RDX crystals with high sphericity prepared by resonance acoustic mixing assisted solvent etching technology
2023-11-11DongjieLioQinLiuChunynLiNingLiuMinghngWngChongweiAn
Dongjie Lio , Qin Liu , Chunyn Li , Ning Liu , Minghng Wng , Chongwei An ,*
a North University of China, Taiyuan 030051, China
b Yichang Testing Technique R&D Institute, Yichang 443099, China
c Xi'an Modern Chemistry Research Institute, Xi'an 710065, China
Keywords:Resonance acoustic mixing Solvent etching RDX Sphericial explosive
ABSTRACT In order to obtain high-quality spherical RDX crystal particles, the RDX crystals were suspended in a mixed solvent of cyclohexanone and cyclohexane, subsequently a solvent etching study was carried out under the action of vibration/acoustic flow coupled flow field, which generated by resonance acoustic mixing.The effects of solvent ratio, temperature, acceleration and experiment time on morphology as well as particle size of RDX crystals were studied.Not only were the morphology, particle size distribution and crystal form of RDX crystals determined, but also the thermal decomposition performance and mechanical sensitivity of spherical RDX were examined and discussed.Results indicated that under the process of solvent/non-solvent volume ratio at 1:2, temperature of 40°C, acceleration of 40 g and experiment time of 4 h, α-type RDX crystal with sphericity of 0.92 can be obtained.Furthermore, the median particle size(D50)of spherical RDX crystals is 215.8 μm with a unimodal particle size distribution(size span 1.34).For one thing, the thermal decomposition peak temperature of spherical RDX is about 2.5°C higher than that of raw RDX, and apparent activation energy reaches 444.68 kJ/mol.For another thing,impact sensitivity and friction sensitivity of spherical RDX are 18.18%and 33.33%lower than that of raw RDX, respectively.It demonstrates that safety of spherical RDX under thermal, impact and friction stimuli has been improved.
1.Introduction
Cyclotrimethylenetrinitramine is the chemical name of a common explosive known as the cyclone explosive or,more popularly,RDX.It is a low-cost, high-energy nitroamine explosive that is widely employed in military and civilian fields.However,due to its relatively high mechanical sensitivity, accidental explosions are common under impact and friction forces.The study of RDX explosive passivation has become a research hotspot in the field of energetic materials.Many studies have revealed that the particle shape of explosives influences particle flowability and is closely related to safety performance.Spherical explosives,in general,offer excellent flowability and low mechanical sensitivity.
Furthermore,using spherical explosives in mixed or propellants can enhance the loading rate and mechanical properties [1,2].So far, domestic and foreign researchers have developed various explosive particle spheroidization methods.Primarily recrystallization method [3,4], spray spheroidization method [5,6],ultrasonic-assisted emulsion method [7], electrostatic spray method [8], jet milling spheroidization technology [9], solvent etching method [10,11], etc., and the spheroidization of various energetic materials such as RDX,HMX,CL-20,ADN,and AP.Among the techniques stated above, the solvent etching method is a spheroidization method with easy operation, cheap cost, and strong adaptability, in which explosive crystals are suspended in a solvent system,and the suspension produces a flow field under the action of stirring(or vibration).The crystal moves irregularly in the flow field,and the solvent continuously erodes,washes,and wears the protruding parts of the crystal(such as edges and corners).This makes the protruding edges and corners of the crystal passivated and smooth, and finally, spherical crystal particles are obtained[12].
The erosion impact of crystal particles in the solvent etching process is not only affected by the type of solvent.However, it is also closely related to the flow field parameters (flow velocity, acceleration, and eddy current).The classic solvent etching process involves creating a flow field in the liquid using the stirring blade's rotating shearing to propel the particles into a three-dimensional turbulent motion.Due to the considerable variances in flow velocity and acceleration characteristics in each section of the flow field, the particles are subjected to unequal force in the flow field,which affects particle uniformity.Furthermore, collisions between the explosive particles and the paddle are unavoidable during the stirring phase of the paddle,affecting the spheroidization effect.As a result, obtaining a paddleless flow field with a higher degree of homogeneity is a critical issue for this technology.The advent of Resonant Acoustic Mixing (RAM) offers a viable solution to this challenge.
Resonance acoustic mixing employs the coupling effect of vibration macro mixing and acoustic flow micromixing.It helps to generate a large circulating flow field and a small microcirculation flow field in the explosive particle/solvent suspension system,resulting in uniform dispersion of the suspension system throughout the field [13-15] and improved force uniformity on each particle.Furthermore,this mixing method has no interposing blade,which can prevent physical damage to explosive particles or dangerous accidents triggered by blade shear or friction impact with the vessel wall [16,17].The benefits of resonance acoustic mixing include high mixing safety, rapid mixing speed, simple container cleaning, and in-situ mixing.It has been used in medicine, food, biology, cosmetics,energetic materials, and other fields[18-21], demonstrating good mixing effects and the benefits of outstripping the traditional method.However, the application of resonance acoustic mixing in energetic materials is represented chiefly in material mixing, and there are few reports on the regulation of energetic material crystal morphology.
The current research attempts to apply resonance acoustic mixing technology to the preparation process of spherical RDX explosives.The coupling effect of macro vibration and lowfrequency acoustic-flow-resonance acoustic mixing was used to provide solvent etching of explosive particles with an overall uniform flow field with a large and small regional cycle.The spheroidization evolution process of RDX explosive particles and its mechanism under various process conditions were investigated.Also,the morphology,particle size distribution of the crystal form,thermal decomposition property, and mechanical sensitivity were tested and characterized.
2.Experimental
2.1.Material
RDX was provided by Gansu Yinguang Chemical Industry Group Co., Ltd.of China.The other reagents such as absolute ethanol,cyclohexanone and cyclohexane in analytical grade used in this work were obtained from Macklin Biochemical Co., Ltd.of China and used without further purification.
2.2.Preparation of spherical RDX
2.2.1.Preparation of explosive crystal/solvent suspension system
The explosive crystal/solvent suspension system was made by blending 1.5 g RDX crystal with 36 mL of cyclohexanone and cyclohexane mixed solvent (1:2) and placing it in a resonance acoustic mixing container.
2.2.2.Preparation of spherical RDX by resonance acoustic mixing assisted solvent etching method
The resonance acoustic mixing apparatus used in the study has a maximum acceleration of 100 g and is equipped with reactors of various specifications that can complete the material mixing experiment with a volume of 1-1000 mL.The resonance acoustic mixing container carrying the RDX crystal/solvent suspension system was attached to the resonance acoustic mixing equipment and connected to the temperature control device for preparing spherical RDX.The temperature was set to 40°C, the resonance acoustic mixing acceleration was set to 40 g, and the resonance acoustic mixing time was set to 4 h.After resonance acoustic mixing according to the established process parameters, the suspension comprising spherical RDX crystal particles was obtained.The suspension was filtered under reduced pressure,washed three times with anhydrous ethanol, and then placed in a water-bath drying oven at 60°C for 2 h to obtain spherical RDX particles.
2.3.Characterization
Surface morphology:Raw RDX and spherical RDX morphologies were examined using a laser confocal microscope (Shanghai Zeiss Co., Ltd.) and a MIRA3-LMH scanning electron microscope (Tescan company, Czech Republic).Sphericity: Bettersize BT-1600 image particle analysis system (Dandong Bettersize Instrument Co., Ltd.)binarized the spherical RDX image in the microscope photo of RDX crystal.Its circularity was examined to define the sphericity of spherical RDX crystal(more details are summarized in S1).Particle flowability: RDX crystal flowability was assessed by the angle of repose;moreover, the angle of repose of RDX crystal particles was determined using the fixed funnel method.Bettersize 2000 E laser particle size distribution equipment was used to study the particle size distribution of RDX crystals (Dandong Bettersize Instrument Co., Ltd.).XRD (DX-2700 X-ray, CHINA) was used to describe the crystal form of RDX crystals using 40 kV and 30 mA Cu-Kα radiation.The crystals were examined in the 2θ range from 5°to 55°with a 0.05°step scan.Thermal stability:DTA552-EX(IDEA SCIENCE,USA)was used to examine the thermal stability of raw RDX and spherical RDX at sample masses of 10 mg and heating rates of 5,10,15, and 20°C/min.Mechanical sensitivity: The impact sensitivity of spherical RDX was analyzed using a BAM impact sensitivity tester(OZM RESEARCH, Czech Republic), and the friction sensitivity of spherical RDX was tested using a BAM friction sensitivity tester(OZM RESEARCH, Czech Republic).
3.Results and discussion
3.1.Effect of process conditions on RDX crystal quality
3.1.1.Effect of solvent ratio
The erosion solvent is often a combination of solvent and nonsolvent, used when the solvent etching process is performed to prepare spherical explosives.When the solvent content is too high,the erosion solvent will completely dissolve the RDX crystal, leaving behind just the explosive solution in place of spherical RDX crystal particles.It is challenging to make spherical RDX crystals when there is a high fraction of non-solvent and low solubility of explosive crystals in the erosion solvent, and the erosion effect on the edges and corners of RDX crystals is weak.In order to successfully etch and dissolve the corners and edges of RDX crystals without dissolving all of them, one of the goals of this work is to develop an erosion solvent.
During the initial stages of the study, cyclohexanone, dimethyl sulfoxide,and acetone were used as solvents,while water,absolute ethanol, and cyclohexane were selected as non-solvents for RDX crystal recrystallization.Later it was discovered that among all these reagents, a mix of cyclohexanone and cyclohexane could be used to form an erosion solvent with the proper solubility.The prepared RDX had fewer corners, making it promising to prepare spherical RDX explosives.Therefore,the erosion solvent employed to produce the spherical RDX was a mixture of cyclohexanone and cyclohexane.
The effects of an erosion solvent with various solvent/nonsolvent volume ratios on the yield and crystal morphology of spherical RDX crystals were investigated with the following parameters: experimental temperature (40°C), resonance acoustic mixing acceleration (40 g), and resonance acoustic mixing time(3 h).The results are shown in Fig.1, and more details are summarized in Section S2.
As shown in Fig.1,the crystal formed with a solvent/non-solvent ratio of 1:5 has sharp corners and edges and a circularity of 0.74 with the worst sphericity.With a solvent/non-solvent ratio of 1:1 and 1:2,the crystal shape was relatively regular;the circularity was 0.91 and 0.89, respectively.An increase in the solvent ratio in the erosion solvent aids in dissolving the edges and corners of the RDX crystal and generating a more circular RDX crystal.However,as the solvent ratio increases, the solubility of erosion solvent in RDX increases,but the yield of spherical RDX drops.The yields of spherical RDX were 11.13% and 56.8% when the solvent/non-solution ratio was 1:1 and 1:2,respectively.The yield of a 1:2 solvent/non-solvent ratio is 45.67% higher than that of a 1:1 solvent/non-solvent ratio.When considering circularity and yield,a solvent/non-solvent ratio of 1:2 is preferable for making spherical RDX.
3.1.2.Effect of temperature
Under the parameters of a solvent/non-solvent ratio of 1:2,resonance acoustic mixing acceleration of 40 g, and resonance acoustic mixing time of 3 h, the effects of varied experimental temperatures(20-60°C)on the formation of spherical RDX crystals were investigated.The results are shown in Fig.2.
As shown in Fig.2, the circularity of a spherical RDX crystal at 20°C is only 0.78.The circularity of RDX crystals generated at 30°C was increased to 0.82 when compared to 20°C, yet some RDX crystals have irregular morphology.The RDX crystals have no sharp edges or corners at 40°C,the particle size is homogeneous,and the circularity is 0.88.When the temperature is between 20-60°C,the sphericity of the RDX crystal increases with increasing experimental temperature, while particle size and yield are also affected by the solvent temperature(Fig.3).
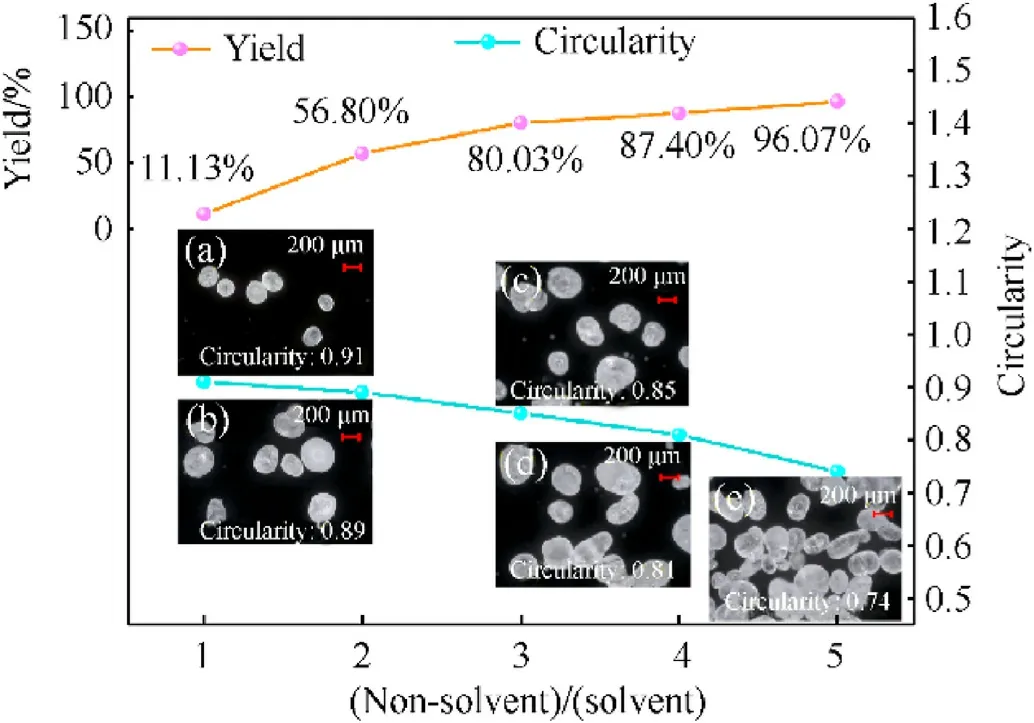
Fig.1.Yield and crystal microscope photos of spherical RDX prepared with various solvent/non-solvent volume ratios: (a) 1:1; (b) 1:2; (c) 1:3; (d) 1:4; (e) 1:5.
According to the experimental data, the crystal size formed at 20°C and 60°C is 220.13 μm and 177.96 μm, respectively.This revealed that the crystal size reduced as temperature increased.Simultaneously, when the temperature was raised, the solute’s solubility also increased,decreasing the spherical RDX yield.When the trial temperature was 40°C, careful consideration was more advantageous for the manufacturing of spherical RDX(more details are summarized in Section S3).
3.1.3.Effect of resonance acoustic mixing acceleration
The resonance acoustic mixing acceleration magnitude has a strictly linear relationship with the energy input to the mixed material by the resonance acoustic mixing equipment [22].It will also directly affect the vibration frequency and amplitude of the container,which significantly impacts the crystallization solution’s flow field.Different resonance acoustic mixing accelerations(20-60 g) were tested during the preparation of spherical RDX crystals under the conditions of a solvent/non-solvent ratio of 1:2,an experimental temperature of 40°C, and a resonance acoustic mixing time of 3 h to investigate the effect of resonance acoustic mixing acceleration on the spheroidization effect of RDX.The experimental results are exhibited in Fig.4.
According to Fig.4, when the resonance acoustic mixing acceleration is 20 g,the formed RDX crystal has sharp edges and corners,and the circularity is 0.68.The edges and corners of the RDX crystal prepared with 30 g acceleration significantly reduced compared to the resonance acoustic mixing acceleration of 20 g.However,some crystals still have apparent edges and corners.The created RDX crystal has no sharp edges or corners when the acceleration is 40 g,and the circularity is 0.86.When the acceleration reaches 50 g,some spherical RDX breaks, reducing the circularity to 0.84.More RDX crystals were broken when the acceleration was raised to 60 g,and many small particles of broken crystals adhered to the spherical crystals.The vibration frequency and amplitude of the container were small when the resonance acoustic mixing acceleration was too small, the relative displacement of RDX crystal particles and solvents was small, and the interface scouring effect between crystal particles and solvents were small.The effect of grinding edges and corners of explosive particles was not apparent.When the acceleration was too high, the container strongly impacted the RDX crystal particles,causing them to collide and rub aggressively against the container wall and neighboring crystals.Due to the violent collision and friction,some crystal particles were fractured, resulting in an inadequate spheroidization effect.When the acceleration was 40 g,prepared RDX had a great sphericization effect.
3.1.4.Effect of resonance acoustic mixing time
One of the most critical factors influencing RDX morphology is resonance acoustic mixing time.The RDX spheroidization effect will be poor if the resonance acoustic mixing time is too short.The manufacturing energy consumption will increase if the resonance acoustic mixing time is too long.The effects of different resonance acoustic mixing durations (2-6 h) on the preparation of spherical RDX crystals were investigated at an experimental temperature of 40°C and a resonance acoustic mixing acceleration of 40 g.The experimental results are shown in Fig.5.
As shown in Fig.5, the circularity of RDX crystals prepared by resonance acoustic mixing for 2 h was only 0.80,and the majority of crystals had edges and rough surfaces.The circularity of RDX crystals created by resonance acoustic mixing for 3 h was 0.88,yet certain crystals had a significant length-diameter ratio.RDX crystals with 4 h of resonance acoustic mixing had no visible edges and corners, consistent crystal particle size, and maximum circularity(0.92).When the resonance acoustic mixing time was 2-4 h, the circularity of RDX crystals increased as the resonance acoustic mixing time increased.The circularity of the crystal was 0.90 after 5 h of resonance acoustic mixing, although a few crystals had cracks.
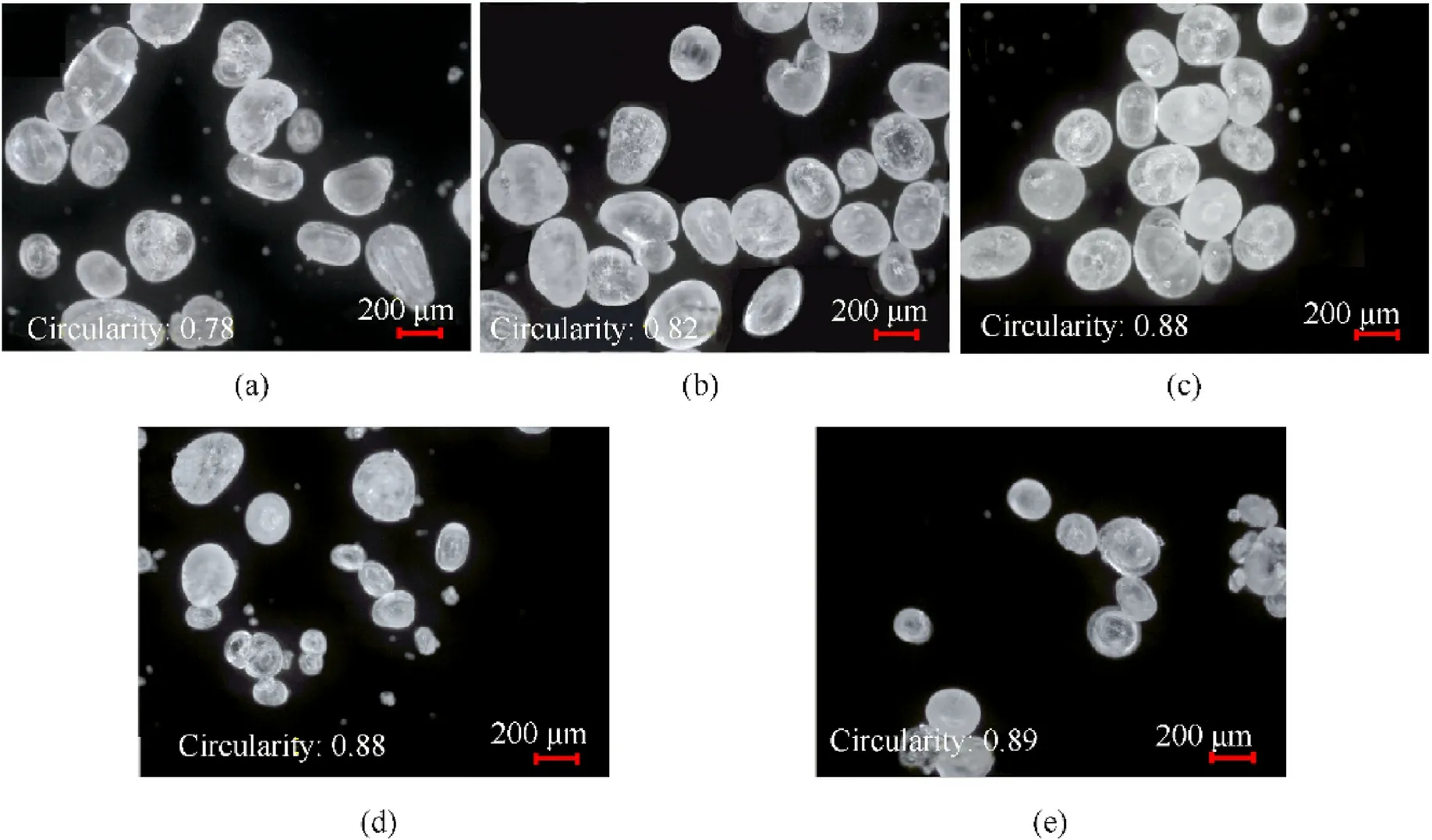
Fig.2.Micrographs of spherical RDX prepared with various temperatures: (a) 20°C; (b) 30°C; (c) 40°C; (d) 50°C; (e) 60°C.
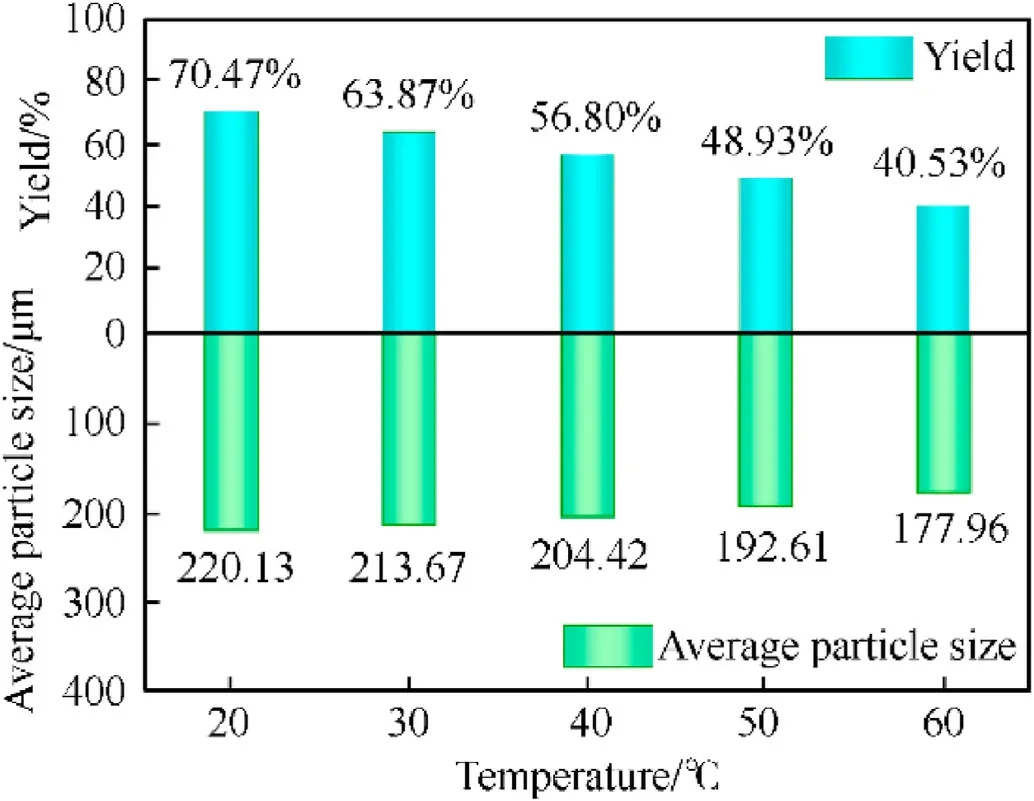
Fig.3.Effect of temperature on the yield and particle size.
Furthermore, after 6 h of resonance acoustic mixing, more crystals broke.The experimental results showed that when the resonance acoustic mixing time is too short, the RDX crystal's spheroidization effect is poor; additionally, when the resonance acoustic mixing time is too long, crystal fracture and energy consumption rise.The results indicate that when the resonance acoustic mixing time is 4 h,RDX crystals with high sphericity can be prepared.
The sphericity, yield, and microscopic morphology of spherical RDX crystals were compared.It was discovered that good sphericity spherical RDX crystals could be prepared under solvent/nonsolvent ratio of 1:2, reaction temperature of 40°C, resonance acoustic mixing acceleration of 40 g,and resonance acoustic mixing time of 4 h.
3.2.Analysis of the spheroidization mechanism of RDX crystals
While using the resonance acoustic mixing assisted solvent etching method to prepare spherical RDX crystal particles, the resonance acoustic mixing platform vibrates up and down according to the acceleration set by the program.It causes the RDX crystal particles/solvent suspension system in the resonance acoustic mixing container to move.At the same time, resonance acoustic mixing causes the solvent to generate a broad circulating flow field and a small circulating flow field in the micro area, in which RDX crystal particles circulate and rotate.As a result, the solvent repeatedly erodes and washes the protruding areas of the crystal,such as edges and corners,leaving the projecting edges and corners passivated and smooth.In addition, the solution has a dynamic crystallization process that involves crystal dissolution and crystallization.Eq.(1) [23] describes the link between the RDX crystal critical nucleus radius Rcand solution supersaturation σ.
In the equation,γ is the surface tension,N∙m-1;V is the volume of 1 mol RDX crystal, m3∙mol-1; R is Avogadro's constant,8.314 J mol-1K-1; T is the thermodynamic temperature, K.
Eq.(1) shows that when the same solute and solvent are used,the edges and corners radii of crystals are shorter,and their surface energy is higher.A higher supersaturation is necessary to maintain a shorter radius.As a result,while the solution’s supersaturation is constant, the edges and corners of crystals with shorter radii, as well as the tiny crystals in raw RDX, dissolve gradually and crystallize on the surface of bigger crystals(Ostwald ripening[24]).As a result, the dissolution of microscopic crystals, including crystal edges and angles,and the formation of bigger crystals occur in the solution simultaneously, resulting in crystal spheroidization and the formation of bigger crystals.Fig.6 depicts a schematic diagram of the process of manufacturing spherical RDX using resonance acoustic mixing assisted solvent etching technology.

Fig.4.Effect of resonance acoustic mixing acceleration on spheroidization of RDX: (a) 20 g; (b) 30 g; (c) 40 g; (d) 50 g; (e) 60 g.
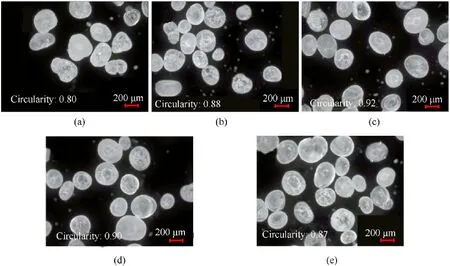
Fig.5.Photographs of crystal microscope obtained at different durations of resonance acoustic mixing: (a) 2 h; (b) 3 h; (c) 4 h; (d) 5 h; (e) 6 h.
3.3.Flowability of RDX crystals
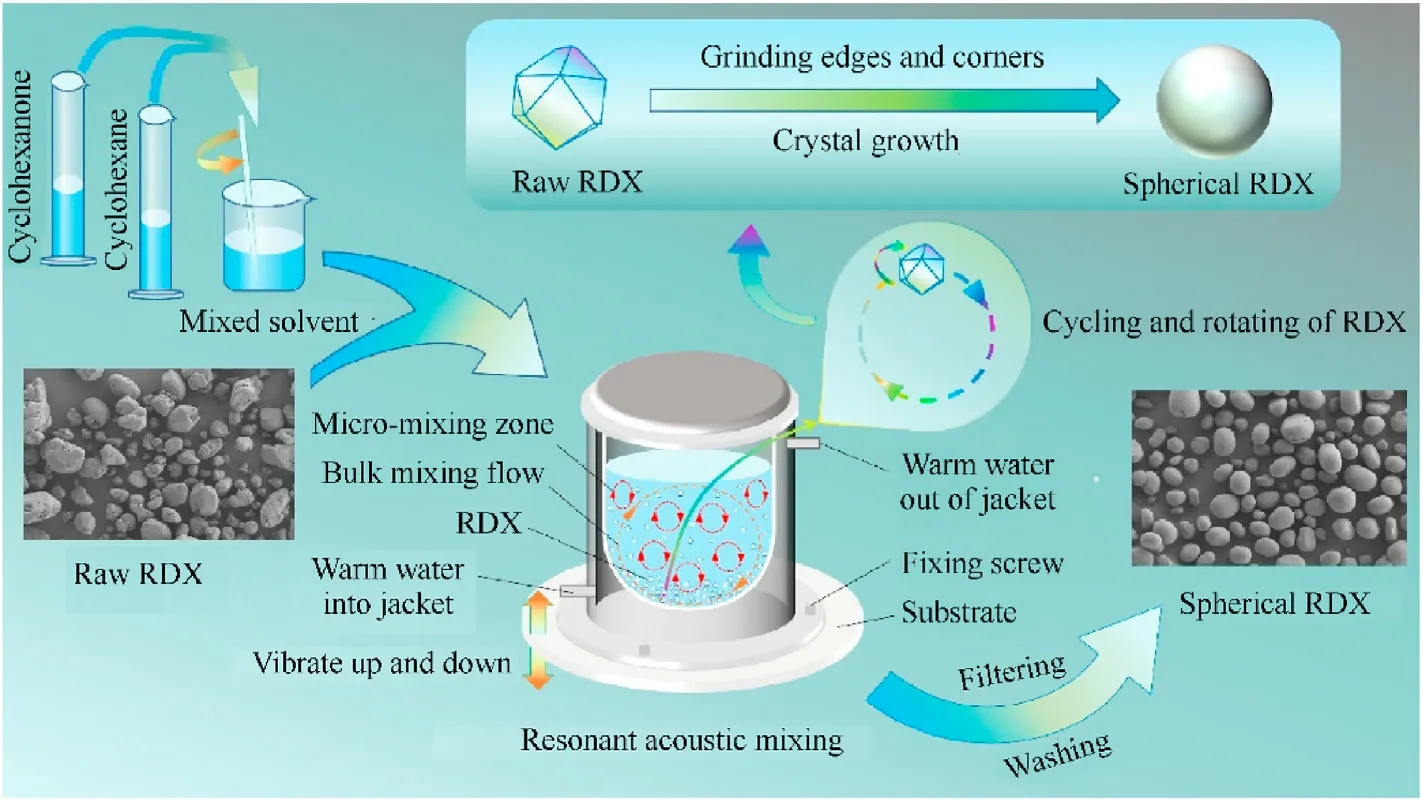
Fig.6.Schematic diagram of the preparation of spherical RDX by resonance acoustic mixing assisted solvent etching technology.
The flowability and the charge performance of the RDX crystal are affected by its morphology and sphericity.Two types of spherical RDX crystals were created using the traditional solvent etching method(RDX1)and the resonance acoustic mixing assisted solvent etching method (RDX2) to investigate the effect of resonance acoustic mixing on the shape and flowability of RDX crystals.Furthermore,the fixed funnel approach was used to obtain a crystal accumulations of spherical and raw RDX crystals.The crystal morphology and photographs of crystal accumulations of spherical RDX crystals and raw RDX were compared (more details are summarized in Section S4).The experimental results are presented in Fig.7.
According to Fig.7, raw RDX crystal exhibits sharp edges and corners, variable particle size, a circularity of 0.45, and crystal aggregation in the shape of a sharp cone.The RDX crystals (RDX1)formed by the solvent etching method had no sharp edges and corners.Furthermore, the crystal particle size was more uniform,the circularity was 0.83, the top morphology of the crystal stack was smoother, and the stacking height was lower than raw RDX.The resonance acoustic mixing assisted solvent etching method produces RDX crystals(RDX2)with uniform particle size,excellent crystal sphericity, the best crystal shape, and the lowest crystal stack height.In addition, the yield difference between RDX1 and RDX2 is minor.The experimental results showed that resonance acoustic mixing assisted solvent etching technology has more potential in manufacturing spherical explosives than classic stirring solvent etching technology.

Fig.8.The angle of repose and apparent densities of raw RDX and spherical RDX: (A)Raw RDX; (B) RDX1; (C) RDX2.
The crystal shape of explosives has a significant impact on the loading rate.The angle of repose and apparent density of three types of RDX crystals were studied further to investigate the influence of crystal shape on loading rate.The experimental results are illustrated in Fig.8.
According to Fig.8,the raw RDX has a repose angle of 42°,while the crystal repose angles of RDX1 and RDX2 are 34°and 28°,respectively.The flowability of crystal particles improves with decreasing the angle of repose[25].As a result,RDX2 crystal has the best flowability,while raw RDX has the worst.The apparent density of explosives is closely related to flowability.In Fig.8,the apparent densities of raw RDX and two spherical RDX crystals are 1.02±0.03 g/cm3,1.13±0.02 g/cm3,1.22±0.02 g/cm3,respectively(more details are summarized in Section S5).The link between the angle of repose and the apparent density of the three RDX crystals demonstrates that the higher the apparent density, the lower the angle of repose of the explosive crystal.It also demonstrates that the greater the sphericity of the explosive crystal, the better the flowability and loading rate.
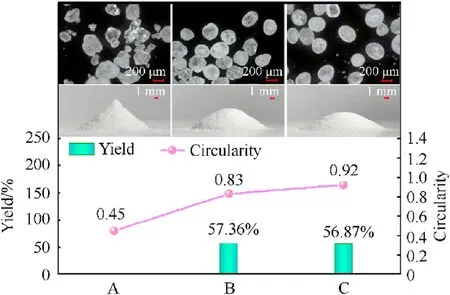
Fig.7.Micrographs and photographs of crystal accumulations:(A)Raw RDX;(B)RDX1;(C) RDX2.

Fig.9.SEM images of (a)-(c) raw RDX, (d)-(f) RDX1, and (g)-(i) RDX2.
3.4.Morphology analysis of RDX crystals
The morphologies of raw RDX crystals and spherical RDX crystals were observed by scanning electron microscopy(SEM),and the SEM images are shown in Fig.9.
As shown in Fig.9,the surface of raw RDX crystal is rough,with evident edges and corners,and there are many fault cracks on the surface of raw RDX crystal; also, the particle size distribution is uneven, with a particle size distribution of about 50-240 μm.The crystal morphology of RDX1 is superior to that of raw RDX but inferior to that of RDX2.RDX2 is spherical, having no discernible edges or corners.RDX2 has a greater average particle size than raw RDX and a more uniform particle size distribution than raw RDX.The particle size distribution of RDX2 is around 100-260 μm.This is because the edges and corners of raw RDX are polished and dissolved by the combined action of resonance acoustic mixing and the solvent, thus improving sphericity.Simultaneously, SEM test findings demonstrate that the average particle size of RDX after spheroidization is bigger, confirming the presence of dynamic crystal development of RDX crystals throughout the spheroidization process.Finally,the RDX2 sphericity was computed up to 0.92 using the Bettersize BT-1600 image particle analysis system.
3.5.Particle size analysis of RDX crystals
Bettersize laser particle size analyzer was used to perform particle size analysis on spherical RDX crystals and raw RDX crystals,and the particle size distribution diagram of spherical RDX crystals and raw RDX crystals is shown in Fig.10.Table 1 shows the particle size and particle size span results for spherical RDX crystals and raw RDX crystals based on the cumulative distribution particle size number,where the particle size span Spis defined as Eq.(2)[26,27].
In Eq.(2),Spis the particle size span,dimensionless;D10,D50and D90are the particle sizes with volume fraction accumulated to 10%,50% and 90% respectively,μm.
According to the test results, the particle size spans of RDX1,RDX2,and raw RDX are 1.51,1.34,and 1.99.The particle size span of RDX1 and RDX2 is less than that of raw RDX, indicating that the particle size distribution of spherical RDX crystals is narrower than that of raw RDX crystals.RDX1 and RDX2 have median particle sizes(D50) of 202.10 μm and 215.80 μm, respectively, which, at 160.40 μm, is substantially bigger than that of raw RDX crystals.This is because there is a huge disparity in particle size between large particle crystals and microscopic crystals in raw RDX crystals.Furthermore, the surface energy of microscopic crystals exceeds that of big particles.According to Eq.(1), if a solution has just reached saturation for large particle crystals in the same solute and solvent, it is unsaturated for microscopic crystals.As a result,microscopic crystals dissolve in the solvent and crystallize on the surface of large particle crystals, resulting in a greater average particle size of spherical explosives.The particle size test results agree with the microscope and scan electron microscopy results.
3.6.Analysis of crystal form of RDX crystals
An X-ray diffractometer (XRD)was used to examine the crystal forms of raw RDX and spherical RDX(RDX2).The test results were compared to the RDX PDF#44-1618 card.As shown in Fig.11, the main diffraction characteristic peaks of spherical RDX and raw RDX explosives appeared at 13.10°,15.46°,16.54°,17.87°,20.41°, 29.36°,and so on, all of which correspond to the main diffraction peak positions of the PDF#44-1618 card,demonstrating that the crystal forms of spherical RDX and raw RDX explosives are all α-type.The intensity of the principal diffraction characteristic peaks of spherical RDX crystals varied due to the influence of RDX crystal plane growth throughout the dynamic crystallization process of spheroidization and crystal morphology modification.
3.7.Thermal stability analysis of RDX crystals
The thermal stability of spherical RDX(RDX2)and raw RDX were tested and analyzed by a differential thermal analyzer (DTA) at heating rates of 5,10,15, and 20°C/min, respectively.The experimental results are illustrated in Fig.12.
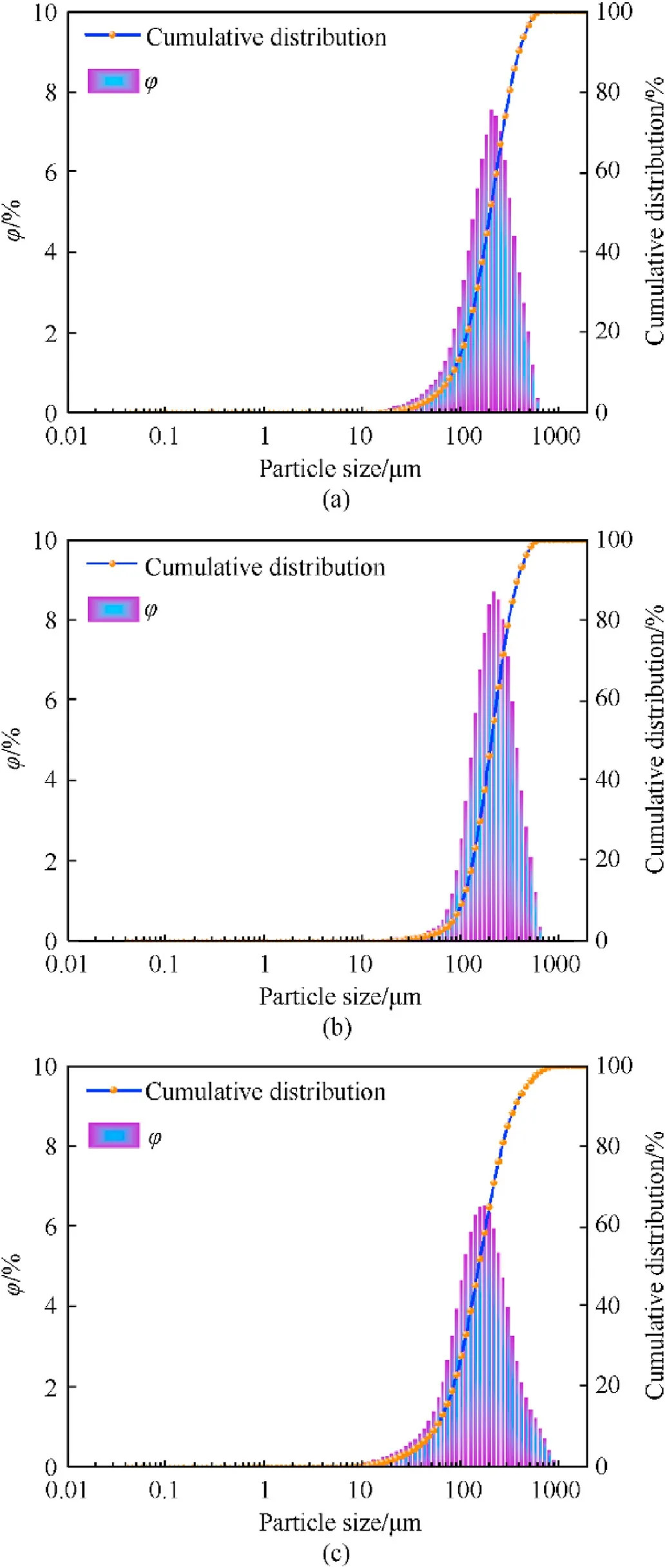
Fig.10.Particle size test results of spherical RDX and raw RDX: (a) RDX1 particle size test chart; (b) RDX2 particle size test chart; (c) Raw RDX particle size test chart.
The DTA curves of spherical RDX (RDX2) and raw RDX crystals showed two peaks at different heating rates(Fig.12).The first is theRDX endothermic peak, and the second is the RDX decomposition exothermic peak,which suggests that an endothermic process will occur prior to the severe decomposition exothermic of RDX explosives.The peak top temperature corresponding to the exothermic decomposition peak gradually increased as the heating rate increased, and the peak shape of the exothermic decomposition peak became sharper, indicating that the faster the heating rate, the faster the exothermic decomposition rate after RDX endothermic.Decomposition exothermic peaks of spherical RDX(RDX2) appeared at 227.22, 230.44, 232.72 and 233.38°C at different heating rates at 5-20°C/min,respectively.Decomposition exothermic peak temperatures of the raw RDX at different heating rates were 224.54,227.69,229.72,and 231.78°C,respectively.While comparing Figs.12(a) and 12(b), it is evident that the decomposition peak temperature of raw RDX and spherical RDX at the heating rate of 5-20°C/min had increased by 2.68, 2.75, 3.00, and 1.60°C respectively,indicating that the temperature resistance of spherical RDX under thermal action had slightly improved.
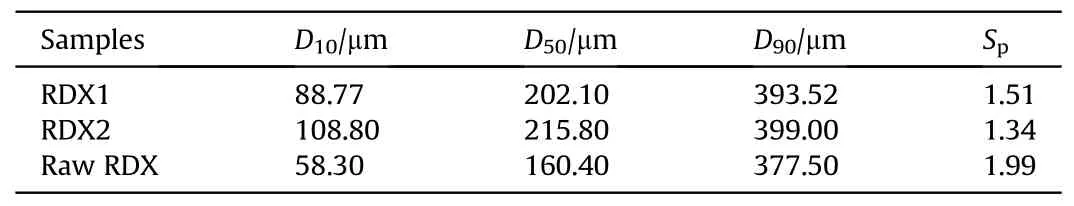
Table 1Particle size and particle size span corresponding to the cumulative distribution particle size number of spherical RDX crystals and raw RDX crystal.
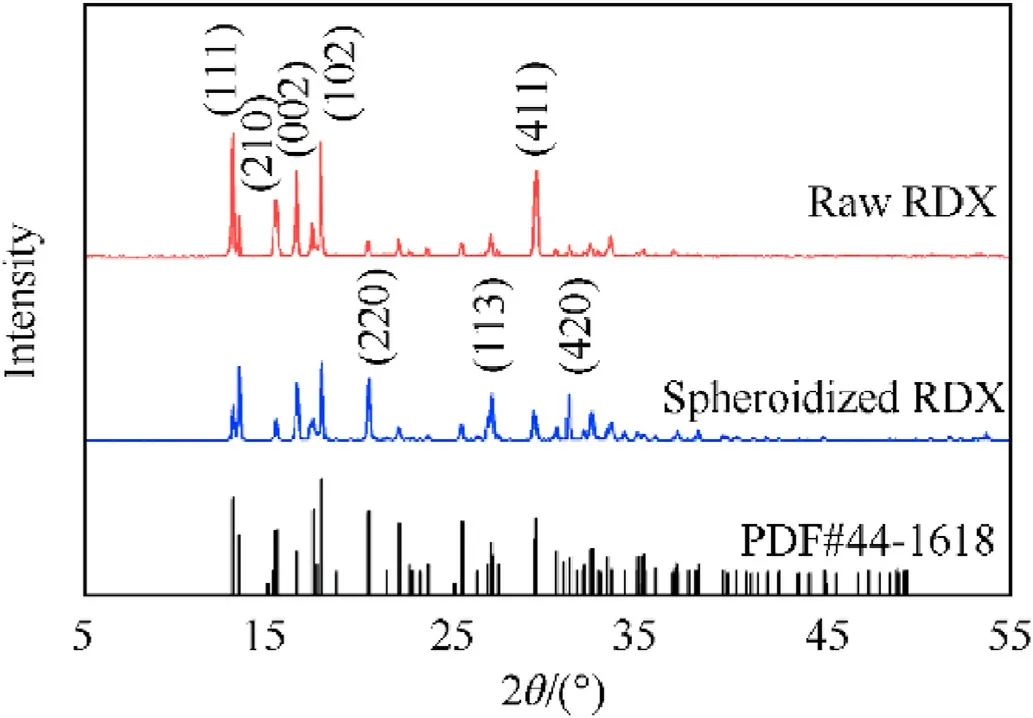
Fig.11.XRD patterns of spherical RDX (RDX2) and raw RDX.
The kinetic parameters of the thermal decomposition of test samples are calculated by the Kissinger equation [28], and the calculation formula is shown in Eq.(3).
In Eq.(3),β is the heating rate,°C/min;Tpis the decomposition peak temperature, K; A is the pre-exponential factor; R is the gas constant, 8.314 J mol-1K-1; Eais the apparent activation energy,J∙mol-1.
The Kissinger equation was used to compute the relative apparent activation energy of spherical RDX (RDX2) and raw RDX based on the decomposition peak temperature of the DTA test results and the linear regression fitting of “ln(β/Tp2)” in Eq.(1) to “1/Tp”was done.The slope and intercept of the equation were used to compute the associated thermal decomposition kinetic parameters.Fig.13 depicts the fitting calculation results,and R2represents the goodness of fit.

Fig.12.DTA test diagram of spherical RDX (RDX2) and raw RDX: (a) Spherical RDX(RDX2); (b) Raw RDX.

Fig.13.Fitting curve of thermal decomposition kinetic parameters of RDX crystals.
The goodness of fit of the“ln(β/Tp2)”to“1/Tp”linear regression of raw RDX and spherical RDX(RDX2)was above 0.989.The value was close to 1, indicating that the thermal decomposition of the two conforms to the Kissinger model.The apparent activation energies of the two are 396.92 kJ/mol and 444.68 kJ/mol,respectively,with an increase of 47.76 kJ/mol.It demonstrates that the thermal stability of spherical RDX has improved over raw RDX.This is because raw RDX has edges,cracks,and many broken tiny particle crystals.The crystal with the smallest particle size has a larger specific surface area and more active molecules on its surface, making it simpler to react under thermal action.The spherical explosive has superior crystal quality, fewer crystal defects, and fewer broken small particles.It indicates that the spherical explosives have a higher thermal decomposition activation energy,a slower response to external thermal stimulation, and a delayed thermal decomposition peak temperature.
3.8.Mechanical sensitivity analysis of RDX crystals
The impact sensitivity and friction sensitivity of spherical RDX(RDX2) and raw RDX were examined by drop weight tester and BAM friction sensitivity tester, respectively, based on GB/T21567-2008 [29] and GB/T21566-2008 [30].Table 2 displays the test results.The test results showed that the minimum impact energy of raw RDX and spherical RDX explosions was 5.5 J and 6.5 J, respectively.The lowest impact energy of a spherical RDX explosion was raised by 18.18%compared to raw RDX.It reveals that RDX's impact sensitivity was effectively reduced by spheroidization.The minimal friction loads for raw RDX and spherical RDX explosion were 108 N and 144 N,respectively.The lowest explosion load of spherical RDX is 33.33%larger than that of raw RDX,showing that spheroidization lessens RDX's friction sensitivity and enhances its safety.
Spheroidization can reduce RDX's impact and friction sensitivity because it reduces the sharp edges and corners of the RDX explosive, reducing the probability of hot spots.Therefore, the impact and friction sensitivity can be effectively reduced, and the safety performance can be improved.
4.Conclusions
(1) In the present study, spherical RDX was prepared using cyclohexanone and cyclohexanes-mixed solvent by a resonance acoustic mixing assisted solvent etching process.The prepared spherical RDX did not have sharp corners,and the sphericity could reach 0.92.The median particle size of D50was 215.8 μm.The average particle size distribution of spherical RDX was smaller than raw RDX.The crystal form of spherical RDX is α type, and the spheroidization process did not change it.(2) The thermal decomposition activation energy of spherical RDX was Ea= 444.68 kJ/mol,which is 47.76 kJ/mol greater than that of raw RDX.In contrast, the minimum impact energy was 6.5 J.The minimum frictional load was 144 N.Spheroidization enhanced the thermal stability of RDX and decreased impact and friction sensitivity, implying that spheroidization improved the safety performance of RDX explosives.(3)The resonance acoustic mixing aided solvent etching method was used to effectively prepare spherical RDX.The output of spherical RDX in this study is of gram grade,demonstrating the feasibility of this process in the preparation ofRDX spheroidization.The resonance acoustic mixing aided solvent etching technique has a strong application potential in the spherical crystallization of energetic materials as a bigger reactor can increase output.
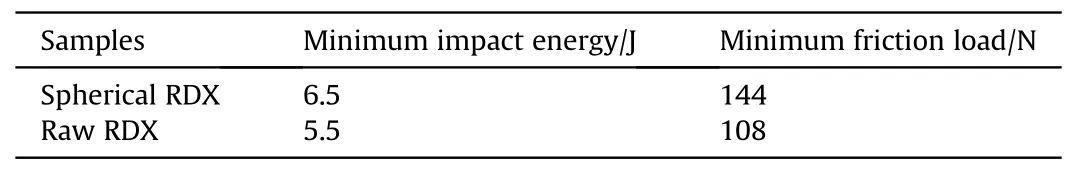
Table 2Mechanical sensitivity test results of spherical RDX (RDX2) and raw RDX.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.01.010.
杂志排行
Defence Technology的其它文章
- Eigen value analysis of composite hollow shafts using modified EMBT formulation considering the shear deformation along the thickness direction
- Synthesis of energetic coordination polymers based on 4-nitropyrazole by solid-melt crystallization in non-ionization condition
- Study of residual stresses and distortions from the Ti6Al4V based thinwalled geometries built using LPBF process
- Modeling and simulation of solvent behavior and temperature distribution within long stick propellants with large web thickness undergoing drying
- Assessment of the ballistic response of honeycomb sandwich structures subjected to offset and normal impact
- Few-shot object detection based on positive-sample improvement
