Spatio-temporal dynamics of cyanobacterial abundance and toxicity in a Mediterranean hypereutrophic lake*
2023-11-10SalahARIFNawelDJEBBARISaberBELHAOUESHassenTOUATIMouradBENSOUILAH
Salah ARIF , Nawel DJEBBARI, Saber BELHAOUES, Hassen TOUATI,Mourad BENSOUILAH
Eco-biology Laboratory for Marine Environments and Coastal Areas, Faculty of Sciences, Marine Sciences Department,University of Badji Mokhtar, Annaba 23000, Algeria
Abstract The Mediterranean basin is considered one of the most vulnerable areas in the world under the impacts of global warming and changes in precipitation patterns.Oubeira, a shallow and polymictic freshwater lake located in northeastern Algeria (36°50′N, 08°23′E), has recently experienced a significant proliferation of harmful cyanobacteria resulting in the generation of toxins.We carried out this study in order to understand the succession patterns of dominant cyanobacteria and cyanotoxin production and the factors driving this in Oubeira Lake.A total of 26 cyanobacterial genera were identified, and among them Microcystis and Planktothrix accounted for more than 60% of the overall cell abundance.The summer/fall period was dominated by Microcystis, Planktothrix, and in lesser extend by Cylindrospermum,Cylindrospermopsis.During the fall/winter transition, Dolichospermum, Pseudanabaena, and Aphanizomenon were the dominant genera.Statistically, the bloom-forming cyanobacteria showed significant differences between months but not between stations.Intracellular microcystins (MC-LR) was detected in all collected samples (0.62- and 19.14-μg MC-LR equivalent/L), but appeared in high concentrations throughout the period of dominance of Microcystis and Planktothrix.Microcystis was more sensitive to nutrients than to temperature.Planktothrix was more dependent on temperature than on nutrients, which explains their coexistence during summer-fall period.However, both genera are positively correlated with MC-LR and would probably be the main producers of microcystins.Pseudanabaena, Dolichospermum,and Aphanizomenon co-occurred at the end of Planktothrix dominance period (December).Aphanizomenon and Pseudanabaena were correlated negatively with temperature and positively with water conductivity.Dolichospermum showed a strong positive correlation with MC-LR.Oubeira Lake, could serve as a model of how cyanobacteria blooms may develop in lakes within Mediterranean climates.
Keyword: Oubeira Lake; cyanobacteria; microcystin; spatiotemporal variations; shallow hypereutrophic lake
1 INTRODUCTION
Lakes play a crucial role in the biogeochemical cycles of the continental watersheds.In lakes, the water residence time is long and the current velocities are generally slow; therefore, lakes provide us with a time-integrated response to the external forcing.That is the case in Oubeira Lake which was considered the main source of drinking water for rural communities in El Tarf Estate until the 1990s, when the lake experienced rapid eutrophication associated with increased cyanobacterial blooms, leading to water shortages for the surrounding local populations (Nasri et al., 2004).
According to Hamouda and Tahar (2012), the watershed of Oubeira Lake has undergone a 12%reduction in its forest estate against a 29%expansion of cultivated land.Moreover, Oubeira Lake receives wastewater discharges from rapidly expanding urban areas (El Kala City) and is subject to dry season withdrawals for the irrigation of peanut crops.
Cyanobacteria development is favoured by nutrient availability, high temperature, solar radiation, a stable water column, and high pH (Paerl and Huisman,2008; Carey et al., 2012; Paerl and Otten, 2013).Other advantages are the ability to regulate their position within the water column through gas vacuoles, to overcome adverse periods by forming akinetes (dormant cells), to fix nitrogen gas through heterocysts (specialized cells) (Komárek and Anagnostidis, 1999, 2005).Moreover, Cyanobacteria can produce numerous secondary metabolites,including cyanotoxins, which are harmful to human and animal health (Codd et al., 2017).Cyanotoxins are found in all continents.In freshwater, microcystins(MCs) (contain cyclic heptapeptides) are among the most common cyanotoxins (Humbert and Fastner,2017; Du et al., 2019), and the most diverse with over 240 different structural variants (congeners)identified to date (Spoof and Catherine, 2017).Numerous cyanobacterial genera are capable of producing MCs; these includeMicrocystis,Dolichospermum,Anabaenopsis,Aphanizomenon,Aphanocapsa,Hapalosiphon,Nostoc,Planktothrix,Pseudanabaena,Gloeotrichia,Synechococcus,andSynechocystis(Huang and Zimba, 2019).These genera have a cosmopolitan distribution, thus making their hepatotoxic metabolites a global concern.The presence of many of these genera has been reported in the waters of Oubeira Lake (Nasri et al., 2004; Zidane et al., 2013; Boussadia et al.,2015).Based on acute toxicity, microcystin-LR(MC-LR) is considered the most potent hepatotoxin(Funari and Testai, 2008).In Oubeira Lake, previous studies on toxins revealed that MC-LR was the most prevalent cyanotoxin, reaching 29.16 μg/L in August 2001 (Nasri et al., 2004).
Future climatic change scenarios predict rising temperatures, enhanced vertical stratification of aquatic ecosystems, and alterations in seasonal and interannual weather patterns (including droughts,storms, and floods); these changes all favour the increase of harmful cyanobacterial blooms in eutrophic waters (Paerl and Huisman, 2008).Some authors consider the Mediterranean basin to be one of the most vulnerable areas in the world to the impacts of global warming and changes in precipitation patterns (Erol and Randhir, 2012).Although Oubeira Lake is the largest freshwater lake in Algeria; there are very few studies on its cyanobacterial community (Souissi et al., 2004;Zidane et al., 2013; Boussadia et al., 2015) and the levels of cyanotoxins (Nasri et al., 2004; Amrani et al., 2014) in its waters.Furthermore, the cyanobacteria genera/species responsible for the production of these cyanotoxins and the environmental factors involved in their biosynthesis remain poorly studied in this shallow Mediterranean lake, which is used for irrigation and fishing.
Thus, the aim of the research presented in this paper was to analyse the temporal and spatial variations of intracellular microcystin content and the structure of the cyanobacteria community in relation to environmental factors.Extra attention was given to the bloom-forming genera (Microcystis,Planktothrix,Pseudanabaena,Aphanizomenon,Dolichospermum, andChroococcus).Such information could be useful for the development of restoration strategies in the region.
2 MATERIAL AND METHOD
2.1 Study site description
Oubeira is a shallow, hypertrophic, and endorheic freshwater lake located in the wetland area of El-Kala National Park, in the far eastern part of northern Algeria (36°50′N, 08°23′E).This site is classified as a natural reserve under the Ramsar Convention (Ramsar Convention Official Website,www.ramsar.org).Oubeira Lake region’s climate is characterised by a typical coastal Mediterranean climate, where the average minimum and maximum temperatures stand at 7 and 31 °C, respectively.Rainy season usually occurs from October to May, with precipitation ranging between 700 and 800 mm/a.Due to its shallow depth, Oubeira Lake has a hydrological regime that is closely linked to the climatic conditions.Four tributaries feed it: Dey Legraa River in the East, Boumerchen River in the northeast, Demet Rihana River in the north, and Messida River or El-Kebir River in the south; these two last ones are the main tributaries (Fig.1).
The watershed of Oubeira Lake occupies an area of 9 728 hm2; According to Hamouda and Tahar(2012), the watershed has undergone a 12% reduction in its forest base versus a 29% expansion of cropland.The intensification of forest rangelands has favoured erosion phenomena that have in turn, amplified the risks of eutrophication (Arfa et al., 2019).
Oubeira Lake receives wastewater discharges from rapidly expanding urban areas and is used in the dry season to irrigate peanut crops.The surface area of Oubeira Lake is approximately 21.73 km2.The depth recorded in the centre of the lake, is about 2.40 m during the wet period and 1.50 m during the dry period.The bottom of the lake is covered with a thick layer of mud, which reaches its maximum(2 m) at the centre.
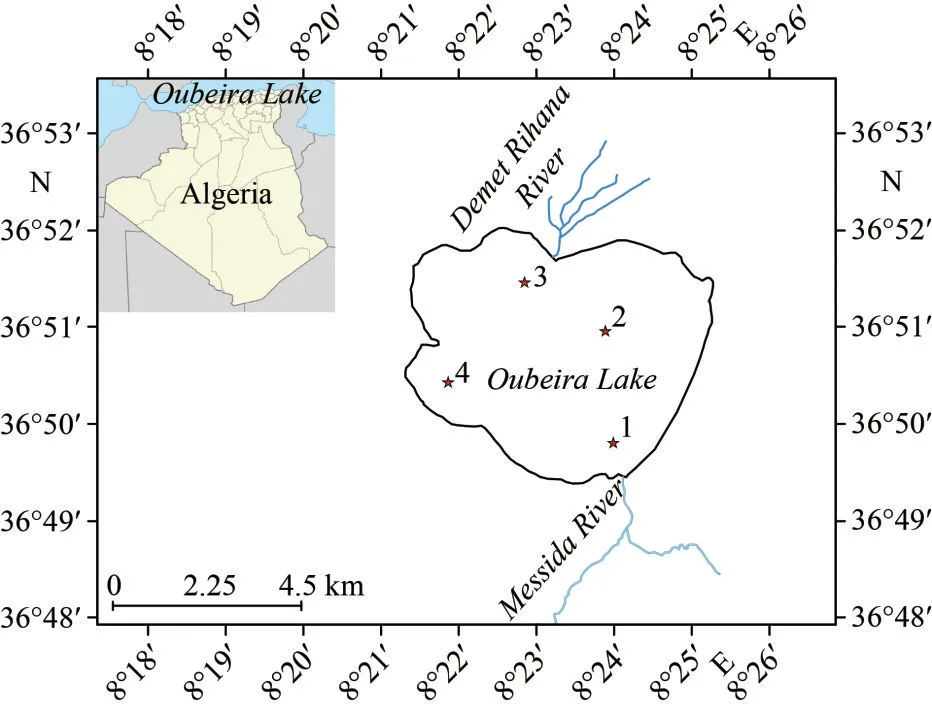
Fig.1 Locations of sampling stations in Oubeira Lake,Algeria
Moreover, an important biodiversity is present in this lake.There are animal species (migratory and sedentary birds, fish, bivalves, and turtles) and plant species (Myriophyllum spicatum,Trapa natans, andNymphaea alba).Although the government has stopped using the lake as a source of drinking water,it is still exploited for aquaculture and agricultural activities.
2.2 Water sampling
The sampling was undertaken once a month from April 2015 to March 2016.A small boat was used to navigate on the lake.To quantify the cyanobacteria,raw water (1 L) was collected from the surface to 1-m depth using a 1-m plastic tube and then concentrated to approximately 100 mL using a phytoplankton net with a 20-μm mesh size.The water samples for physical and chemical analysis and chlorophyllaand microcystindetermination were taken in 1.5-L plastic bottles and transported to the laboratory in a cooler, at 4 °C in the dark to ensure a proper conservation.To determine spatial variation in the abundance of cyanobacteria and intracellular microcystin four stations were sampled in the lake.Stations 1 and 3 are close to the mouths of the two major rivers that feed the lake, namely Messida River and Demet Rihana River (Fig.1).Station 2 is located in the central area of the lake where the maximum depths were recorded.Station 4 is located in the most sheltered area, from the prevailing winds, in the lake’s western part.The set of stations sampled and the months in which the sampling was carried out are a random factor because no prior information is known about the level of expression of the phenomenon studied.
2.2.1 Measurements of abiotic factors
Eleven abiotic factors were analysed during the study period.Water temperature (WT), pH, dissolved oxygen (DO), and water conductivity (Cond) were measured in situ at each sampling station using a multi-parameter probe (WTW, Model 340i).The transparency (Trans) of water was measured using a Secchi disc (SD).
Suspended solids (SS) concentrations were determined according to the European standard (NF EN 872 June 2005/T90-105), by filtering 200–400 mL of water sample through combusted, prerinsed, and pre-weighed glass microfiber filter of 47-mm diameter (Whatman GF/CTM, GE Healthcare Ltd.).The SS concentration was determined from the difference between the weight of the filter before and after filtration (Afnor, 2005).
The concentrations of nitrate nitrogen (NO3-N),nitrite nitrogen (NO2-N), ammonium nitrogen (NH4-N), orthophosphate phosphorus (PO4-P), and chlorophylla(chla) were determined in the laboratory by spectrophotometry following the procedures described in American Public Health Association et al.(1998).
2.2.2 Cyanobacteria microscopic determination
To concentrate cyanobacteria, the raw water was filtered in situ through the plankton net with a mesh size of 20 μm, and the filtrate was recovered and immediately fixed with a 5% formaldehyde solution.Once in the laboratory the samples were filtered through washable polycarbonate filters of 5-μm pore size (CYCLPR PC 47 mm, Germany) to ensure further concentration of cyanobacteria.The polycarbonate washable filters were rinsed with 1 mL of the initial filtrate extracted in the phytoplankton net collector.The composition of cyanobacterial community was determined based on morphoanatomical criteria under an optical microscope according to the following taxonomic literature(Komárek and Anagnostidis, 1999, 2005; Komárek et al., 2014).The cyanobacteria cells were enumerated using an optical microscope (Carl Zeiss, Germany)equipped with a UI-1240 SE camera (IDS, Germany),and a Nageotte counting cell (ISOLAB, 0.5-mm depth) as described in Brient et al.(2008).
Briefly, this quantification was performed on 50 μL of each concentrated sample.For colonial species, the cellular abundances (Ncell=the number of cells) were calculated based on the colonies (S1) and of single cells (S2), using the following equation:Ncell=(S1/S2)–A, whereS1is the colony surface andS2is the cell surface (S2=πr2) andris the radius of the spherical cell (Sun and Liu, 2003).Arepresents the empty part of the colony.It is calculated asA=(S1/S2)×(x/100)wherexis the percentage of the empty parts of the colony estimated visually (e.g., 20% of empty part).Cellular abundance of filamentous cyanobacteria was estimated by dividing the length of the filaments by the mean lengths of the cells (mean cell length was estimated by measuring the length of 50 cells and calculating their mean).
2.3 Analysis of microcystin content by ELISA assay
For the MC analysis, the water samples (500 mL)were filtered through a microfiber glass filter (GF/F).The filters were frozen (-20 °C) until the subsequent toxin analysis.The MC retained by the filters were extracted by placing them in a methanol/water (10 mL) solution (80v/20v), followed by ultrasonic treatment during 5 min.The aqueous fraction remaining after removal of the organic solvent by a rotary vacuum evaporator (40 °C) was subjected to an enzyme-linked immunosorbent assay (ELISA).The intracellular MC concentrations were analysed using ELISA kit (ABRAXIS, USA)according to the manufacturer’s recommendations.All measurements were carried out in triplicate using a microplate ELISA photometer (MindrayMR-96A).MC-LR was used as a standard and therefore results were expressed as micrograms equivalent of MC-LR per litter (μg eq MC-LR/L).
2.4 Statistical analysis
The statistical analyses were performed with R software (3.1.2).First, the normality condition of the sample distributions was checked before by applying the Shapiro-Wilk test.Then, the inter-months and inter-stations comparisons were performed using the non-parametric Kruskal Wallis test.In addition,the principal component analysis (PCA) was carried out using the R package FactoMineR.The aim of this exploratory analysis is to define the typology of the time cycle on the basis of biological (illustrative)and environmental (active) variables.The explanatory variables retained for this analysis were water temperature (T, C°), pH, DO, Cond, Trans, NO3-N,NO2-N, NH4-N, and PO4-P, chla, MC-LR.Microcystis,Planktothrix,Pseudanabaena,Aphanizomenon,Dolichospermum, andChroococcusabundances were included in the analysis as supplemental data.Finally, the correlations in our set of data were evaluated by the non-parametric Spearman correlation coefficient to analyse the intensity of relations between variables.
3 RESULT
3.1 Limnological characteristics and cyanobacteria diversity in Oubeira Lake
To determine the physicochemical characteristics of the water body, we calculated, for each physicochemical variable, the following statistical parameters: the arithmetic mean, median, range,standard deviation (SD), and the coefficient of variation (CV) (Table 1).
Monthly variation in water temperature was observed during the study, with the highest temperature recorded in August (31.21 °C) and the lowest in March (11.6 °C).The lake waters were well oxygenated, recording a peak at the end of the cold period (11.83 mg/L, in March).The pH values varied between 7.99 and 9.82 reflecting an alkaline condition.Conductivity showed a general trend towards higher values during the rainy season than during the dry season, recording more than 500 μS/cm in the September-March period; transparency and suspended solids were highly variable.High availability of NH4-N and PO4-P were recorded during the summer season, reaching, respectively 25.84 μmol/L and 32.98 μmol/L, in August at Station 3 (Fig.2).NO2-N and NO3-N were less plentiful, with maximum levels recorded in December (2.37 μmol/L, Station 2) and August(11.03 μmol/L, Station 3).Finally, chl-avalues varied between 4.74 and 158.11 μg/L with two peaks in September (142.3 μg/L) and November (158.11 μg/L)that coincided with the cyanobacteria bloom period and maximum microcystin concentration, respectively.MC-LR values varied between 0.62 and 19.14 μg/L with two peaks, recorded in Station 3, in September(18.09 μg/L) and November (19.14 μg/L).
Using a Kruskal-Wallis test (Table 2), significant differences were found in NH4-N, PO4-P, and NO3-N concentrations between the four sampling stations,while the other parameters remained relatively synchronous (P>0.05).Figure 2 clearly shows that the lowest NO3-N and PO4-P levels are recorded at Station 4.
Twenty-six genera of cyanobacteria composed mainly of potentially toxic taxa were identified in Oubeira Lake during the period extending fromApril 2015 to March 2016 (Table 2).Based on relative abundance,Microcystis(44.51%),Planktothrix(16.27%),Pseudanabaena(8.38%),Aphanocapsa(6.06%),Cylindrospermum(3.98%),Cylindrospermopsis(3.93%),Synechococcus(3.73%),andAphanizomenon(3.65%)were the most abundant cyanobacterial genera, accounting for more than 90% of the total cyanobacterial abundance observedthroughout the year (Table 3).Regarding the results at the species level, it was established thatMicrocystis aeruginosa,Planktothrix agardhii,andPseudanabaena limneticadominated the cyanobacterial assemblages.
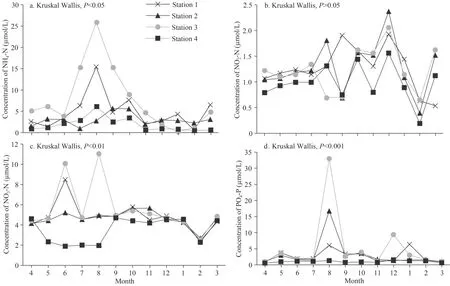
Fig.2 Monthly fluctuations of nutrient concentrations recorded in the 4 stations sampled in Oubeira Lake from April 2015 to March 2016
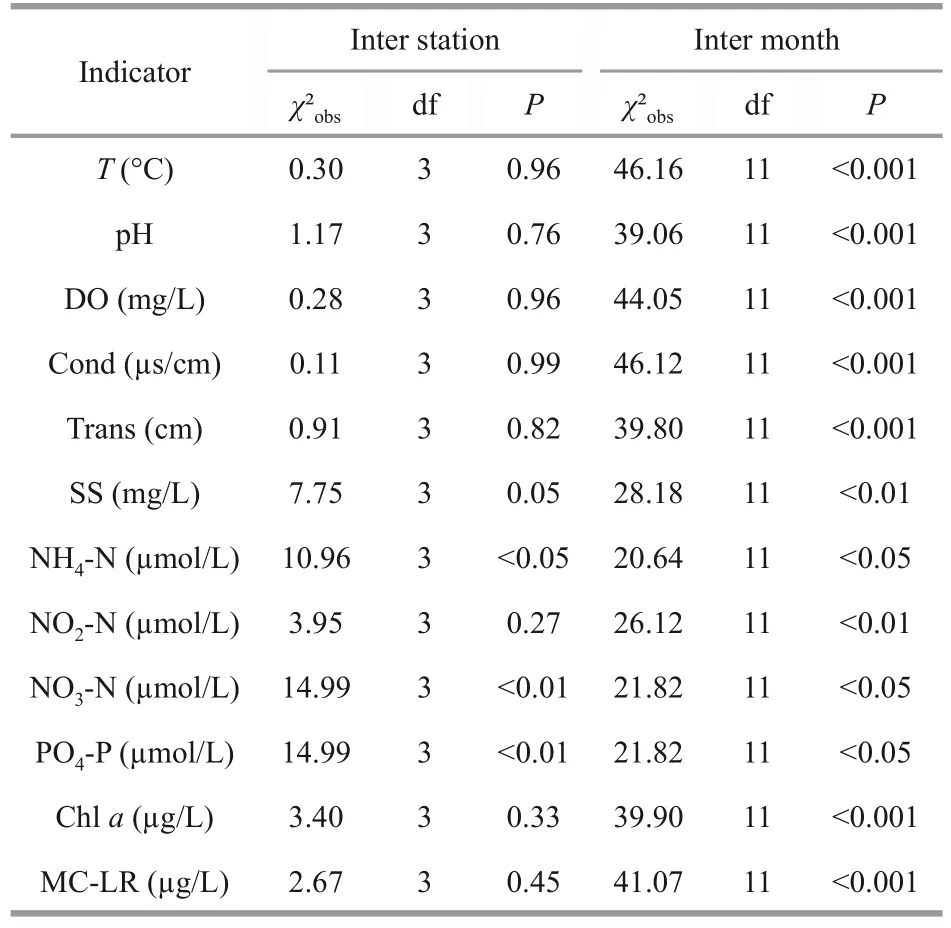
Table 2 Median values of physico-chemical parameters sampled in 4 stations situated in Oubeira Lake between April 2015 and March 2016 compared by Kruskal Wallis test at the level of significance α=0.05
Temporal variability in the cyanobacteria abundance was evident (Fig.3a), with the lowest values reported in winter/spring period and the highest values in summer/fall period.The warm period (July, August, September), when there was an incidence of cyanobacteria blooms, was dominated byMicrocystis,Planktothrix,Cylindrospermum, andOscillatoria.It should be noted that during the summer/fall period we encountered significant blooms ofMicrocystis, with the presence of scums as well as a change in the colour of the lake’s water but without any noticeable odour.
During the fall/winter transitionSynechococcus,Dolichospermum,Pseudanabaena, andAphanizomenonwere the dominant genera, whileWoronichiniaoccurred preferentially in winter.The spatial variation in cyanobacterial abundance (Fig.3b)shows a relatively small difference between Stations 1, 2, and 3, except forDolichospermumandAphanocapsawhere the highest values were recorded in the central area of the lake (Station 2).A much lower density of cyanobacteria was observed in Station 4.

Table 3 Frequency of occurrence, relative abundance, and dominant species of cyanobacteria genera identified in Oubeira Lake from April 2015 to March 2016
3.2 Temporal and spatial dynamics of bloomforming cyanobacteria
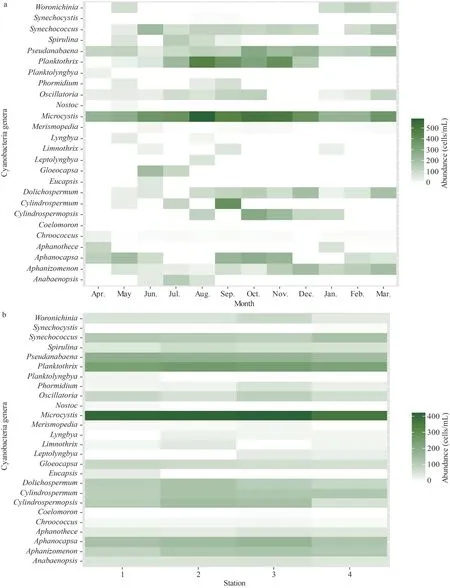
Fig.3 Temporal (a) and spatial (b) heat map of cyanobacteria abundance (cells/mL, square root transformed) identified in Oubeira Lake from April 2015 to March 2016
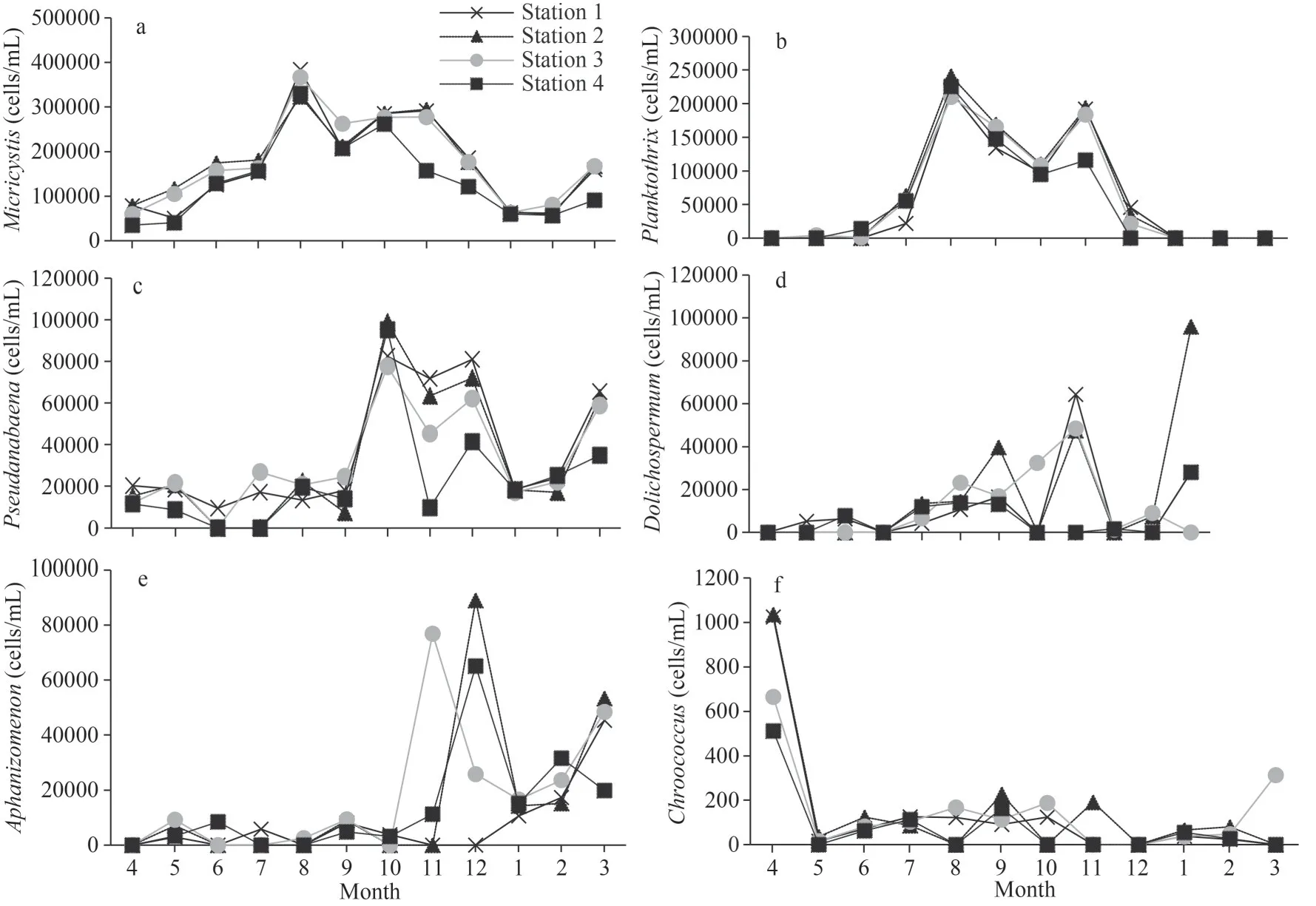
Fig.4 Monthly fluctuations of bloom-forming cyanobacteria abundances recorded in the 4 stations sampled in Oubeira Lake from April 2015 to March 2016
Six genera of cyanobacteria,Microcystis,Planktothrix,Pseudanabaena,Dolichospermum,Aphanizomenon, andChroococcusoccurred frequently in Oubeira Lake (Fig.4).MicrocystisandPlanktothrixabundance increased after June 2015, reaching peaks in August of 384 412 cells/mL and 240 740 cells/mL,respectively.The two genera became very dominant rapidly, accumulating more than 85% of the relative abundance until November.Pseudanabaena limneticaaccounted for more than 8% of the total cyanobacteria cell abundance.Peaks of more than 50 000 cells/mL were recorded from October to December 2015 and in March 2016.Pseudanabaenapeaked in October(98 880 cells/mL) and remained at a high level until the end of the year with relative abundances of approximately 20%.AphanizomenonandDolichospermumstarted to grow during the fall period reaching respectively 88 967 and 64 428 cells/mL in December.Their combined relative abundances fluctuated between 20% and 25% for the period from December 2015 to March 2016.Dolichospermumalso proliferated considerably in March, but only in Station 2 with a peak of 95 908 cells/mL.Finally,Chroococcusremained at a low level not exceeding 1 100 cells/mL throughout the study period.Chroococcushad a peak in April with only 1 036 cells/mL.
According to the Kruskal Wallis test (Table 4), the inter-month variations of bloom forming cyanobacteria have shown significant differences.These were very highly significant (P<0.001) forMicrocystis,Planktothrix,Pseudanabaena,and Aphanizomenon,and highly significant (P<0.01) forChroococcusandDolichospermum.However, the inter-station variations remained statistically insignificant (P>0.05).
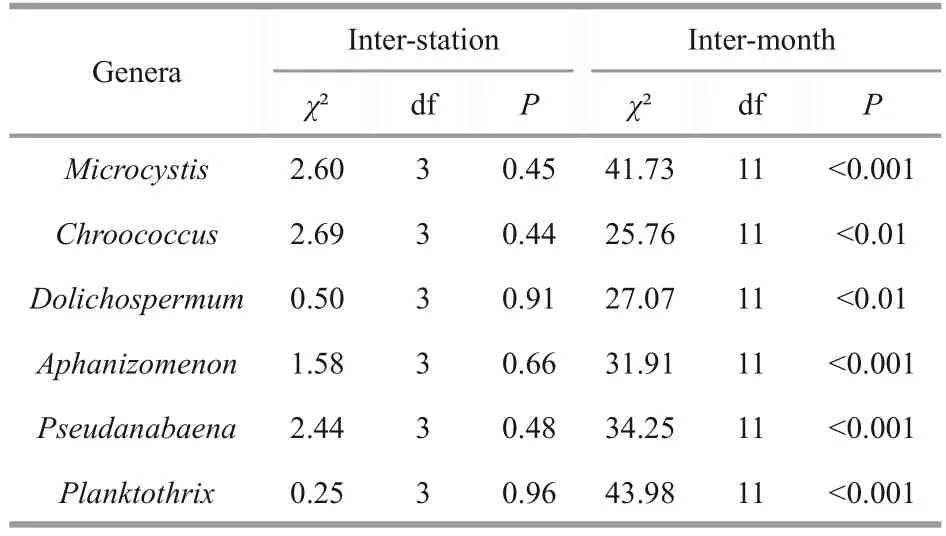
Table 4 The inter-station and inter-month comparison of median values of bloom-forming cyanobacteria abundances recorded in 4 sampled stations situated in Oubeira Lake from April 2015 to March 2016 by Kruskal Wallis test at the level of significance α=0.05
3.3 Intracellular microcystin analysis
Microcystin was found at measurable levels in all the collected samples with contents ranging from 0.62- to 19.14-μg eq MC-LR/L (Fig.5).These levels exceeded the World Health Organization guidelines(1.0 μg eq MC-LR/L) for drinking water by several times.The highest microcystin content (19.14 μg eq MC-LR/L) was found at Station 3 in November and the lowest (0.62 μg eq MC-LR/L) at Station 4 in May.Statistically, highly significant differences (P<0.001) were noted between months for microcystin median values (Table 2).Our results also showed that two distinct peaks characterized Station 3 close to river openings (Demet Rihana).The first peak(18.09 μg eq MC-LR/L) was noted in late summer,after the appearance of the highest densities recorded in August byMicrocystisandPlanktothrix;the second peak, reaching a maximum value of intracellular MC (19.14 μg eq MC-LR/L), was detected in fall (November).
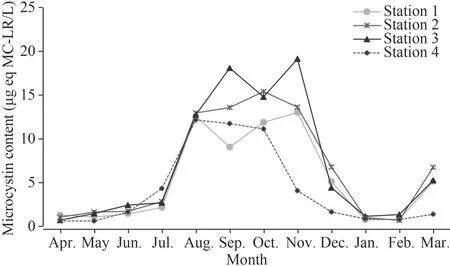
Fig.5 Monthly fluctuations of intracellular microcystin concentrations (μg eq MC-LR/L) recorded in the 4 stations sampled in Oubeira Lake from April 2015 to March 2016
Microcystin concentration as a whole increased much more rapidly from July onwards, coinciding with the rapid growth ofMicrocystisandPlankthotrix.This was confirmed by the strong positive correlations noted between microcystin concentration andMicrocystis(P<0.001) andPlanktothrix(P<0.001)abundance (Table 5).In this current study, microcystin concentrations were found to be positively correlated withDolichospermum(P<0.001) andPseudanabaena(P<0.01) but not correlated with bothAphanizomenonandChroococcus(P>0.05).In addition, the microcystin concentrations measured in the four stations (Table 2) showed no significant difference(P>0.05).
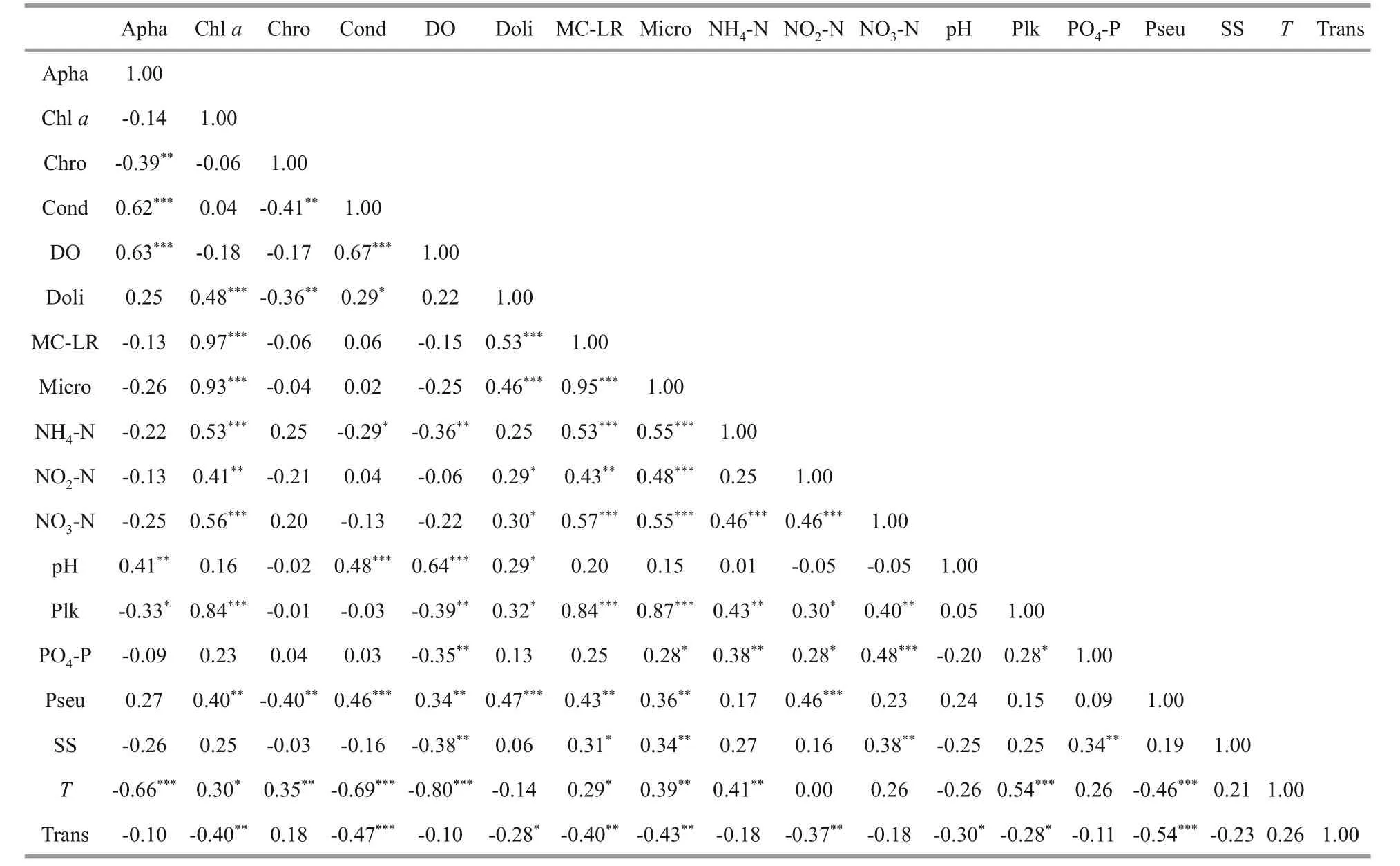
Table 5 Spearman correlation coefficients
The use of principal component analysis (PCA)as a preliminary and exploratory descriptive approach helped visualizing the structure of the temporal and spatial variation in Oubeira Lake.The aim of this exploratory analysis is to define the typology of the time cycle on the basis of biological (illustrative)and environmental (active) variables.
The PCA is performed on a global matrix of 18 variables.Twelve measured physicochemical parameters (active variables): chla, MC-LR,T, pH,DO, Cond, Trans, SS, NO3-N, NO2-N, NH4-N, and PO4-P, and 6 supplementary (illustrative):Microcystis,Planktothrix,Pseudanabaena,Aphanizomenon,Dolichospermum,Chroococcusabundances, and 48 lines (12 months×4 stations).
The first plane (Dim1×Dim2) of the PCA explained 55.3% (Fig.6) of the total variability of the projection cloud of the raw matrix.
The first axis explained 31.5% of the total variability (inter-month variability) and was positively correlated with NH4-N (R=0.76), chla(R=0.72), PO4-P (R=0.69), MC-LR (R=0.68),T(R=0.66), NO3-N (R=0.64) and negatively correlated with DO (R=-0.68).On the positive side of this axis,were projectedMicrocystis(R=0.79) andPlanktothrix(R=0.73).This positioning ofMicrocystisandPlanktothrixalong the first axis of the PCA suggested that these two cyanobacteria were directly associated with nutrient and temperature variables.Moreover, the PCA suggested that there is a positive correlation betweenMicrocystis,Planktothrix, chla,and MC-LR.On the positive pole of axis 1 are projected the months of August (R=0.39) and October (R=0.18) during which theTand nutrients are relatively high to favourMicrocystisandPlanktothrixblooms.On the negative pole are projected the months of February (R=-0.22), January(R=-0.17) when the temperature of water is low enough to avoid the growth of thePlanktothrixgenus.
The second axis explained 23.8% of the total variation (inter month variability); this axis was positively correlated with water conductivity and, to a lesser degree, with pH (R=0.81 andR=0.62 respectively) and negatively correlated with Trans(R=-0.76).On the second axis, were projectedPseudanabaena(R=0.64),Dolichospermum(R=0.51)andAphanizomenon(R=0.50) on the positive side andChroococcus(R=-0.36) on the negative one.This positioning ofPseudanabaenaandAphanizomenonalong the positive side of the second axis of the PCA suggested that these genera are directly associated with water conductivity which show high values at this time of the year.On the negative pole are projected the months of May (R=-0.30), and April(R=-0.24), during which transparency values are high enough to allow the presence ofChroococcus.
In conclusion, the first component defines the expression of a gradient of increasing nutrients and eutrophication and the generaMicrocystisandPlanktothrixare the most representative of this situation in Oubeira Lake.The second component reflects the expression of a gradient of Trans andTcharacterized by the presence of the genusChroococcus, which is opposed to the DO and Cond gradient that favours the presence of the generaAphanizomenon,Pseudanabaena,andDolichospermum.
The results of Spearman’s rank correlation analysis between cyanobacterial genera and environmental variables are shown in Table 5.MicrocystisandPlanktothrixshowed a strong positive correlation with chla, MC-LR.MicrocystisandPlanktothrixshowed positive correlation with NH4-N, NO3-N,NO2-N, and PO4-P.PlanktothrixandMicrocystiswere positively correlated withT.Pseudanabaenawas negatively correlated with Trans andT.In addition,it was positively correlated with Cond and NO2-N.Aphanizomenonwas correlated negatively withTand positively with DO and Cond.Dolichospermumwas correlated positively with MC-LR and chla.In the present study, statistical analysis results show that MC-LR is correlated with nitrogen (NH4-N,NO3-N, and NO2-N) concentration.
4 DISCUSSION
This study identified 26 genera of cyanobacteria by microscopic analysis in Oubeira Lake, compared to 11 genera in 2001 (Souissi et al., 2004) and 17 genera in 2009 (Boussadia et al., 2015), revealing a greater number of cyanobacterial genera.The generaSynechocystis,Anabaenopsis,Aphanothece,Coelomoron,Limnothrix,Planktolyngbya,Nostoc,andEucapsiswere observed, for the first time.Furthermore, it is important to note the presence, in high abundance, of the speciesCylindrospermopsis raciborskii, which was identified for the first time in 2004 (Bouaicha and Nasri, 2004) in this lake,confirming its successful establishment.Nostocales cyanobacteria are considered potentially invasive organisms due to a number of competitive advantages(ecological plasticity, N2fixation, formation of resting stages and dispersal forms under adverse conditions)that may favour their ability to colonize new environments especially under the scenario of global warming (Sukenik et al., 2012).The fact thatCylindrospermopsis raciborskiihas become part of the annual cyanobacterial assemblage requires special attention in terms of health and environmental concerns, as this species is known as a potential producer of cylindrospermopsin.This cyanotoxin has cytotoxic and hepatotoxic effects, sometimes lethal, on humans and many animals (Mathe et al., 2017).
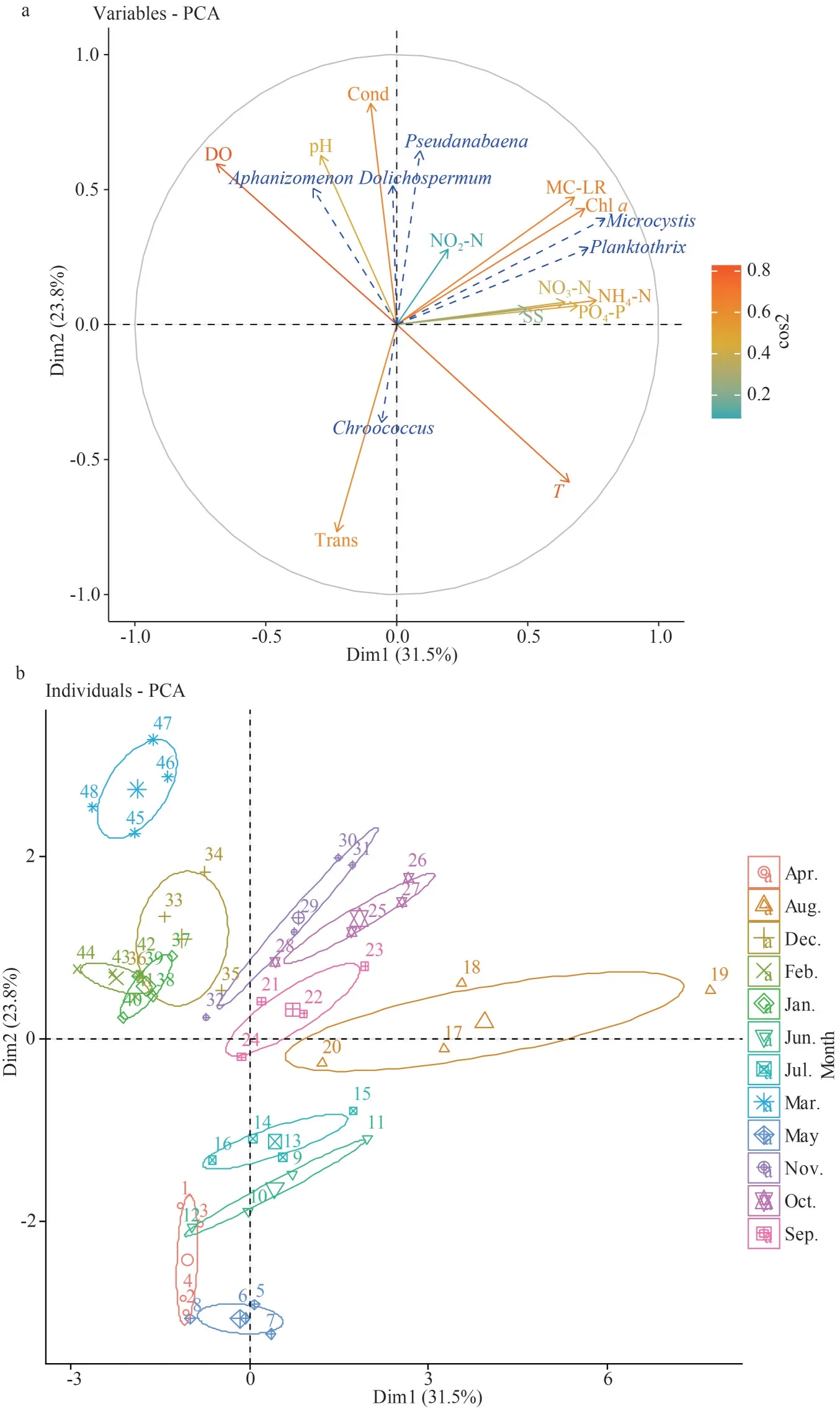
Fig.6 Principal component analysis based on monthly variations of biotic and abiotic variables, in Oubeira Lake, during the period of April 2015 to March 2016
In terms of both frequency of occurrence and relative abundance, the genusMicrocystis, dominated by the speciesM.aeruginosa, was the main component of the cyanobacterial population,contributing up to 44.51% of the total cyanobacterial abundance.The abundance and persistence ofMicrocystiswere a common phenomenon in Mediterranean lakes and reservoirs (Romo et al.,2013; Gkelis et al., 2014), including those of Algeria(Bouhaddada et al., 2016; Touati et al., 2019) and the two main factors that were generally cited to explain such a trend were the warm Mediterranean climate and the advanced eutrophication.In shallow Mediterranean lakes, internal nutrient recycling from the sediment was increased by temperature(Søndergaard et al., 2003), and nutrients could be concentrated in the water column by evaporation(Özen et al., 2010); that may favourPlanktothrixblooming.The coexistence betweenMicrocystisandPlanktothrixwas common in eutrophic freshwater systems and depended on a wide variety of environmental factors, of which light availability was an essential element (De Araujo Torres et al.,2016).In Zit Emba, a reservoir close to our study site, the average proportion ofMicrocystisto total cyanobacteria population was 43%, andPlanktothrix16% (Touati et al., 2019).
Previous studies (Glibert et al., 2016; Gobler et al., 2016; Chaffin et al., 2018) showed that nondiazotrophs, such asMicrocystisandPlanktothrix,were highly competitive for chemically reduced nitrogen forms, such as NH4-N and urea, which is in good agreement with our statistical results.The abundance of both genera in Oubeira Lake would be related to nutrients and temperature.However,Microcystisshowed greater correlation with nutrients,rather than temperature.In contrast,Planktothrixshowed greater dependence on temperature compared to nutrients.Their correlation with these environmental factors may explain their coexistence in the summer-fall period.
In Oubeira Lake,DolichospermumandAphanizomenonbloomed in late fall and winter,when nutrients and water temperature were greatly reduced.Previous studies (Chia et al., 2018) showed that low nitrogen in the environment would lead to the dominance ofDolichospermumoverMicrocystis,the reverse will occur under high nutrients (nitrogen)and low phosphorus conditions.According to Pearl and Otten (2013), the succession between nondiazotrophicMicrocystisand filamentous N2-fixingDolichospermumorAphanizomenonwas the most common cyanobacterial assemblage pattern noted in temperate or subtropical eutrophic lakes.Recent studies showed that the presence of toxicMicrocystiscould suppress the proliferation ofDolichospermum(Chia et al., 2018) andAphanizomenon(Ma et al.,2015) via allelopathy.
It has been inferred that onlyA.flos-aquaehad a competitive advantage overMicrocystiswhen the temperature was under 15 °C (Wu et al., 2008).In our pond, the genusAphanizomenonis mainly represented byA.flos-aquae, which records its highest densities in late autumn and winter when the water temperature is quite low.Statistical results showed thatAphanizomenonwas correlated negatively with water temperature.Mehnert et al.(2010) reported that onlyAphanizomenon gracile/flos-aquaecan grow around (or below) 10.8 °C; according to Uvëges et al.(2012),A.flos-aquaeblooms in winter under the ice of Stechlin Lake (Germany).
The bloom-formingPseudanabaena limneticawas also an important component in Oubeira Lake,accounting for more than 8% of the total cyanobacteria cell abundance.In this water body,peaks of more than 50 000 cells/mL were recorded when waters showed a decrease in temperature and transparency and an increase in conductivity.In Fengqing Lake (China), Zhao et al.(2015) reported thatPseudanabaena limneticahad a wide tolerance range to environmental changes and could adapt and grow even under low water temperature, low phosphorus concentrations, and low water transparency.According to Reynolds et al.(2002),Oscillatorials such asPlanktothrixagardhiiandPseudanabaenapreferred mixed and turbid waters with limited accessibility to light.This seemed to be the case for Oubeira Lake, as both genera showed, a negative correlation, with transparency.
In the current study, we found that despite a high frequency of occurrence (67%),Chroococcusrepresented only 0.03% of the total relative abundance.In the same water body, Boussadia et al.(2015)reported that the genusChroococcuswas considered accessory with a frequency of occurrence (F%=44%)and had a relative abundance close to that recorded in the present study.The results of the statistical analysis showed that the genusChroococcuswas negatively correlated with conductivity.It tended to proliferate in relatively clear waters.We noted, in fact,that the highest densities were recorded during April when the conductivity values were the lowest(<393 μs/cm).
In Oubeira Lake, intracellular MC-LR content was found in the whole set of collected samples; but the highest concentrations of MC-LR were most often detected during the period from August to November.This heavy production of microcystins observed at this period was attributed to the high cyanobacteria densities recorded at this same period of the year.The strong relationship found between MC-LR, chla,Microcystis, andPlanktothrixsuggested that MC biosynthesis results from a synergistic and/or additive effect of these two cyanobacteria.Studies have shown that in periods of drought, water retention time increased by 45%,leading to a 2-fold increase inMicrocystis aeruginosaabundance and microcystin concentration (Romo et al.,2013).According to Taranu et al.(2017), quantities of these toxins were expected to increase under eutrophic conditions in relation with high levels of nitrogen elements, turbidity, and cyanobacterial biomass.
Previous studies of natural cyanobacterial populations have found that elevated nitrogen concentrations led to the dominance of potentiallytoxicMicrocystisstrains (Davis et al., 2010; Gobler et al., 2016) and elevated microcystins concentrations inPlanktothrix-dominated communities (Davis et al.,2015).In the present study, statistical analysis results showed the strong relationship of MC-LR with nitrogen content.According to Lee et al.(2000), Van de Waal et al.(2010), and Harke and Gobler (2013),high inorganic nitrogen levels were needed to synthesize the N-rich MCs and to promote higher cellular quotas of MCs in the non-diazotrophsMicrocystisandPlanktothrix.According to Chaffin et al.(2018), assimilation of nutrients was required for cell function and expression of mcy genes,which are responsible for microcystin production in toxicMicrocystisandPlanktothrixstrains.Other studies (Lee et al., 2015; Bouhaddada et al., 2016;Chaffin et al., 2018) have shown that toxin production was largely due to changes in the composition of cyanobacterial strains/genotypes rather to changes in cyanobacterial biomass.Feng et al.(2019) suggested that the genotypes of toxinproducing cyanobacteria exceeded those of nontoxin-producing cyanobacteria under unfavourable environmental conditions.
5 CONCLUSION
It appears from this research that the main environmental parameters that impact the structure of the cyanobacterial community found in Oubeira Lake (from April 2015 to March 2016) are water temperature, nutrients (ammonium, nitrate, and nitrite)and the electrical conductivity of the water.Nutrients(ammonium, nitrate, and nitrite) and temperature were positively correlated withMicrocystisandPlanktothrixas well as chlaand MC-LR concentration.Pseudanabaena,Dolichospermum,andAphanizomenonco-occurred, at the end of thePlanktothrixdominance period (fall/winter period) and showed positive correlation with water conductivity and a negative one with water temperature.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We are grateful to the staff of Oubeira Lake for the excellent assistance provided during the study period.Special thanks to Ms.Kachabia El Khansa,Mr.Samar Faouzi, and Mr.Merad Tarek for their technical assistance.
杂志排行
Journal of Oceanology and Limnology的其它文章
- A new decomposition model of sea level variability for the sea level anomaly time series prediction*
- Pharmacokinetics of fucoidan and low molecular weight fucoidan from Saccharina japonica after oral administration to mice*
- Diel, seasonal, and annual variations of fish assemblages in intertidal creeks of the Changjiang River estuary*
- Motion simulation of moorings using optimized LSTM neural network*
