Photoluminescence Enhancement of Aluminum Ion Intercalated MoS2 Quantum Dots
2023-11-08YnminKungWenliHeZhihoZhuYruChenDongweiXiojunWngLijunGuoYuluHeZhenChiXiRnLuogngXie
Ynmin Kung,Wenli He,Zhiho Zhu,Yru Chen,Dongwei M,Xiojun Wng,Lijun Guo,Yulu He,Zhen Chi,Xi Rn,Luogng Xie
a.School of Physics and Electronics,International Joint Research Laboratory of New Energy Materials and Devices of Henan Province,Henan University,Kaifeng 475004,China
b.School of Physics and Electronic Engineering,Zhengzhou University of Light Industry,Zhenzhou 450002,China
c.Key Laboratory for Special Functional Materials of Ministry of Education,Henan University,Kaifeng 475004,China
Low photoluminescence (PL) quantum yield of molybdenum disulfide (MoS2) quantum dots (QDs) has limited practical application as potential fluorescent materials.Here,we report the intercalation of aluminum ion (Al3+)to enhance the PL of MoS2 QDs and the underlying mechanism.With detailed characterization and exciton dynamics study,we suggest that additional surface states including new emission centers have been effectively introduced to MoS2 QDs by the Al3+ intercalation.The synergy of new radiative pathway for exciton recombination and the passivation of non-radiative surface traps is responsible for the enhanced fluorescence of MoS2 QDs.Our findings demonstrate an efficient strategy to improve the optical properties of MoS2 QDs and are important for understanding the regulation effect of surface states on the emission of two dimensional sulfide QDs.
Key words: Molybdenum disullfide,Quantum dot,Photoluminescence enhancement,Exciton dynamics
I.INTRODUCTION
As a kind of 2D layered materials,molybdenum disulfide (MoS2) has been studied widely for their excellent electronic and optical properties [1,2].Unlike few or single layer nanosheet forms,MoS2quantum dots(QDs) with atomic thickness and nanoscale diameter have high specific surface area,plentiful edge sites,and unconventional optical properties,which demonstrate a great prospect to be applied in many fields such as catalysis [3,4],fluorescence sensing [5],bioimaging [6],etc.In the past decade,many strategies and methods for preparing MoS2QDs have been reported,such as hydrothermal synthesis [3],chemical ion intercalation[7,8],and ultrasound-assistant liquid exfoliation [9].Among them,the liquid exfoliation based on alkali metal intercalation provides an effective way to obtain largescale production of transition metal dichalcogenides(TMDC) QDs with a monolayer structure [7,8,10,11].However,the alkali metal intercalation is very sensitive to the environmental conditions and is difficult to be carried out in ambient conditions [12].Although the hydrothermal or solvothermal method possesses some advantages of low-cost,easy operation,and large scale synthesis [13],the prepared MoS2QDs usually exhibit very low photoluminescence (PL) quantum yields(QYs).So far,it is still a challenge to develop a simple and feasible strategy in the preparation of MoS2QDs with high PL QYs.
Besides the preparation methodologies,modifying the surface states or defect sites to regulate the exciton dynamics provides an alternative way to improve the PL properties of MoS2QDs.The introduced defects can be expected to regulate the energy levels and density of trap states,and act as traps or recombination centers of excitons,affecting the PL properties of MoS2QDs [14].Therefore,the surface states have an effect on the properties of TMDCs.Defects usually play a role in non-radiative exciton recombination pathway to result in poor QY of TMDCs,which restricts their applications in optoelectronic devices.Hence,it is an effective method to introduce surface states and passivate defects to promote the optical performance of monolayer TMDCs.In previous studies,many efforts have been devoted to promoting the PL properties of TMDCs,such as plasma and laser irradiation,surface chemical route,alloying,and substitutional doping.It has been proposed that the PL intensity of monolayer MoS2could be enhanced through defect engineering of mild plasma irradiation [15].So far,most of previous studies have focused on TMDC nanosheets,and few works reported on the TMDC QDs with strong quantum confinement and edge effects.In addition,the role of surface states and underlying mechanism in regulating the PL property of MoS2QDs have not been fully understood and need further study.
Herein,we report an effective intercalation of aluminum ions (Al3+) to modilfy MoS2QDs with improved PL properties and investigate the involved mechanism.The intercalated Al3+greatly increases the exfoliation efficiency and induces more surface states,and enhances the PL QY of MoS2QDs to be 7.5%.The exciton dynamics from time resolved PL indicates that PL enhancement mainly originates from the radiative exciton recombination assisted by the surface states of MoS2QDs.
II.Methods
A.Synthesis of MoS2 QDs
MoS2powder (20 mg) and AlCl3(0,0.4,0.8,4,or 8 mg) were mixed inN,N-dimethylformamide (DMF,40 mL).Subsequently,the sloution was sonicated for 6 h to exfoliate MoS2,then the mixture was heated at 200 ℃ for 12 h in a Teflon-lined stainless steel autoclave (50 mL).When the mixture was cooled down to room temperature,the suspension of MoS2QDs was obtained by centrifuging the mixture at 10000 r/min for 10 min.The resulting suspension was further purified by a 0.22 µm microporous membrane,finally the target yellow MoS2QDs solution was collected and stored under 4 ℃ in dark for further measurements.
B.Characterization of MoS2 QDs
The absorption and PL spectra for MoS2QD dispersed in DMF were obtained by Cary 5000 UV-Vis-NIR spectrophotometer and PerkinElmer LS 55 spectrometer,respectively.The PL decay curves were measured by a lifetime setup (Picoharp300,Picoquant),and the samples were excited with fs-pulsed laser at 340,350,370 nm (repetition frequency of 10 MHz,Coherent).
The morphological images of MoS2QDs were acquired by a high resolution transmission electron microscope (HRTEM) (JEOL JEM-2010) operated at an accelerating voltage of 200 kV.The MoS2QDs were dripped onto copper grid coated with carbon film for the TEM measurement.
The thickness of MoS2QDs was confirmed by atomic force microscopy (AFM) (NT-MDT SOLVER P47H)under tapping mode.The samples for the AFM measurement were prepared by spin coating the MoS2QDs solution onto a mica substrate.
The elemental analysis was conducted using X-ray photoelectron spectroscopy (XPS) (PHI 5000 VersaProbe,Japan).The C 1s peaked at 284.6 eV was used as reference for binding energy calibration.
C.Density functional theory (DFT) calculations
DFT calculations were performed using projector augmented wave (PAW) potentials as implemented in the Vienna Ab initio Simulation Package (VASP).The exchange correlation potential is approximated by generalized gradient approximation (GGA) using PBE functional (see the Supplementary materials (SM) for details).
III.RESULTS AND DISCUSSION
In this study,the bulk MoS2powder was used to prepare monolayer MoS2QDs by direct exfoliation through sonication together with the solvothermal treatment in the presence of Al3+ions.FIG.1illustrates the strategy for preparing MoS2QDs with Al3+intercalation.

FIG.1 Illustration of the preparation process for MoS2 QDs with Al3+ intercalation in solvothermal method.
The as-prepared MoS2is monodispersed and uniform in shape and size,which can be seen from the typical TEM image inFIG.2(a).The diameter distribution inFIG.2(b) indicates that the MoS2QDs are ultrasmall and the average size is about 2.6 ± 0.3 nm.The HRTEM image (inset inFIG.2(a)) with characteristic of lattice fringes spacing of~2.3 Å reveals that the MoS2QDs are highly crystalline,which can be assigned to the (103) plane of MoS2.AFM imaging is further employed to check the thickness of MoS2QDs.FIG.2(c,d)show that the height of MoS2QDs is determined to be~0.7 nm,suggesting a monolayer feature and the efficient exfoliation of MoS2QDs in the solvothermal method with Al3+intercalation.In order to observe the effect of Al3+intercalation on the geometric structure,the TEM characterization and AFM analysis of the pristine MoS2QDs prepared without Al3+intercalation are performed (FIG.S1 in SM).The pristine MoS2QDs demonstrate the very similar average size,morphology,fringe spacing,and monolayer thickness,indicating that the introduced Al3+cations mainly regulate the defects and surface states of MoS2QDs.
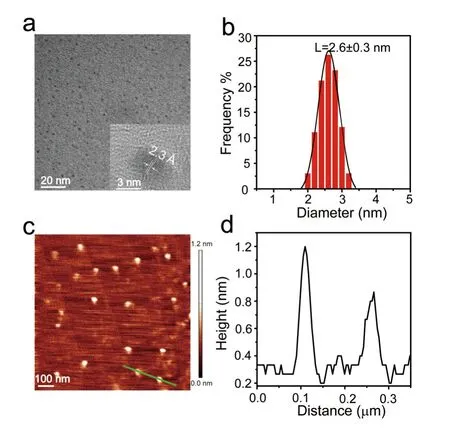
FIG.2 (a) Typical TEM image of MoS2 QDs.HRTEM image in the inset shows the lattice spacing of MoS2 QDs.(b) Diameter distribution of 200 MoS2 QDs.(c) Typical AFM image of MoS2 QDs dispersed on mica substrate.(d) Height profile for the green line marked in (c).
The elementary analysis of MoS2QDs based on highresolution XPS was also conducted.FIG.3shows the typical XPS response in the Mo 3d,S 2p and Al 2p.Two peaks inFIG.3(a) located at 232.7 and 229.4 eV correspond to Mo4+3d3/2and Mo4+3d5/2,respectively.The peaks of S 2p1/2 and S 2p3/2 at 163.5 and 162.3 eV shown inFIG.3(b) indicate that the crystal structure of MoS2QDs is 2H phase [12,16].The atomic ratio of Mo/S is 1:2.36 based on the compositional analysis in detail,which is attributed to the unsaturated S atoms at the MoS2QDs edge [17].It should be mentioned that the XPS spectrum of Al 2p at 74.2 eV (FIG.3(c))proves the presence of Al in MoS2QDs.The position of the Al 2p peak suggestes that Al is bonded to S [18,19].The amount of Al in MoS2QDs prepared with 750 µmol/L Al3+intercalation is calculated to be 10.73% of atomic percentage.These results indicate that Al3+may form a complex with S atoms at MoS2QDs edges without affecting structure nature,which is consistent with the reports on the MoS2nanomaterials modified by other metal cations [20,21].

FIG.3 XPS spectra of MoS2 QDs prepared with 750 µmol/L Al3+.High-resolution peak fitting for (a) Mo 3d,(b) S 2p,and (c) Al 2p,respectively.The solid line shows the fitting result of experimental data.
In order to further confirm the formation of Al-S bonds at the edge of MoS2,we carried out systematic DFT calculations on pristine and Al-adsorbed MoS2.Our DFT calculations indicate that Al ion tends to be attached at the MoS2edge with the binding energy of-4.82 eV.The Al-S bonds are formed with the formed lengths of~2.30 Å.The corresponding densities of states(DOS) and projected DOS (PDOS) in FIG.S2 (SM)show that the band gap of MoS2decreases by 0.27 eV and some additional peaks occur in the band gap of MoS2upon the adsorption of Al ion at the MoS2edge.From the analysis of PDOS,the surface states in the vicinity of fermi energy are contributed by the adsorbed Al ion.These surface states can act as recombination centers for photoexcited carriers.
The Al3+intercalation is effective to improve exfoliation efficiency of MoS2.FIG.4(a,b) show the photographs of MoS2QDs dispersed in DMF prepared with different amounts of Al3+under daylight and UV light illumination.The yield and PL intensity significantly increase for the MoS2QDs prepared with the addition of Al3+.The colour of the solution turns from yellow to brown with increasing the Al3+concentration from 0 µmol/L to 750 µmol/L,while excess Al3+(1500 µmol/L) may lead to the decomposition of MoS2QDs resulting in the decrease of MoS2QDs concentration.To attain the quantitative concentration of the MoS2QDs dispersion,we carefully measured the weight of the samples after filtration and drying,then dispersed the MoS2QDs powder in 2 mL DMF,respectively.As shown inFIG.4(a),the mass concentrations of these dispersions are 2.53,3.14,3.82,8.43,and 3.67 mg/mL for MoS2QDs prepared by Al3+intercalation of 0,75,150,750,and 1500 µmol/L,respectively.Thus,it can be concluded that Al3+intercalation is effective for MoS2exfoliation.It is worth mentioning that a smaller PL enhancement is also observed for the K+,Na+,Mg2+,Mn2+treated MoS2sheets,indicating similar regulation effects of metal cations on the MoS2exfoliation (FIG.S3 in SM).The highest PL enhancement for MoS2QDs suggests that the Al3+cation has the proper atom size and high charge density to be inserted into the interlayer space of MoS2sheets and promotes the exfoliation of MoS2QDs during the sonication and solvothermal processes [22,23].

FIG.4 Photographs of MoS2 QDs dispersed in DMF prepared with different amounts of Al3+ (0,75,150,750,and 1500 µmol/L) under (a) daylight and (b) UV light illumination,respectively.(c) UV-Vis absorption of MoS2 QDs synthesized with different amounts of Al3+ (0,75,150,750,and 1500 µmol/L).(d) PL spectra of the samples with the 350 nm excitation.(e) Corresponding PL quantum yields of samples based on the data in (a,b).(f) PL decay curves of samples under the fs-pulsed excitation at 350 nm.
The optical characterizations including absorption,PL spectra and PL decay dynamics of MoS2QDs dispersed in DMF were carried out to explore the regulation effect on the optical properties of MoS2QDs with Al3+intercalation.Compared with pristine MoS2QDs,the absorption edge of MoS2QDs with the introduction of Al3+extend to longer wavelengths (FIG.4(c)),suggesting the surface states are formed.Meanwhile,the PL intensity of MoS2QDs significantly increase compared with the pristine MoS2QDs.With the increasing concentration of Al3+,both the absorption and PL intensity of MoS2QDs increase and reach their maxima at the concentration of 750 µmol/L,but decrease with further increasing the Al3+intercalator (FIG.4(d)).
The corresponding PL QY of MoS2QDs was determined relative to quinine sulfate acid aqueous solution(54%) and calculated according to the following equation:
where Φunkis the radiative quantum yield of the sample;Φstdis the radiative quantum yield of the standard;IunkandIstdare the integrated emission intensities of the sample and standard,respectively;AunkandAstdare the absorbance of the sample and standard at the excitation wavelength,respectively;and ηunk and ηstd are the index of refraction of the sample and standard solutions,respectively [24].The maximal PL QY of MoS2QDs with the Al3+intercalation is determined to be 7.5%,around 3 times that of 2.7% for pristine MoS2QDs without the assistance of Al3+(FIG.4(e)).In order to explore whether the enhancement of PL QYs originate from the increased MoS2QDs concentrations or the passivation of defect on the edge of MoS2QDs,we conducted the control experiment to investigate the concentration dependence on PL QY of MoS2QDs.MoS2QDs solution prepared with 750 µmol/L Al3+intercalation was diluted to different concentrations to measure the PL QYs.FIG.S4 in SM shows the UV-Vis absorption and PL spectra of MoS2QDs at concentrations of 1.42 mg/mL,1.05 mg/mL,and 0.84 mg/mL.The calculated PL QYs are 7.4%,7.5%,7.4% for these three cases,which are independent of MoS2QDs concentration.Meanwhile,the result of another control experiment exhibits little change in fluorescence when 750 µmol/L Al3+is mixed with the pristine MoS2QDs solution (FIG.S5 in SM),excluding the contribution of Al3+physisorption on the MoS2QDs to the fluorescence enhancement.We infer that the enhanced PL QYs originate from the passivation of non-radiative defects by the formation of Al-S bonds.Such a PL enhancement was also observed in other TMDC nanomaterials prepared with ion intercalation and was attributed to the edge effects or surface states [8,12,16,21,25].
The enhanced PL by introducing Al3+into the intercalation procedure probably originates from the regulated exciton dynamics due to the modified surface states.To prove this,the time-resolved PL measure-ments were performed for MoS2QDs prepared with different concentration of Al3+intercalator.The PL decay curves of MoS2QDs samples consist of two competitive decay processes for the exciton deactivation(FIG.4(f)),with the time constants of τ1around 1.7 ns and τ2around 8 ns (the fitting parameters are shown in Table I).The similar biexponential PL dynamics have been reported in carbon nanodots,MoS2,and WS2[26-28],and the two different exciton decay pathways via radiative recombination are related to surface states.The short-lived decay component has been attributed to direct exciton recombination on the surface states,while the long-lived decay is a relaxation of carriers from MoS2core onto the surface states,respectively[26,27,29].

TABLE I PL decay lifetime components and relative amplitudes with a bi-exponential fitting for samples with different concentrations of Al3+.
The evolution of these two components (τ1,τ2) and their relative percentages (A1,A2) with different concentrations of Al3+are shown in Table I.We can observe that with the increase of Al3+,the contribution of fast decay component (A1) gradually decreases,and the relative contribution from slow decay (A2) increases.However,the exciton decay curves remain unchanged when the concentration of Al3+increases from 750 µmol/L to 1500 µmol/L,indicating that the density of surface states is saturated.The increased contribution from long-lived emission τ2suggests that the surface states introduced by Al3+affect the PL QYs of MoS2QDs.It has been reported that the exfoliation efficiency of TMDCs and their electronic and optical properties can be improved with intercalated ions [7,8,10,11].During the sonication and solvothermal treatments,the Al3+could intercalate the MoS2sheets and expand the layer-layer distance,which may produce more defects in MoS2sheets,such as S/Mo vacancy and boundaries [30].These defects make the MoS2sheets fragile for effective exfoliation and promote the formation of MoS2QDs [31].The XPS analysis and DFT calculations suggest that Al is bonded to S at the edge of MoS2QDs to passivate the defect.Hence,the concentration of Al3+is a key parameter to affect the density of surface states.In the meanwhile,the PL QYs of the MoS2QDs peak around 750 µmol/L.If the concentration is further increased,the MoS2QDs may be decomposed,resulting in fluorescence quenching [25].Consequently,the PL QYs of MoS2QDs decrease once the concentration of Al3+is more than 750 µmol/L,while the exciton decay curves remain unchanged.
In order to understand these two decay pathways of exciton deactivation in depth,we further study the PL decay dynamics of the MoS2QDs prepared with 750 µmol/L Al3+intercalation.Under 350 nm excitation,the PL decay curves of MoS2QDs were measured with varying detected wavelengths of 390,400,and 410 nm (FIG.5(a)).The slopes of PL decays in MoS2QDs remain unchanged with increasing detected wavelength,indicating the emission center is isolated on the surface site,which is in agreement with DFT calculations.Similar reports have been mentioned in the previous studies of diethylenetriamine-doped WS2QDs and surface functional groups capped MoS2and WS2QDs[27,28].In the meanwhile,the PL decay curves appear at the same emission peak of 410 nm when MoS2QDs are excited by different wavelength of 340,350,370 nm(FIG.5(b)).The contribution from short-lived decays is gradually enhanced when the excitation wavelength increases from 340 nm to 370 nm,and the fitting parameters are listed in Table S1 in SM.We know that the surface state is a lower energy state,which can be excited if using a long-wavelength excitation with smaller energy.As a result,the contribution from direct exciton recombination on surface states is enhanced.In addition,the excitons generated from MoS2QDs core reduce with the excitation wavelengths increasing.Hence,the fast decay (~1.7 ns) and slow decay (~8 ns) are assigned to a direct exciton recombination on surface site and relaxation of photogenerated carriers from MoS2QDs core onto the surface states,respectively.
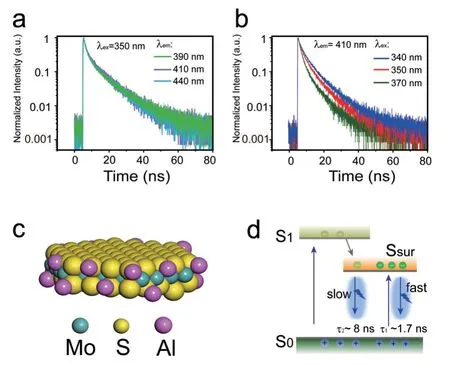
FIG.5 PL decay curves of MoS2 QDs (a) at detection wavelength of 390,410,and 440 nm excited by 350 nm laser;(b) at detection wavelength of 410 nm excited by 340,350,and 370 nm laser,respectively.(c) A schematic diagram of structure model for MoS2 QDs,yellow balls:sulfur,green balls: molybdenum,purple balls: Al at the Sedge,respectively.(d) Schematic diagram showing the exciton relaxation channel in MoS2 QDs.S0,S1,and Ssur represent ground state,excited state of MoS2 QDs core,and surface state,respectively.
For clarity,a structure model and a schematic of the PL mechanism are plotted inFIG.5(c) and (d) to interpret the effect of Al3+intercalator on the optical properties of MoS2QDs.With this model,we expect to explain the exciton relaxation in detail from the two dynamic pathways.On one hand,Al3+intercalator could improve the exfoliation efficiency of MoS2nanosheet.On the other hand,Al is bonded to S atoms at the edge of MoS2QDs (shown in the proposed model inFIG.5(c)),which can introduce new surface states as the additional emission centers,resulting in enhanced fluorescence of MoS2QDs [29,30].FIG.5(d) shows a simplified diagram of excitons relaxation processes,which can be proposed to understand the deactivation pathway of photogenerated carriers of MoS2QDs.The ground state,excited state of MoS2QDs core,and surface states are represented by S0,S1,and Ssurrespectively.The decays of carrier can be related to fast delay pathway 1 (~1.7 ns,direct exciton recombination on the surface states) and slow delay pathway 2 (~8 ns,the relaxation of carriers from MoS2QDs core to the surface states).For pristine MoS2QDs,due to the strong Coulomb interaction,the energy of excitons is mainly deactivated through nonradiative process including the energy transfer from one exciton to the other,and the exciton radiative recombination is weakened,leading to a lower quantum yield [32].For the MoS2QDs produced with Al3+intercalator,more surface states are introduced to serve as the addition emission centers.The carriers generated in MoS2QDs core can fall into the surface states after photoexcitation,then electron-hole radiative recombination via surface state assists emission to increase the contribution of slow delay pathway,resulting in an enhancement of PL QY.
IV.CONCLUSION
We report a facile exfoliation technique to improve the fluorescence of MoS2QDs in solvothermal process with Al3+intercalation.The dependence of photophysical properties for MoS2QDs on the Al3+concentration have been systematically investigated to deeply understand the regulation effect and the involved mechanism.The intercalation of Al3+not only improve the exfoliation of MoS2,but more importantly regulate the surface states of MoS2QDs.Some of the surface states can serve as additional emission centers for the exciton relaxation,resulting in the enhanced fluorescence of MoS2QDs.Therefore,these introducing additional emission centers can effectively compete the dominant non-radiative deactivation process in the pristine MoS2and improve the opticl properties of MoS2QDs.These findings are useful for understanding the photophysical mechanism of weakly emissive MoS2QDs and developing the optoelectronic devices based on monolayer TMDs QDs.
Supplementary materials:The detailed characterization of TEM and AFM of the pristine MoS2QDs,DFT calculations on pristine and Al-adsorbed MoS2,PL spectra of MoS2QDs prepared with different metal ions,UV-Vis absorption and PL spectra of MoS2QDs in DMF solutions with different concentration,PL spectra of pristine MoS2QDs with and without Al3+ions,and fitting parameters for PL decay curves of MoS2QDs when excited at 340,350,and 370 nm are avaible.
V.ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No.12004101,No.61905066,No.22103024,No.61805070,and No.22105063),the Natural Science Foundation of Henan Province(No.202300410065) and the Open Project of the State Key Laboratory of Crop Stress Adaptation and Improvement.
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
- Controllable Modulation of Morphology and Property of CsPbCl3 Perovskite Microcrystals by Vapor Deposition Method
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
