; Other authors participated in the article polishing and formatting.
All authors declare no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- Advances in cellular and molecular mechanisms of oral submucosal fibrosis
- Research progress of natural products in treating polycystic ovary syndrome
- Research progress of related signal pathways in the prevention and treatment of heart failure with traditional Chinese medicine
- Quality of life of hospitalized patients after lung cancer operation and analysis of influencing factors
- Meta-analysis of the efficacy and safety of extended right liver transplantation versus whole liver transplantation
- Clinical study of different modes of repetitive transcranial magnetic stimulation in the treatment of post‑stroke executive dysfunction
