Fabrication of alginate-based microspheres with cellular structure for tuning ammonium dinitramide performance
2023-11-11DunjuWngXuZhouYofengMoXinWngYemingHungRuihoWngweiZheng
Dun-ju Wng , Xu Zhou , Yo-feng Mo , Xin Wng , Ye-ming Hung ,Rui-ho Wng , D-wei Zheng
a Co-Innovation Center for New Energetic Materials, Southwest University of Science and Technology, Mianyang, 621010, China
b China Academy of Engineering Physics, Institute of Chemical Materials, Mianyang, 621900, PR China
Keywords:Ammonium dinitramide Sodium alginate Microspheres High reactivity Energetic materials
ABSTRACT Recently,an emerging category green of energetic material ammonium dinitramide(ADN)has exhibited promising application in propellants due to its outstanding merits in energy release and environmental friendliness.It can be considered to substitute traditional oxidizer of ammonium perchlorate (AP) in military systems and aerospace.In this paper,a novel spherical energetic composite ADN/copper alginate(CA) with a microporous structure was designed and prepared by the W/O gel emulsion method, and a desirable porous microsphere structure was obtained.Multiple characterization techniques were used to investigate the structure and properties of ADN/CA composites.The results showed that ADN crystals were homogeneously encapsulated in an alginate-gel matrix.Thermal decomposition temperature was reduced to 151.7 °C compared to ADN, while the activation energy of them was reduced from 129.73 kJ/mol (ADN) to 107.50 kJ/mol (ADN/CA-4).In addition, as-prepared samples had lower impact and frictional sensitivity than ADN.The mechanism of sensitivity reduction and decomposition are also discussed.Constant-volume combustion tests show that peak pressure of the ADN/CA-4 achieves 253.4 kPa and pressurization rate of 2750.4 kPa/s.Hence, this has a promising application in improving the combustion performance and safety performance of solid propellants.
1.Introduction
Composite solid propellants have been essential propulsion sources for aerospace delivery vehicles missile propulsion systems.As military science advances and modern warfare evolves, novel strategic and tactical targets present additional requirements for the functionality and performance of solid propellants composite[1].Specifically, high energy density [2], high-oxygen content,excellent combustion characteristics[3],weak characteristic signal as well as environmentally friendly [4] behavior, as green solid propellant has attracted steadily growing attention around the world[5].Until now,Ammonium perchlorate(AP)is common and extensively utilized as an oxidizer for conventional propellants[6,7].AP base composite solid propellants have been extensively used in thrusters, rockets, launch vehicles and missiles.However,the chloride element of AP would be reduced to hydrogen chloride gas (HCl) in practical applications, which contaminates surrounding environments [8], biological impacts [9], corrodes armaments,as well as is detrimental to covert launching positions due to its intense characteristic signal.
In recent decades, ammonium dinitramide (ADN) has emerged as a new opportunity for the further development of solid propellants[10],since ADN is made up of anionic N(NO2)2-and cationic NH4+without halogen in molecule [11], apart from it has high oxygen content, high energy density [12], and high burning rate[13,14].The decomposition products are relatively less harmful to the surrounding environment,personnel,and devices.Hence,ADN is promising to be one of the candidate oxidizers to replace AP for the future generation propellants with a low signature signal [15],which are suitable for aerospace propulsion and missile power systems that require low signature signal and low pollution.Nevertheless, ADN safety and hygroscopicity issues hinder its further commercial application and need to be settled urgently[16,17].On the one hand,researchers have been focusing on topical issues, such as how to reconcile the balance between high-energy and insensitivity of single energy materials [18,19], since ADN shows lower thermal stability and poor sensitivity compared to AP[13], which is prone to autocatalytic decomposition and directly impacts the safety issues of ADN in applications[20].On the other hand,owing to the inherent properties of ADN itself with strongly polar [16], NH4+on the crystals surface acts as attract H2O through electrostatic force to hydroxylate the surface.After absorbing moisture, ADN will spontaneously decompose in the storage process to generate highly hygroscopic compound ammonium nitrate(AN), which will further exacerbate the hygroscopic of ADN [16].
So far,in terms of improving the comprehensive performance of ADN,researchers devoted a lot of endeavor and time to investigate melt prilling [21], emulsion crystallization [22], spherical crystallization,surface coating of hydrophobic substances to prepare ADN composite [23,24].For example, Li et al.[25] developed a gentle procedure to preparation micro spherical ADN crystals by ultrasound-assisted solvent-antisolvent recrystallization without introducing any additives.Yan et al.[26] reported that ADN was encapsulated in alkylated graphene by the Pickering emulsion method, it exhibited excellent thermal decomposition performance, meanwhile ADN@AmGO showed an approximately decrease 60%in water adsorption.Despite some achievements have been reported,there are still challenges in preparing and designing ADN composites with excellent performance.The hygroscopicity of ADN is decreased by turning the crystal morphology into spherical shape, but due to that ADN crystals are sufficiently exposed to the atmosphere, NH4+still bonds with H2O and has significant hygroscopicity.Further studies have shown that coating with insensitive and hydrophobic materials on the surface of crystals,it is proved to be an effective way to reduce mechanical sensitivity and decrease the hygroscopicity of ADN [23,27,28].Nevertheless, the core-shell structure often imposes spatial confinement to energy release of ADN particles.In addition, since most conventional encapsulation routes were often operated in the ADN molten state,which suffers from uncontrolled thermal decomposition, resulting in potential safety concerns during the melting process [29].Given these reasons,it is significant and challenging to construct unique structures and rational compositions to enhance comprehensive performance and broaden the application environment of materials, especially for safety, lower hygroscopicity and energy release capabilities.
Sodium alginate (SA) hydrogel is one of natural biomaterials with significant potential [30], whose unique three-dimensional network structure enables it to capture drugs as carriers, and prevents their decomposition or reaction with external reagents,and it facilitates the release of drugs in specific situations [31].SA is polysaccharide with two constituent monomers,α-L-guluronic acid(G units) and β-D-mannuronic acid (M units) linked together by a β-1, 4-glycosidic bond (Fig.1(a)).Adding divalent metal ions like copper ions induces the cross-linking of alginate chains to form gels[32], this process is explained by the typical “egg-box” model as shown in Fig.1(b) [33].Importantly, divalent cations polymerized with hydroxyl and carboxyl groups of different chain segments from alginate hydrogels in internal and external to molecule.Polysaccharide chains are bound in a highly cooperative manner,each sodium alginate chain polymerizes and as a result combine with other chains to form a gel network [34].The drug is being uniformly dispersed in polymer networks by this encapsulation method [35-37].It exhibits mesoporous structure after drying,which benefits to energy release of energetic materials.Hence,metal-alginate exhibits promising applications in improving propellant pyrolysis performance and high explosives desensitization.In particular,copper ions have significant catalytic property during thermal decomposition of ADN[38],incorporating natural product alginate and ADN is a promising method to obtain spherical particles.
In response to the above problems, this study designed and prepared ADN/copper alginate (ADN/CA) via the water-in-oil emulsion diffusion method, where ADN has been assembled in gel network of alginate.It is worth mentioning that copper ions were chosen as additives due to the significant catalytic effect on thermal decomposition of ADN,on the other hand,copper alginate(CA) has excellent mechanical properties which diminish the mechanical sensitivity of ADN.The water-in-oil emulsion diffusion method was developed for this work to assemble ADN/CA microspheres with porous structure, as well as to investigate different mass fractions of SA on the morphology and properties of ADN/CA microspheres.Therefore, alginate can serve as a template for promoting the comprehensive performance of ADN/CA composites,not only enhancing energy release efficiency but also promoting mechanical safety.
2.Experimental
2.1.Materials
Sodium alginate (SA), paraffin oil and cupric chloride (CuCl2)were purchased from Chengdu Kelon Chemical Reagent Factory.Ammonium dinitramide (ADN) obtained from Xi’an Modern Chemistry Research Institute, China.All reagents were used as received without further purification.
2.2.Preparation of CA/ADN microspheres
ADN/copper alginate (ADN/CA) microspheres were presented by water-in-oil (W/O) emulsification-diffusion method, Fig.1(d)shows our synthesis protocol.Firstly, ADN (Fig.1(c)) and SA with different mass ratios were added into deionized water with constant magnetic stirring at 50°C water bath to obtain homogeneously 1.0 g/mL ADN/sodium alginate (ADN/SA) mixed hydrogel.And then 0.5 g of CuCl2was dissolved in 10 mL isopropanol solution by ultrasound-assisted CuCl2dissolution.Then, the obtained mixture solution of 1 mL ADN/SA was slowly dripped into mixture of 10 mL of liquid paraffin and 0.1 g Span 80 at magnetic stirring rate of 1000 rpm.Stirring at the same speed for 10 min to produce homogeneous W/O emulsion,1 mL CuCl2(0.05 g/mL)solution was dripped into the aforementioned W/O emulsion and stirred for 2 h.Finally, the light blue suspension was filtered, washed with petroleum ether, and then freeze-dried for 24 h, the ADN/CA microspheres can be obtained.To easily distinguish these obtained samples, which were denoted as ADN/CA-2, ADN/CA-3, ADN/CA-4 and ADN/CA-5 which corresponding the mass ratio of ADN:SA with 98:2,97:3,96:4 and 95:5 respectively,and the raw material of ammonium dinitramide is marked as ADN.
2.3.Characterization
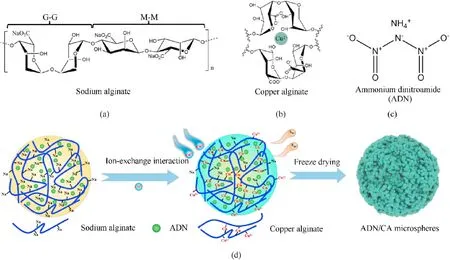
Fig.1.(a) Molecular structure of sodium alginate; (b) The copper alginate of the “egg-box” model; (c) The molecular structures of ADN; (d) Protocol for ADN encapsulation by alginate.
The structure and surface morphology of sample was imaged by the field emission scanning electron microscopy(FE-SEM,Ultra-55,Carl Zeiss, Germany) at an acceleration voltage of 10 kV after gold sputtering coating under a vacuum of degree 10-6Pa for 50 s.The surface element analysis of samples was characterized by an energy dispersive x-ray spectroscopy (EDS) equipped on the FE-SEM.The composition of samples was investigated by Fourier transform infrared (FT-IR) which spectra were recorded on a Nicolet-5700 FTIR spectrometer using KBr pellets.X-ray diffraction (XRD, X′Pert pro, Panacco, Netherlands) was obtained on a Bruker D8-Advance diffractometer equipment at Cu Kα radiation(λ=0.15405 nm),the sample was scanned in the 2θ range from 5°to 80°.X-ray photoelectron spectroscopy(XPS)was obtained on an ESCALAB 250 type electron spectrometer under Al Kα irradiation.Simultaneous thermal analysis (STA 449-F5, NETZSCH, Germany)analyzed the thermal decomposition of sample particles at aluminum pan.The measurements were carried out with 60 mL/min N2ambient purge in the temperature range of 50-500°C with a sample mass of about 2.0 mg.The heating rates were maintained at 5°C/min, 10, 15 and 20°C/min.The thermal decomposition mechanism of ADN and ADN/CA-4 composite was investigated via real-time FTIR spectroscopy (NetzschSTA409, BruckerV70) in the N2atmosphere and the heating rate was 10°C/min.The impact and friction sensitivities were performed on a BAM fall hammer BFH12 and a BAM friction apparatus FSKM-10 (OZM Research, Czech Republic).The prepared sample was placed in constant-volume combustion cell (330 mL),the 100 mg sample was ignited under air atmosphere by nickel-chromium wire load voltage with 7 V voltage from DC power supply.Piezoresistance pressure sensor (PCB Piezotronics, 113B26)was used to record the combustion pressure evolution.
3.Results and discussion results
3.1.Morphology and microstructure of samples
As can be observed in Fig.2,the apparent difference in size and morphology can be found by comparing the SEM images of asprepared samples (Figs.2(a)-d).All as-prepared samples show spherical morphology with the increase of alginate concentration(from 2 to 4wt%), the particles dispersion increases and agglomeration decreases.For samples with SA concentrations of 2wt%and 3wt% (Figs.2(a) and (b)), its average particle size is approximately 10 μm and 3 μm,respectively.They exhibit elliptical particles with low sphericity, and further magnification reveals that it is significantly agglomerated,where adjacent particles were glued together by polymers.Upon increasing the content of SA to 4wt%,as seen in Fig.2(c), the particles exhibit spherical with nanopores and dispersion increases.However,it continues to increase the content of SA to 5wt%(Fig.2(d)),the number of irregular spherical particles increased, and their sizes increased to approximately 38 μm.It could draw a conclusion that this particle integrity is extremely dependent on the concentration of sodium alginate.This observation can be elucidated by the effect of the viscosity of solution alginate [39], which increases with increasing alginate concentration, enhancing the interfacial tension of the ADN/SA solution.Therefore, the change of alginate solution concentration affects particle morphology.At 2wt% and 3wt% alginate concentrations,small microsphere particles with low surface energy were formed,leading to agglomeration[40].It is attributed to the sparse hydrogel network by low concentration of polymer molecular chains,which makes the particles susceptible to deformation by shear stress during ionic cross-linking process.Hence ADN/CA particles shrank more easily after filter drying.In contrast, for ADN/CA-5, the interfacial tension between alginate droplets and oil phase increases [41], resulting in the ineffective emulsification and dispersion of alginate solution in the oil phase.The high viscosity alginate solution is sheared into irregular fragments by the shear stress from magnetic stirring, and portions of fragments agglomerate into rough and irregular microspheres in the vortex.Relatively speaking,the sphericity of ADN/CA-4 is better than other samples.The magnified image in Fig.2(c)clearly shows that these spherical particles have porous surfaces where small pores of micro-nano scale are observed, which implies the possibility of porous structures in the particles.The cross-sectional images (Fig.2(e)) of the particles were captured and prove that these samples possess porous structure inside.This is probably due to expansion and extrusion of ice crystals during freeze-drying process.Unanticipated,there are no significant ADN crystals detected on the surface and inside of microspheres.To investigate how ADN is integrated with alginate,porous microspheres were immersed into ethanol for washing the ADN and then followed by the filtered dry process.As shown in Fig.2(e), these microspheres possess coral-like skeletal structure and rough surface.This result indicates that ADN crystals may be encapsulated in copper alginate.These results would be further demonstrated by FTIR, XRD, XPS.Besides, in order to analyze the surface composition of ADN/CA-4, the SEM images of ADN/CA-4 and corresponding mapping images are shown in Figs.2(g)-(i).The characteristic elements of ADN and copper alginate correspond to N and Cu, respectively.The N and Cu elements were uniformly distributed on the surface of microspheres,which indicates that ADN is uniformly mixed with alginate and ADN is embedded in the network of alginate after recrystallization.
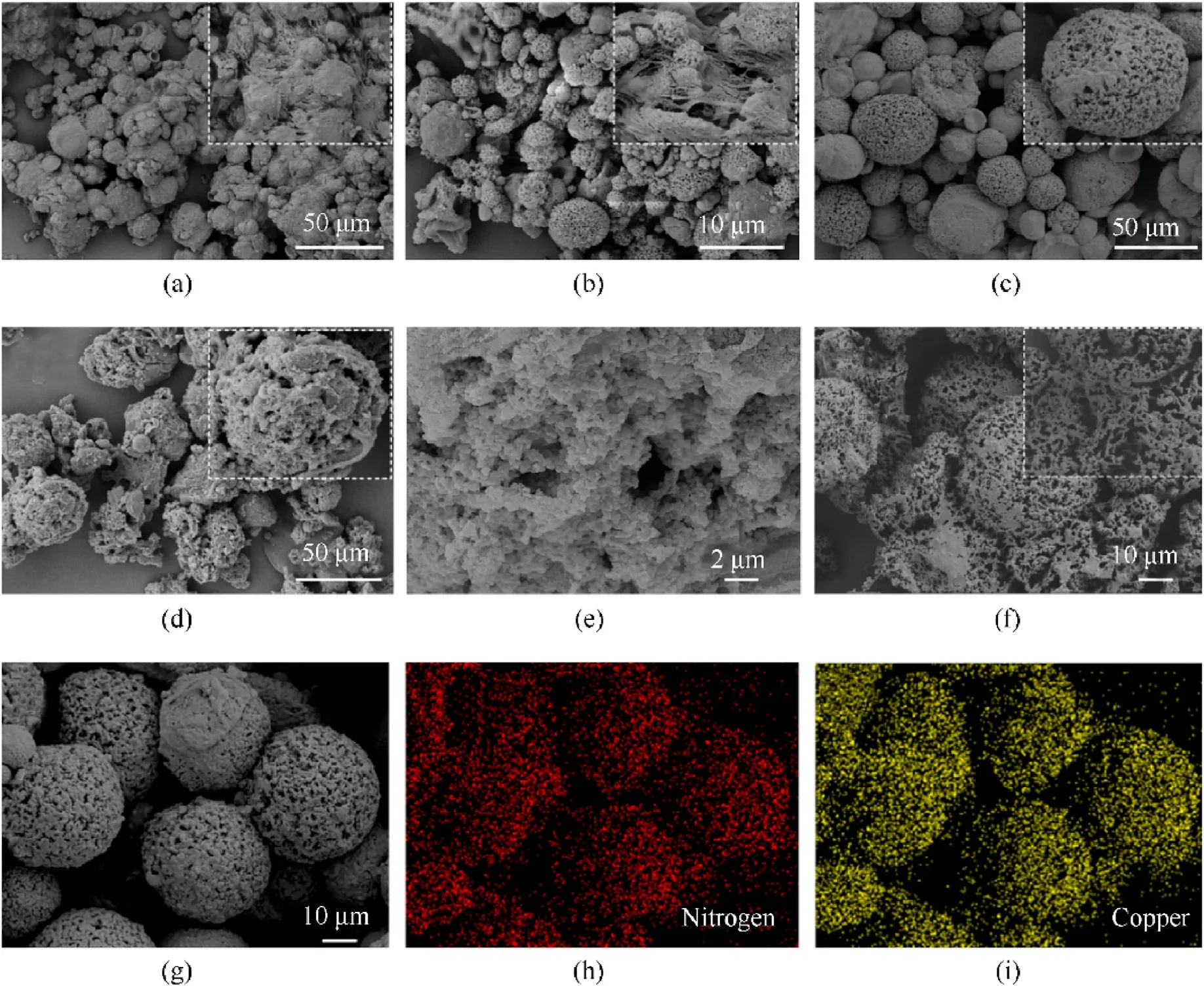
Fig.2.SEM images of ADN/CA microspheres with different mass ratio: (a) ADN/CA-2; (b) ADN/CA-3, (c) ADN/CA-4; (d) ADN/CA-5; (e) Cross-section of ADN/CA microsphere; (f)ADN/CA-4 microspheres after being washed with ethanol; (g)-(i) elemental mapping images of ADN/CA-4.
3.2.Composition analysis of samples
The composition of as-prepared samples was determined by FTIR spectroscopy analyses, this result is displayed in Fig.3(a).For sodium alginate,the adsorption peak at 2925,1612 and 1417 cm-1belong to the aliphatic C-H stretching vibration, asymmetric COO stretching vibration (i.e., vasym(COO-) and symmetric COO stretching vibration(vsym(COO-))in sodium alginate spectroscopy[42].Copper alginate exhibited similar characteristic absorption peaks.However, the characteristic peak of the COO-shifts toward lower frequency, which is attributed to alginate with abundant hydroxyl and carboxyl active groups coordinated with Cu2+.The vasym(COO-)characteristic peaks move to higher frequencies(from 1612 to 1623 cm-1), which was probably caused by the high electron density owing to Cu2+adsorption on neighboring hydroxyl groups [43].As for raw material ADN, the absorption peak located at 3136 cm-1corresponds to stretching and bending vibration of N-H bond in NH4+, while the peaks at 531 cm-1and 1344 cm-1,which was assigned to asymmetric stretching and symmetrical stretching vibration of-NO2.The peak at 1032 cm-1is assigned to the characteristic absorption of N-N-N stretching vibration [44].In addition, as shown in Fig.3(a), the characteristic peaks of ADN and CA appeared simultaneously in ADN/CA, indicating that ADN/CA was successfully prepared.
XRD is presented for analysis the crystalline phases of ADN,CA,SA, and ADN/CA-4 composites as shown in Fig.3(b).As can be observed, there are no obvious diffraction peaks for SA and CA,except a specific diffraction peak for SA at 2θ of approximately 14°,indicating that it belongs to the category of semi-crystalline amorphous polymer.It is noticeable that the capping packet at about 14°disappeared after adding copper ions, perhaps because the crystallinity of SA was destroyed by the addition of copper ions.The XRD pattern of raw material ADN was obtained in diffraction angle range 5°≤2θ ≤80°.The sharp peaks obtained at 2θ=12.99,14.97, 17.57, 26.18, 26.93, 27.56, 29.14, 29.98, 39.68, 54.46 can be assigned to (1,0,0), (0,2,0), (0,1,1), (1,3,0), (1,2,1), (0,3,1), (2,1,-1),(0,4,0), (0,3,2), (2,5,-2) crystal planes [25], respectively.All of the diffraction peaks could be indexed to standard pattern of ADN(PDF No.48-1188).For ADN/CA-4, the positions of typical diffraction peaks have not been altered compared to the original ADN,but the intensity of ADN characteristic peaks has been reduced significantly, this indicates that ADN is already encapsulated in CA.Another interesting phenomenon can be observed that the relative intensities of diffraction peaks between different crystal planes in ADN/CA-4 are obviously different compared to original ADN, even some of the diffraction peaks disappear,such as((1,0,0)and(2,5,-2)crystal planes.This is attributed to that ADN crystal growth process is limited by surrounding alginate, and then part of crystal face grows into edges or vertices, even disappeared in the final ADN morphology [25].

Fig.3.The compositions analysis of raw materials ADN, CA, SA, and obtained ADN/CA-4: (a) FT-IR spectra; (b) XRD patterns.
XPS analysis was performed to evaluate the surface chemical elemental composition of ADN/CA.According to the survey scan,XPS spectrums exhibited a strong C1s peak, N1s peak, O1s and a weak peak Cu2p (Fig.4(a)).Three board peaks at around 406.12,401.9 and 400.19 eV are observed in the high-resolution N1s,which can be assigned to ADN.As shown in Fig.4(c),six peaks were fitted into the C1s pattern.They are C-C (284.27 eV), C-H (284.80 eV),C-O (286.01 eV), C-OH (286.49 eV), C═O (287.90 eV), O-C═O(289.03 eV) bonds can be attributed to copper alginate.Within high-resolution Cu2p spectrum of Fig.4(d), two peaks located at 954.5 eV and 934.8 eV can be observed, which can be assigned to Cu2p1and Cu2p3of the copper ion, respectively.In the previous experimental results,it was shown that no new bonds were formed in ADN/CA composite, proves that the ADN was encapsulated in copper alginate successfully.
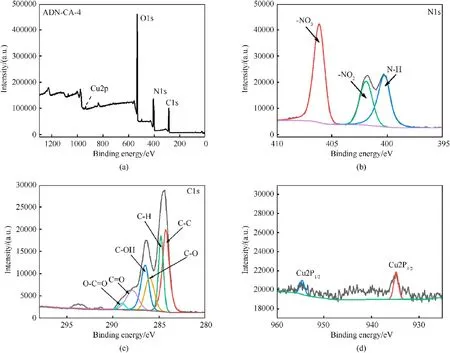
Fig.4.XPS survey spectrum of (a) ADN/CA-4, (b) N1s, (c) C1s, and (d) Cu2p.
3.3.Thermal decomposition properties analysis of samples
TG-DSC is an important characterization means to study the thermal decomposition properties and decomposition reaction kinetics of energetic materials.Fig.5(a) and (b) show the thermal decomposition behavior of ADN/CA with different sodium alginate contents in nitrogen atmosphere at 10 K/min heat rate.It is not hard to find that the thermal properties of as-prepared ADN/CA samples show great differences,compared with DSC and TG curves of ADN.For original ADN [45], one endothermic peak is located at 90.4°C which is attributed to material conversion from crystalline phase to the liquid phase, another exothermic peak occurs at 191.7°C and the ADN decomposes completely to produce NO2, NO, NH3, H2O,etc.which are in general consistent with reports[46].As shown in Fig.5(a), when the content of SA was 2, 3, 4, 5wt%, the corresponding exothermic peak temperatures were 149.5,155.8, 150.7,165.9°C,respectively,which were reduced by 42.2,35.9,41.0,25.8 compared to original ADN.The exothermic peak temperatures of ADN/CA-2, ADN/CA-3 and ADN/CA-5 increased as the alginate content increased, these were all lower than exothermic peak temperature of ADN.These results indicate that CA contributes to catalyzing the thermal decomposition of ADN.However, an abnormal phenomenon has noticed that difference from the case of other DSC curve, that exothermic peak temperatures of ADN/CA-4 is lower than ADN/CA-3 and ADN/CA-5.On the one hand,because alginate reduces the ADN thermal decomposition peak temperature, on the other hand benefited from the distinctive porous structure of ADN/CA-4.As shown in Fig.5(b), ADN/CA-2,ADN/CA-3 and ADN/CA-5 with two weight loss step is due to structural differences.Specifically, the first weight loss step is complete exothermic decomposition of ADN and decomposition of the first stage of copper alginate [47], where copper alginate loses crystal water, a small amount of decarboxylation reaction occurs and copper carbonate is produced.The second weight loss step is copper alginate main decomposition stage[48].We speculate that the reason for two weight loss steps is that ADN/CA-2, ADN/CA-3 and ADN/CA-5 does not have good porous structure of ADN/CA-4,and the particles are poorly dispersed and even stick together.This results in ADN decomposition gas is slowly release from copper alginate during weight loss at first step,which may be captured by partially decomposed copper alginate, retained in the copper alginate skeleton,and thus participated in decomposition of second weight loss step.The opposite is that ADN/CA-4 benefits from its porous structure,sample decomposition is done almost in one step.
In order to further investigate the detailed parameters of thermal decomposition,the Kissinger method was utilized in this study to calculate activation energy(Ea)of ADN and as-prepared ADN/CA samples[49],where Tprepresents the peak temperature in the DSC curve and β is the heating rate and based on these parameters line fitting the ln (β/Tp2) versus 1/Tpcurve (Fig.5(c)).The activation energies of ADN and as-prepared samples are shown in Fig.5(d).It can conclude that the Eaof ADN/CA-2(129.66 kJ/mol)and ADN/CA-3 (130.70 kJ/mol) are almost unchanged compared to the raw material ADN (129.73 kJ/mol).Except for the Eaof ADN/CA-5(147.78 kJ/mol) has been improved compared with raw ADN crystal, demonstrating ADN/CA-5 composites are much difficult to be activated than that of original ADN.Besides, another interesting phenomenon is that ADN/CA-4 reached lowest activation energy(107.50 kJ/mol), which also confirms that ADN/CA-4 is the most reactive composite among other samples.It can be attributed to the fact that ADN/CA-4 has microporous channels in its interior causing it to exhibit high reactivity.In terms of heat release,the DSC-TG test shows that the ADN thermal decomposition heat release was 1872 J/g(Fig.5(d)).When alginate was applied to encapsulate ADN crystal,the heat release of as-prepared samples increased gradually with the increase of alginate content,the heat release of ADN/CA-2,ADN/CA-3 and ADN/CA-4 corresponded to 1173,1344 and 1679 J/g,respectively.ADN/CA-4 exhibits lower decomposition temperature,higher reactivity and greater heat release compared with ADN/CA-2, ADN/CA-3 and ADN/CA-4.These results demonstrate that alginate catalyzed thermal decomposition of ADN.ADN/CA-4 may be the optimal content mass ratio and structure.

Fig.5.(a) DSC and (b) TG curves of ADN and as-preparate samples; (c) Based on non-isothermal DSC data linear fit of plotted ln (β/Tp2) against 1/Tp by the Kissinger method for calculation activation energy of ADN and as-prepared samples; (d) the heat release and activation energy of ADN and the as-prepared samples.
3.4.Decomposition mechanism of ADN/CA-4
In order to analyze the thermal decomposition mechanism of ADN and ADN/CA-4 composite microspheres, the DSC-TG instrument was coupled with FTIR instrument to evaluate gaseous products from thermal decomposition (Fig.6).The threedimensional (3D) plot shows the infrared absorption intensity of gaseous product as a function of wavelength/temperature.With reaction progresses,the gas is captured and this result is shown in 3D plot exhibiting four intensive absorption peaks for the ADN(Fig.6(a)) and ADN/CA-4 (Fig.6(b)).The results of preliminary analysis indicate that the main evolved gases by ADN and ADN/CA-4 were determined to be N2O (IR absorbance: 2250-2100 cm-1,and 1350-1200 cm-1) and NO2(IR absorbance:1650-1550 cm-1)[50].
Comparing Fig.6(c)and(e),it can be concluded that the gaseous products of raw material ADN (from 166.84 to 223.94°C) undergo time from the generation to disappearance during thermal decomposition is longer than that of ADN/CA-4 (from 138.08 to 173.64°C).In addition to this the decomposition stage has been reduced from about 200 to about 150°C,which has been confirmed in thermal performance section of sample.Further details of sample decomposition without and with copper alginate were discussed in the reaction process,and IR spectra of samples gaseous products at the peak exothermic temperature were obtained.From Fig.6(c), it shows clearly that initially ADN decomposition produces N2O,H2O(166.85-177.22°C)and the further gaseous decomposition product is NO2(187.60°C)which is detected by FTIR.According to previous reports on the thermal decomposition of ADN [46], the main pathway of ADN dissociation decomposition is NH4NO3(AN) and N2O,and then decomposition of AN at high temperature to produce the gases N2O and H2O,in addition to some co-products of gas NO2.However, the thermal decomposition process of composite microspheres(Fig.6(d)) was slightly different from ADN,the generation of gas products is being accelerated.One possible thermal decomposition mechanism of ADN catalyzed by copper alginate was postulated based on real-time FTIR results.According to acidcatalyzed mechanism, an abundant amount of CO2, CO accumulates in multi-porous channels of microspheres at the initial stage,CA catalyzes ADN decomposition.Subsequently, an abundant amount of N2O, NH3accumulates in multi-porous channels of microspheres, NH3and copper ions may form coordination compounds, besides porous microspheres with large specific surface area increase residence time of N2O, as well as the increase mass and thermal transfer efficiency.In addition,the absorption peak in FTIR spectrum located around 950 cm-1was assigned to NH3characteristic peak, which may be attributed to that [Cu(NH3)4]2+was acidified by intermediate product HNO3to produce NH3,replacing the side reaction of NH3during ADN decomposition.Therefore, these results cause thermal decomposition reaction to being compressed into shorter reaction time, accelerating thermal decomposition reactions and releasing large amounts of energy.
3.5.Sensitivity analysis
The impact and friction sensitivities are also studied for the obtained samples, which are important parameters for assessing safety performance of energetic materials.As shown in Fig.7(a),the raw material ADN needs lower impact energy and friction force be triggered than other samples.After ADN was encapsulated in CA,the impact sensitivity of as prepared samples exhibits a decreasing trend with increasing content of SA.At SA contents of 2, 3 and 4,5wt%, the samples were triggered by applying frictional forces of 80, 84,108 and 80 N,respectively.These results may be attributed to the reduced particle size of ADN crystals, the distinctive morphology and interfacial interactions of ADN/CA composite.In general, it is known that the formation of hot spots is the main reason for detonation of explosives.The ADN is encapsulated in CA network structure to obtain ADN/CA composite, therein micronano ADN was uniformly dispersed in CA, which effectively hinders the generation of hot spots.On the other hand, the resilient copper alginate skeleton absorbed most of energy from impact stimuli.Even though the remaining stimuli may arrive in an ADN crystal interior, these stimuli may not produce hot spots due to lacking enough energy.These desensitization mechanisms are also applicable to frictional sensitivities,the difference is that ADN/CA-4 exhibits optimal frictional sensitivities due to its structural advantages that unique porous structure and spherical particle morphology.Under friction shear, particles with porous structure can withstand more intense deformation to offset the mutual friction between crystals[51],while spherical particles facilitate the shear slip between particles reducing frictional heat generation efficiency, further reducing the frictional sensitivity of ADN/CA composite.Benefiting from this reason, ADN/CA exhibits excellent security properties in terms of impact and friction sensitivities,especially, for ADN/CA-4 microspheres with porous structure.
3.6.Hygroscopicity test
ADN acts as a strong polar inorganic salt, dissolving 357 g in 100 g of water, when ADN molecules are directly exposed to the atmosphere,NH4+located on the surface of ADN crystals can easily combine with water molecules from the environment by hydrogen bonding, leading to moisture absorption and agglomeration of the ADN particles.Thus, it is particularly important to test the hygroscopicity of ADN.ADN and as-prepared samples were placed in an open glass container, and then exposed to atmospheric relative humidity of 75%and temperature was 20°C,hygroscopicity curves of samples were obtained by monitoring the change in sample mass over time.As shown in Fig.7(b), the raw material ADN has completely turned into aqueous after being placed in atmospheric with relative humidity of 75%for 3 h,the water uptake rate of this sample increased to 30% and exhibited extreme hygroscopicity.However, for increasing the content of SA 2, 3, 4, 5wt%, the corresponding water uptake rate of as-prepared ADN/CA samples were decreased to 24.54%, 27.51%, 18.97%, 18.85%, respectively, which resulted in reduction significantly in terms of hygroscopicity compared with raw material ADN.Moreover,the hygroscopicity of samples evolved longer time from increasing towards plateauing compared with raw materials ADN,and all ADN/CA samples do not turn into aqueous after 48 h of moisture absorption.These results indicate that ADN/CA composite obtained by the W/O emulsification-diffusion method has shown a positive effect on reducing the water uptake rate.
3.7.Pressure output performance
Pressure output performance of ADN/CA was evaluated using constant-volume combustion test, from which peak pressure and the pressurization rate were obtained [52,53].The pressures-time curve of ADN and different ADN/CA composites with various mass fraction of alginate were shown in Fig.8(a).The pressure increases slowly during the combustion reaction, reaches maximum and then slowly decreases with time.However, as the alginate content increases, the pressure output peak of samples becomes sharper.Therefore, alginate can promote the combustion of ADN as combustible.The peak pressure and pressurization rate of samples increased gradually as shown in Fig.8(b).Clearly,ADN/CA-5 has maximum peak pressure compared with other formulations.On the one hand, the combustion of ADN/CA-5 releases massive heat, which causes the gas to expand, as well as a large number of gaseous products generated from the combustion process.The sample with 4wt%alginate had the highest pressurization rate,due to porous structures.The porous structures increase heat and mass transfer rates of combustion process,resulting in pressure in the combustion cell attains its peak in extremely short time.Thus, the pressure output performance of ADN/CA is well correlated with the sample structure and alginate content, of which ADN/CA-4 and ADN/CA-5 exhibit better pressure output performance.
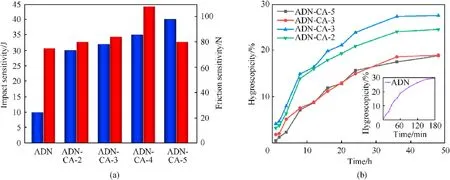
Fig.7.(a) Impact sensitivity and friction sensitivity of samples; (b) Hygroscopicity curves of samples.

Fig.8.(a) Pressure output curve; (b) Pressurization rate and peak pressure (Pmax) of samples with different alginate ratio.
4.Conclusions
In conclusion,this work reports a favorable fabrication strategy of porous structured composite microspheres ADN/CA, wherein ADN crystals were encapsulated in copper alginate framework employing the W/O emulsion method.The structure and composition have been investigated and presented a satisfactory result with ideal spherical morphology.Furthermore, the thermal decomposition kinetics and thermodynamics of composites were calculated and analyzed.It was demonstrated that ADN/CA-4 microspheres with porous structure could improve energy release efficiency and reactivity of composite energetic materials.The activation energy (107.50 kJ/mol) was obtained by Kissinger method,the thermal decomposition temperature was decreased by 41°C.The safety performance of composite was also significantly enhanced, especially ADN/CA-4 has a significant reduction in friction and impact sensitivities of 108 N and 35 J respectively.The thermal decomposition and desensitization mechanisms are explored.Besides,ADN/CA-4 and ADN/CA-5 exhibit better pressure output performance.Hence, this fabrication strategy would no doubt exhibit promising applications in improving propellant pyrolysis performance and desensitization the high explosives.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No.22005253).
杂志排行
Defence Technology的其它文章
- Eigen value analysis of composite hollow shafts using modified EMBT formulation considering the shear deformation along the thickness direction
- Synthesis of energetic coordination polymers based on 4-nitropyrazole by solid-melt crystallization in non-ionization condition
- RDX crystals with high sphericity prepared by resonance acoustic mixing assisted solvent etching technology
- Study of residual stresses and distortions from the Ti6Al4V based thinwalled geometries built using LPBF process
- Modeling and simulation of solvent behavior and temperature distribution within long stick propellants with large web thickness undergoing drying
- Assessment of the ballistic response of honeycomb sandwich structures subjected to offset and normal impact
