A meta-analysis of risk factors for epilepsy after acute ischaemic stroke and the development of a predictive model
2023-11-14YANGYihaoCHENShihuiLIZongjunJIADandanZOUQinCaiYiLIQifu
YANG Yi-hao, CHEN Shi-hui, LI Zong-jun, JIA Dan-dan, ZOU Qin, Cai Yi, LI Qi-fu✉
1.Department of Neurology, the First Affiliated Hospital of Hainan Medical College, Haikou 570102, China
2.Key Laboratory of Tropical Brain Science Research and Translation in Hainan Province, Haikou 570102,China
3.Department of Medical Psychology, the First Affiliated Hospital of Hainan Medical College, Haikou 570102,China
Keywords:
ABSTRACT Objective: To screen risk factors for epilepsy after acute ischaemic stroke based on meta-analysis and cohort study and to establish a predictive model.Methods: Computer searches of MEDLINE, Embase, Cochrane library, Web of Scinence, PubMed, CNKI, and WanFang Data data were conducted to collect literature on epilepsy after in acute ischemic stroke, from database creation to September 1, 2022.The RRs and their 95% confidence intervals(CI) for risk factors for post stroke epilepsy were extracted for each study, and pooled estimates of the RRs and 95% CIs for each study were generated using either a random-effects model or a fixed-effects model.Beta coefficients for each risk factor were calculated based on the combined RR and their corresponding 95% CIs.The beta coefficients were multiplied by 10 and rounded.Results: Ten articles were identified for final inclusion in this meta-analysis,with a total of 141 948 cases and 3 702 cases of post stroke epilepsy.The risk factors included in the final risk prediction model were infarct size (RR 4.67, 95% CI 1.41~15.47; P=0.01), stroke recuRRence (RR 2.48, 95% CI 2.01~3.05; P<0.000 01), stroke etiology (RR 1.70, 95% CI 1.34~2.15; P<0.000 01), stroke severity (RR 1.70, 95% CI 1.34~2.15; P<0.000 01), and stroke risk.stroke severity (RR 1.53, 95% CI 1.39~1.70; P < 0.000 01), NIHSS score (RR 2.91, 95%CI 1.64~5.61; P = 0.000 3), early-onset epilepsy (RR 5.62, 95% CI 5.08~6.22; P < 0.000 01),cortical lesions (RR 3.83.95% CI 2.23~6.58; P < 0.000 01), total anterior circulation infarction(RR 18.94, 95% CI 10.38~34.57; P < 0.000 01), partial anterior circulation infarction (RR 4.39,95% CI 2.29~8.40; P < 0.000 01), cardiovascular events (RR 1.78, 95% CI 1.59~1.99;P < 0.000 01).Conclusion: Based on a systematic review and meta-analysis, we developed a simple risk prediction model for late epilepsy in baseline ischemic stroke that integrates clinical risk factors, including infarct size, stroke recurrence, stroke etiology, stroke severity,NIHSS score, early onset epilepsy, cortical lesions, stroke subtype, and cardiovascular events.
1.Introduction
Post-stroke seizures have been reported to occur in approximately 6% to 8% of adults with ischemic stroke[1], and for older adults over 65 years of age, the leading cause of newly diagnosed epilepsy is stroke, which has been shown to account for nearly 50% of newly diagnosed epilepsy in this age group[2].In contrast, approximately 87% of all strokes are ischemic strokes, 10% are intracranial hemorrhages, and 3% are subarachnoid hemorrhages[3].Although all forms of stroke are associated with an increased risk of seizures,with a higher incidence after hemorrhagic stroke compared to ischemic stroke, the actual total seizure burden in this group is higher due to the much higher incidence of ischemic stroke[4],with seizures or post-stroke epilepsy adding an additional burden to stroke patients[5].Both seizures and post-stroke epilepsy may increase the morbidity and mortality of stroke[6].Seizures shortly after stroke can lead to increased metabolic stress and cell death, resulting in increased infarct size, mortality and negative functional outcomes[7], and recurrent seizures may lead to injury, impaired cognitive function and loss of ability to work or operate a vehicle, and reduced quality of life[8].Seizures after stroke are classified as acute symptomatic and remote symptomatic.Acute symptomatic seizures (also known as early seizure (ES)) occur within 7 days of infarction and are thought to be caused by toxic or metabolic effects of stroke.Remote symptomatic seizures (also known as post-stroke seizure (late seizure (LS)) are unprovoked seizures that occur more than 1 week after stroke[9].The risk of seizure after an acute symptomatic seizure is approximately 30% and therefore acute symptomatic seizures are not considered epilepsy because of the low risk of recurrence[10-12].In contrast, a single remote symptomatic seizure after stroke has a > 60% risk of seizure,which is sufficient to diagnose epilepsy in this setting[13].
Few studies have examined post-stroke epilepsy after ischemic stroke based on meta-analysis, and most of this study focused on the analysis of risk factors for post-stroke epilepsy in acute ischemic stroke, along with the development of a predictive model that can be used in a convenient clinical setting.A comprehensive assessment of these risk factors, as well as early detection and individualized intervention for those at risk, may be the most effective strategy to prevent such disorders.
2.Materials and Methods
2.1 Inclusion and exclusion criteria
2.1.1 Type of Research
(1)Studies examining risk factors for post-stroke epilepsy and reporting risk ratios (RR) and corresponding 95% confidence intervals (CI) for these risk factors.(2)Cohort studies, including prospective and retrospective cohort studies.
2.1.2 Research Subjects
(1)Comply with the diagnosis of acute ischemic stroke in the China Acute Ischemic Stroke Diagnosis and Treatment Guidelines 2018;(2)age 18 year; (3)Meet the diagnostic criteria for post-stroke epilepsy proposed by the International League Against Epilepsy in 2014.
2.1.3 Closing indicators
Post-stroke epilepsy is defined as epilepsy occurring more than 1 week after stroke that meets the diagnostic criteria of the International League Against Epilepsy.
2.1.4 Exclusion Criteria
(1)With dementia, cognitive impairment and other mental system disorders; (2)Combined with serious heart, liver, kidney, blood and other system diseases; (3)Abnormal blood clotting function; (4)Combined brain tumor, traumatic brain injury, immune-related intraand extra-brain diseases.
2.2 Literature Search Strategy
Computer searches of MEDLINE, Embase, Cochrane library,Web of Scinence, PubMed, CNKI, and WanFang Data data were conducted to collect literature on post-stroke epilepsy in acute ischemic stroke, all with a search time frame from the date of database creation to September 1, 2022.In addition, references to the included literature were retrospectively included to supplement access to relevant literature.The search was conducted with a combination of subject terms and free words, with the Chinese search terms acute ischemic stroke, epilepsy, risk factors, and cohort studies; the English search terms included: Ischemic Stroke,Epilepsy, risk factors, and cohort studies.
2.3 Literature screening and data extraction
Two investigators independently selected the cohort study, which was later collated and discussed when differences of opinion arose.If two people had not obtained a unified opinion after discussion,a third person was involved and gave a unified opinion.When duplicate studies of the same study emerged, only the most recent study, or the study with the longest follow-up period, was included.Two researchers read the full text of the 10 included articles, details of the study design, study site and year of published study, patient demographic characteristics (age and sex), number of patients enrolled and number of morbidities, duration of follow-up, study outcomes, identified risk factors and their corresponding RR at 95% CI.assessment of the quality of the included articles, using the modified Newcastle-Ottawa scale (NOS ) was used to evaluate the quality of the studies.The modified NOS is a 10-point scoring system based on 9 items.The higher the score, the higher the quality of the study.
2.4 Risk of bias evaluation of included studies
A funnel plot was used to evaluate publication bias.
2.5 Statistical Analysis
Risk Ratio (HR) and Relative Risk Ratio (RR) were approximated to be the same and were collectively referred to as RR in this study.considering the reliability of the results, only risk factors involving more than two cohort studies were included in the meta-analysis.The RR and its 95% CI for post-stroke epilepsy risk factors were extracted for each study, and pooled estimates of the RR and 95% CI for each study were generated using either a random-effects model or a fixed-effects model.Heterogeneity across studies was determined using the Cochrane Q test and I2.I2values > 50% or Cochran Q test P-values < 0.10 were significant for heterogeneity, and a randomeffects model was used; conversely, a fixed-effects model was selected.Subgroup analysis was performed by stratifying each risk factor.To test the robustness of our results, we performed sensitivity analyses by omitting one study in each round.All tests were twosided and differences were considered statistically significant at P < 0.05, whereas for heterogeneity assessment, differences were considered statistically significant at P < 0.10.Statistical analyses were performed using R software 4.1.1 and Review Manager software version 5.4.
2.6 Modeling
The modeling cohort was derived from a systematic review and meta-analysis of 7 prospective cohorts and 3 retrospective cohorts.A total of 141,948 patients with acute ischemic stroke were from Europe (including Italy, Norway, Sweden, and Finland), Asia(Taiwan, China), and the Americas (United States).All studies reported risk ratios (RRs) for risk factors and corresponding 95%CIs to develop a risk prediction model based on the point system proposed by Sullivan et al[14].The combined RR values of each risk factor were extracted from all risk factors included in the prediction model selected from the systematic review and meta-analysis described above, and the beta coefficients of each risk factor were calculated based on the combined RRs and their corresponding 95%confidence intervals.The beta coefficients were multiplied by 10 and rounded off.All risk factors in the prediction model were classified according to meta-analysis and clinical practice guidelines, and scores were assigned to each category of risk factors.The scores of all components in the prediction model were summed to calculate the total score.P < 0.05 was considered a statistically significant difference.
3.Results
3.1 Literature screening process and results
We identified 707 potentially relevant articles, removed 399 duplicate articles from each database, and performed preliminary title and abstract screening of the remaining 308 studies, excluding 258 (e.g., cases of basic research, combined early-onset epilepsy,case reports, conference articles, etc.).The remaining 50 articles were read in full, and 10 articles were identified for final inclusion in this meta-analysis (see Table 1), for a total of 141 948 cases and 3 702 cases of post-stroke epilepsy (see Figure 1).All 10 studies included in the meta-analysis were high quality studies with scores of 8 or more according to the Newcastle-Ottawa scale (see Table 2)
3.2 Analysis of risk factors
Nine risk factors were identified in a systematic review and metaanalysis.These factors were infarct size (4.67), stroke recurrence(2.48), stroke etiology (1.70), stroke severity (2.11), NIHSS score(2.59), early-onset epilepsy (7.20), cortical lesions (3.83), stroke subtype (9.57), and cardiovascular events (1.78) in that order.details of the 9 risk factors and forest plots are shown in Table 3-4 and Figures 2-10.-4 and Figures 2-10.Considering the feasibility of clinical practice, a subgroup analysis and sensitivity analysis were performed for each risk factor, and a crude observation of the funnel plot was made.

Fig 1 Flow chart of literature search and study selection for risk factors for post-stroke epilepsy in patients with acute ischaemic stroke

Tab 1 Baseline characteristics of the 10 cohorts included in the meta-analysis

Tab 2 Newcastle-Ottawa quality assessment scales for the 10 cohort studies
3.3 Modeling
The risk factors included in the final risk prediction model were infarct size (RR 4.67, 95% CI 1.41-15.47; P=0.01), stroke recurrence(RR 2.48, 95% CI 2.01-3.05; P<0.000 01), stroke etiology (RR 1.70,95% CI 1.34-2.15; P<0.000 01), stroke stroke severity (RR 1.53,95% CI 1.39-1.70; P < 0.000 01), NIHSS score (RR 2.91, 95% CI 1.64-5.61; P = 0.000 3), early-onset epilepsy (RR 5.62, 95% CI 5.08-6.22; P < 0.000 01), cortical lesions (RR 3.83, 95% CI 2.23-6.23; P <0.000 01).95% CI 2.23-6.58; P < 0.000 01), total anterior circulation infarction (RR 18.94, 95% CI 10.38-34.57; P < 0.000 01), partial anterior circulation infarction (RR 4.39, 95% CI 2.29-8.40; P < 0.00001), cardiovascular events (RR 1.78, 95% CI 1.59-1.99; P < 0.000 01).Subgroup and sensitivity sensitivity analyses are shown in Table 5.Table 6 shows the involved studies, sample sizes, combined RR values (95% CI), beta coefficients, and risk scores for the risk factors included in the risk prediction model.A simple risk prediction model is shown in Table 7.
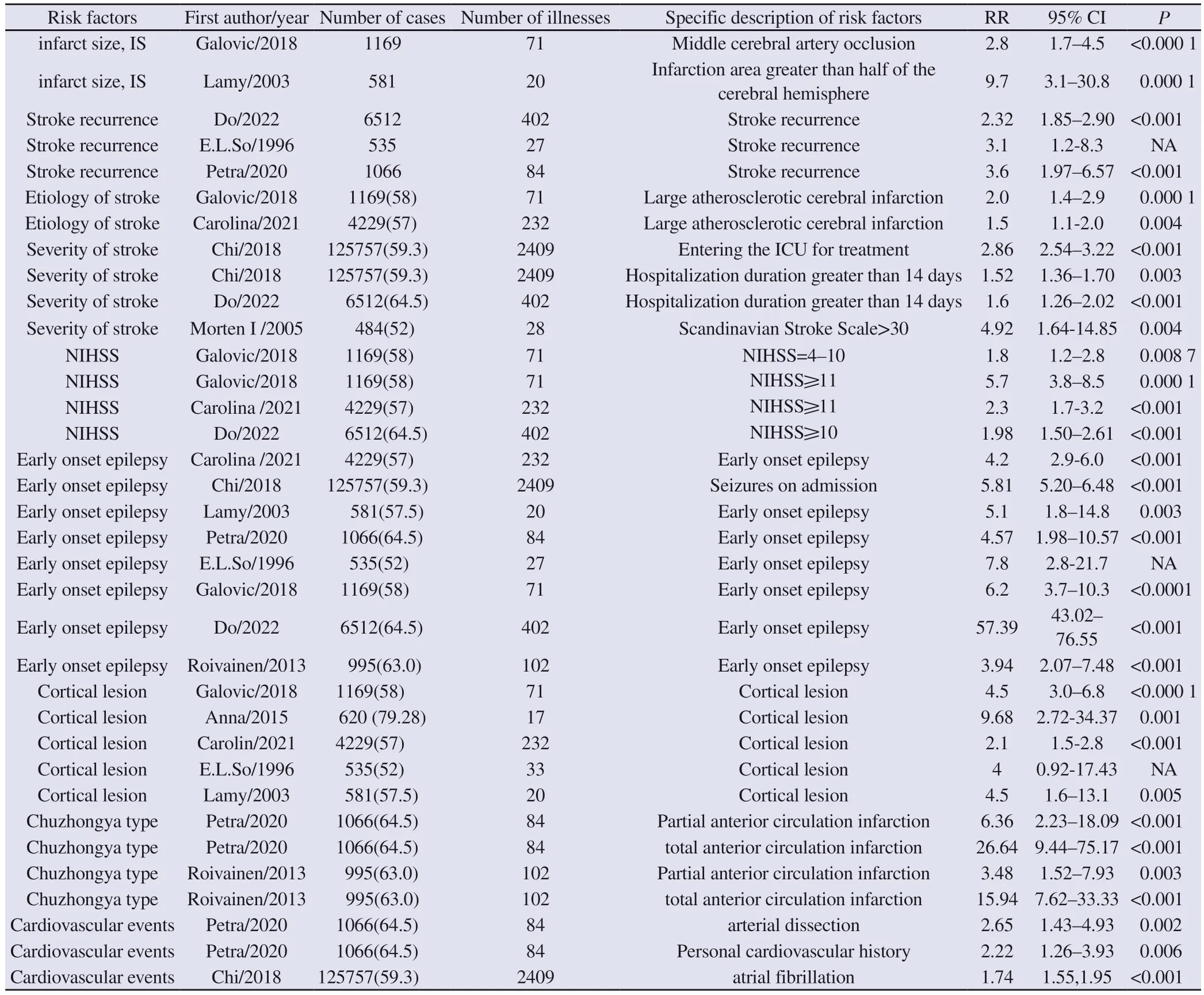
Tab 3 Nine risk factors included in the meta-analysis

Tab 4 Overall RR values of risk factors for post stroke epilepsy and their 95% confidence intervals

Fig 2 Relationship between infarct size and post stroke epilepsy
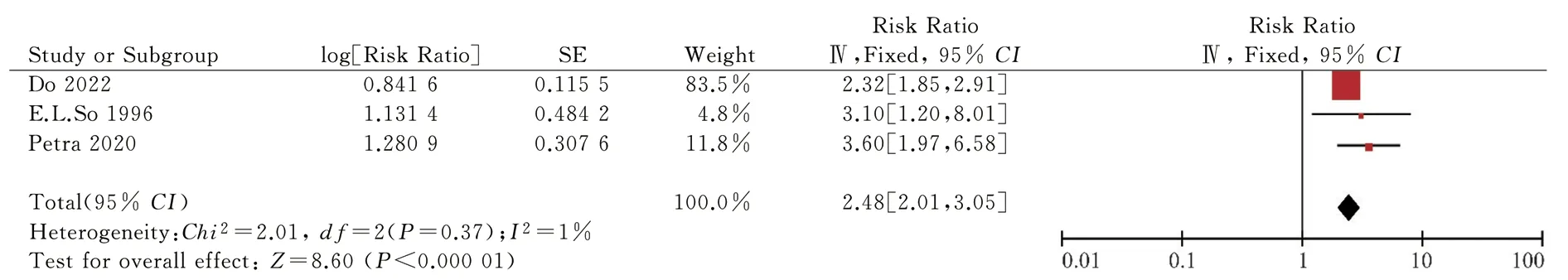
Fig 3 Relationship between stroke recurrence and post stroke epilepsy

Fig 4 Relationship between stroke etiology and post stroke epilepsy

Fig 5 Relationship between stroke severity and post stroke epilepsy

Fig 6 Sensitivity analysis of the relationship between stroke severity and post stroke epilepsy

Fig 7 Relationship between NIHSS scores and post stroke epilepsy
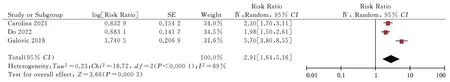
Fig 8 Subgroup analysis of the relationship between NIHSS scores and post stroke epilepsy

Fig 9 Relationship between early-onset epilepsy and post stroke epilepsy
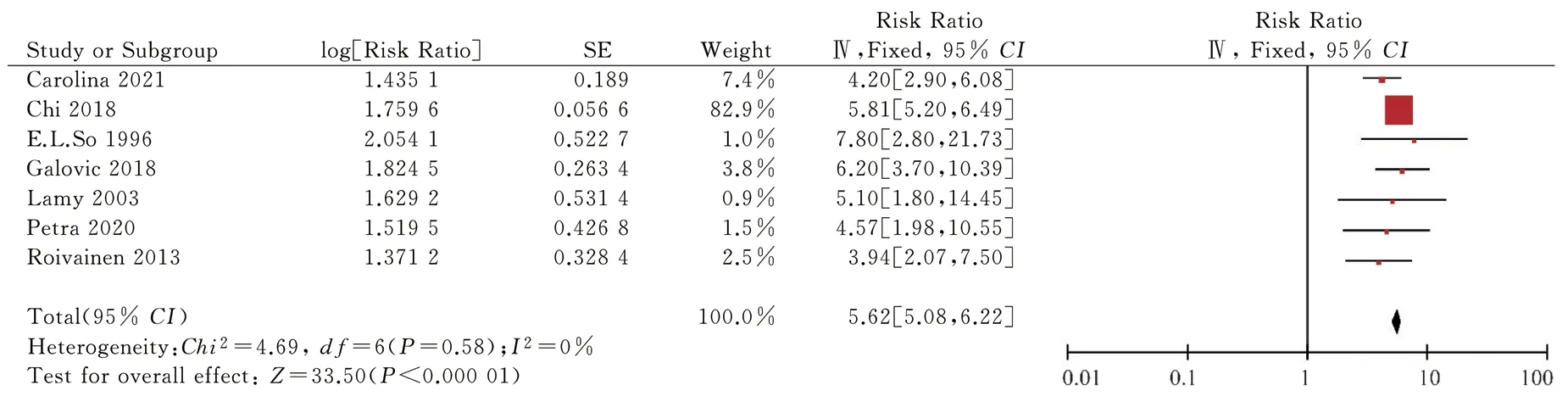
Fig 10 Sensitivity analysis of the relationship between early-onset epilepsy and post stroke epilepsy

Fig 11 Relationship between cortical lesions and post stroke epilepsy

Fig 12 Relationship between subtype of stroke and post stroke epilepsy

Fig 13 Subgroup analysis of the relationship between stroke subtype and post stroke epilepsy (partial anterior circulation infarction)

Fig 14 Subgroup analysis of the relationship between stroke subtype and post stroke epilepsy (total anterior circulation infarction)

Fig 15 Relationship between cardiovascular events and post stroke epilepsy

Fig 16 Funnel plot of the relationship between cortical lesions and post stroke epilepsy

Fig 17 Funnel plot for sensitivity analysis of the relationship between early-onset epilepsy and post stroke epilepsy

Fig 18 Funnel plot of the relationship between early-onset epilepsy and post stroke epilepsy
4.Conclusion
Current research on post-stroke risk factors is mainly from cohort studies with sample sizes ranging from a few hundred to several thousand cases Many existing cohort studies have suggested risk factors based on their own studies, such as stroke subtype, cortical involvement, large infarcts, stroke severity, and vascular risk factors[15-25].These studies were derived from different cohort studies, the results of which were not completely consistent, and the method of integrating these studies by systematic evaluation and meta-analysis.Systematic evaluation and meta-analysis is a validated method located at the top of the evidence pyramid, which allows for high quality and statistical efficiency, resulting in highly credible results.This study included a total of 141,948 cases through 10 highquality cohort studies combining 9 risk factors: infarct size, stroke recurrence, stroke etiology, stroke severity, NIHSS score, earlyonset epilepsy, cortical lesions, stroke subtype, and cardiovascular events.With knowledge of risk factors, targeted and timely treatment will delay or even prevent the development of post-strokeepilepsy and reduce the economic burden on the individual and the nation.For the mechanism of post-stroke epilepsy, it has been noted that loss of neurovascular unit integrity, blood-brain barrier disruption, neurotransmitter release, local gliosis, inflammation and synaptic sprouting may promote epileptogenesis, that ironcontaining heme deposits can induce a sustained increase in neuronal excitability, and that a combination of factors leads to epilepsy[26].

Tab 5 Subgroup analysis or sensitivity analysis of risk factors for late epilepsy

Tab 6 Number of studies included in the risk prediction model, pooled RR (95%CI), beta coefficients, and risk scores for risk factors
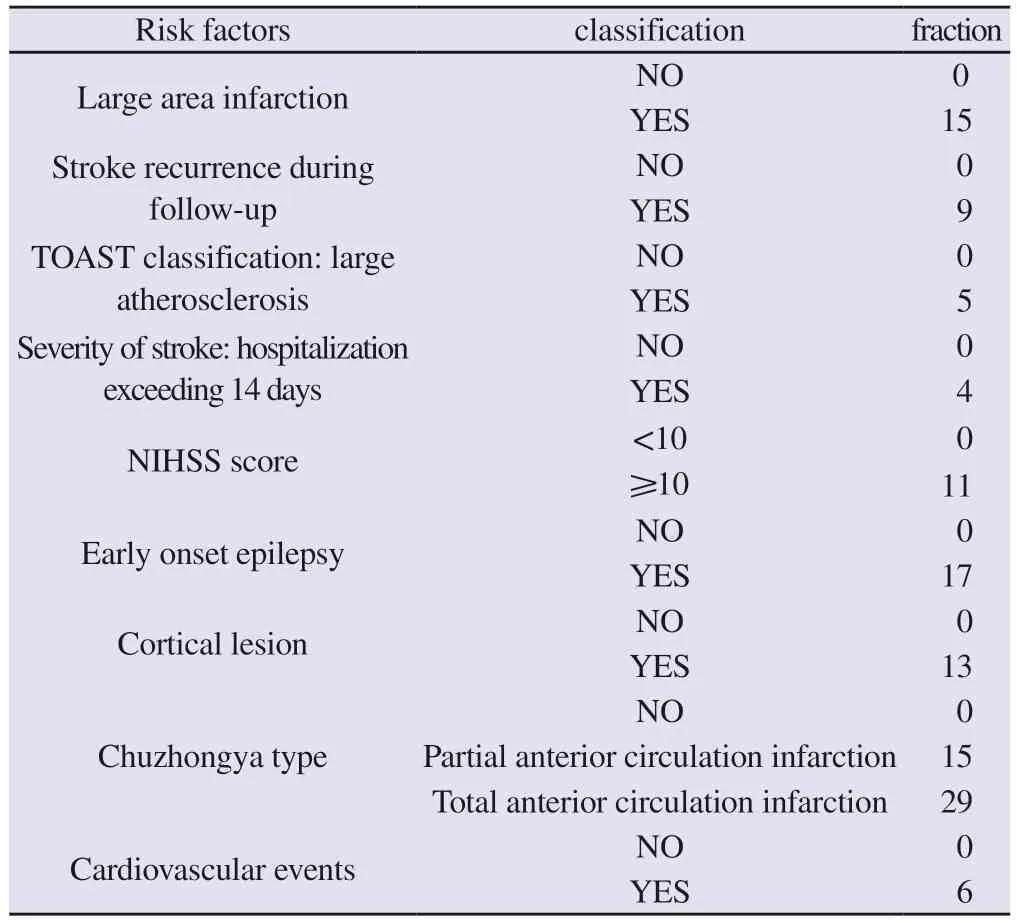
Tab 7 Risk prediction model for late epilepsy
In the present study, risk factors mostly focused on the severity of stroke itself, and existing studies for stroke subtypes focused between hemorrhage and ischemia, whereas the present study typed stroke by the OCSP typing method proposed by the Oxfordshire Community Stroke Plan[27], UK.A high probability of stroke in patients with total anterior circulation infarction can be seen, which may be related to the size of the infarct and the site of the infarct.Some evidence suggests that post-stroke epilepsy also varies depending on the cortical area affected.Involvement of the parietal temporal cortex,the superior limbic gyrus and the superior temporal gyrus seems to be associated with the occurrence of post-stroke epilepsy[28].This also explains the correlation between cortical involvement and poststroke epileptogenesis, with some brain imaging studies showing that large infarcts involving the cortex, especially in the middle cerebral artery region, may be associated with a higher risk of seizures[29].The most relevant risk factor for post-stroke epilepsy is early-onset seizures, and early seizures suggest an increased susceptibility to seizures after stroke[30].Also the length of hospital stay may be directly related to seizures.Early seizures increase the length of hospital stay.However, the length of hospital stay is mainly related to the severity of the stroke.So for more severe stroke patients the risk of late onset epilepsy is also high, which in turn may have a higher risk of early onset seizures[25].In contrast, the severity of stroke is often evaluated in a variety of ways, from NIHSS score,infarct size, infarct etiology and hospital days.
Some studies have developed predictive models for post-stroke epilepsy after stroke[18, 21, 31].However, most of these prediction models have been developed based on small cross-sectional studies or post hoc analyses of randomized controlled trials.Therefore,we developed a new risk prediction model based on a systematic review and meta-analysis of 10 high-quality cohort studies, which greatly improved statistical performance.A simple prediction model was developed including easily available clinical data on infarct size, stroke recurrence, stroke etiology, stroke severity,NIHSS score, early-onset epilepsy, cortical lesions, stroke subtype,and cardiovascular events.The risk prediction model in this study does not require complex calculations and is more convenient and intuitive for both patients and clinicians.
However, this study has several limitations.First, there was inevitable heterogeneity in the methods of systematic evaluation and meta-analysis due to differences in study design and methodology,as well as the different ethnic and gender composition of the cohorts included in the study.However, heterogeneity can be reduced by further investigation of sources and by conducting subgroup and sensitivity analyses.Secondly, the included ethnic groups are European and Asian, with a lack of data for predominantly black regions such as Africa, and baseline risk may vary by study population, so model implementation may need to be adjusted for each population to improve performance in new populations[32].Finally, our prediction model lacks a validation group, and further validation is still needed for the model’s adaptability and generalizability.
Based on a systematic review and meta-analysis, this study developed a simple risk prediction model for post-stroke epilepsy in baseline ischemic stroke that integrated clinical risk factors,including infarct size, stroke recurrence, stroke etiology, stroke severity, NIHSS score, early-onset epilepsy, cortical lesions, stroke subtype, and cardiovascular events.
Description of authors’ contribution: Yihao Yang: responsible for topic selection, collection of articles and inclusion of data, statistical analysis and processing, and article writing; Qifu Li, Qin Zou, Yi Cai: assessment of feasibility of topic selection, article revision, and article quality assessment; Shihui Chen, Zongjun Li, and Dandan Jia: inclusion of article quality control and data collection.
Conflict of interest: The content of the article does not involve a relevant conflict of interest.
The author declares that the article is original, the content is true,the data is accurate, the content does not involve leaks, there is no double submission, no plagiarism, no content plagiarism, no authorship disputes, the text is responsible for itself.
杂志排行
Journal of Hainan Medical College的其它文章
- Research progress of traditional Chinese medicine in the treatment of complications related to spinal cord injury
- Research progress on the safety of nail placement in adolescent idiopathic scoliosis surgery
- Study on the protective mechanism of Yizhiren regulating lipid metabolism in mice with diabetic nephropathy
- Differential expression analysis of coronary heart disease related genes in Hainan residents
- Experimental study of the anti-inflammatory activity of some compounds in Berchemia lineata(L.)DC
- Mechanism of Qiliqiangxin capsule on the regulation of IP3Rs/GRP75/VDAC1 gene in myocardial infarction rat heart
