Energy Transfer Dynamics between Carbon Quantum Dots and Molybdenum Disulfide Revealed by Transient Absorption Spectroscopy
2023-11-08RuixiangWuXinLiuXiaoshuaiWangJingjingLuoBinLiShengzhiWangXiangyangMiao
Ruixiang Wu,Xin Liu,Xiaoshuai Wang,Jingjing Luo,Bin Li,Shengzhi Wang,Xiangyang Miao
Key Laboratory of Spectral Measurement and Analysis of Shanxi Province,College of Physics and Information Engineering,Shanxi Normal University,Taiyuan 030031,China
Zero-dimensional environmentally friendly carbon quantum dots(CQDs) combined with two-dimensional materials have a wide range of applications in optoelectronic devices.We combined steady-state and transient absorption spectroscopies to study the energy transfer dynamics between CQDs and molybdenum disulfide (MoS2).Transient absorption plots showed photoinduced absorption and stimulated emission features,which involved the intrinsic and defect states of CQDs.Adding MoS2 to CQDs solution,the lowest unoccupied molecular orbital of CQDs transferred energy to MoS2,which quenched the intrinsic emission at 390 nm.With addition of MoS2,CQD-MoS2 composites quenched defect emission at 490 nm and upward absorption,which originated from another energy transfer from the defect state.Two energy transfer paths between CQDs and MoS2 were efficiently manipulated by changing the concentration of MoS2,which laid a foundation for improving device performance.
Key words: Energy transfer,Transient absorption spectroscopy,Carbon quantum dot,Molybdenum disulfide
I.INTRODUCTION
Zero-dimensional (0D) quantum dot (QD) nanomaterials have remarkable optical properties that include high photostability,strong absorption,broad excitation,and narrow size-dependent emission [1-3].QDs combined with two-dimensional (2D) materials like graphene [4,5] and molybdenum disulfide (MoS2) [6,7]can control photoluminescence (PL) flexibly [8-10].0D-2D composites take advantage of large QD absorption cross-sections [11,12],high carrier transport of 2D materials [13,14],and they have unique potential for applications in optoelectronics [15-17],field effect transistors [18],photodetectors [19,20],and sensors [21,22].
Depositing graphene QD solution onto MoS2monolayers quenches the PL intensity of the QD/MoS2heterostructure and redshifts due to the enhanced electronelectron interactions [23].The fluorescence biosensor of graphene QD and MoS2nanosheets has been used for cell protein detection [24].Electrical control of excitonic absorption of MoS2resulted in a~75% modulation of PL intensity in CdSSe QDs induced by the efficient near-field energy transfer from QDs into MoS2rather than charge transfer [25].After black phosphorus QD doping,the back-gated monolayer MoS2field-effect transistors were fabricated with high electron concentrations [26].
Our previous works described the effective manipulation of energy transfer between QDs and graphene oxide by laser irradiation [27-29],via using CdSe/ZnS QDs or Mn2+-doped ZnS QDs.Recently,carbon QDs(CQDs) have attracted wide attention due to water solubility,low toxicity,environmental friendliness,easy surface functionality,and ease of synthesis on a large scale [30-33].Here,we investigated the excited state interaction in a CQD-MoS2composite by transient absorption (TA) spectroscopy.Intrinsic emission and defect emission of CQDs were quenched with higher MoS2concentrations.TA measurements of CQD-MoS2composites were performed to understand the energy transfer dynamics involving the intrinsic excited state and defect state of CQDs,and a TA spectral evolution was analyzed from the dynamic trajectory.
II.MATERIALS AND METHODS
CQDs dispersion (XF253-1) and MoS2nanosheet suspensions (XF209) were purchased from Jiangsu XFNANO Materials Tech.Co.,Ltd.CQDs were diluted to 0.1 g/L in anhydrous ethanol.A 40 µL MoS2suspension (1 g/L) was mixed with CQDs solution to form 4 mL MoS2solution with concentration of 10 mg/L.The CQD-MoS2composite was shaken by vortex mixer for 5 min.Sequentially adding 40 µL MoS2(10 mg/L) to the solution gradually increased the concentration of MoS2suspension to 100 mg/L.
PL spectra of CQD-MoS2ethanol solutions were measured by a spectrofluorometer (Duetta,Horiba).PL peaks of CQDs appeared at 490 nm (see FIG.S1 in Supplementary materials (SM)).Transient absorption(TA) spectra were acquired using a nanosecond laser flash photolysis instrument (LFP-100,Dalian Institute of Chemical Physics).The pump source was a 355 nm harmonic pulse generated from a Q-switched Nd:YAG laser (3 Hz,Nimma-900,Beamtech Optronics Corp.).A probe light was adopted to a xenon lamp (XBO 450W/OFR,OSRAM),and the excited state dynamics in CQD-MoS2was detected from 360-700 nm.In this work,the single pulse energy of the pump laser was~10 mJ.
III.RESULTS AND DISCUSSION
FIG.1shows TA spectra of 0.1 g/L CQDs solutions excited by a 355 nm pulse laser.The TA plots of CQDs inFIG.1(a) consisted of stimulated emission features(SE,ΔA<0,370-690 nm) and photoinduced absorption features (PIA,ΔA>0,430-550 nm).SE and PIA concentrated on 490 nm together.The TA signal at 490 nm was faster than that at the two PL sidebands.InFIG.1(b),the TA kinetic traces of three typical probe wavelengths at 390 nm,490 nm,and 620 nm were extracted from the TA plot.At 490 nm,the PIA feature decreased quickly but turned into SE.The TA trace involving the above two features was well fitted by the following two-exponent decay equation:
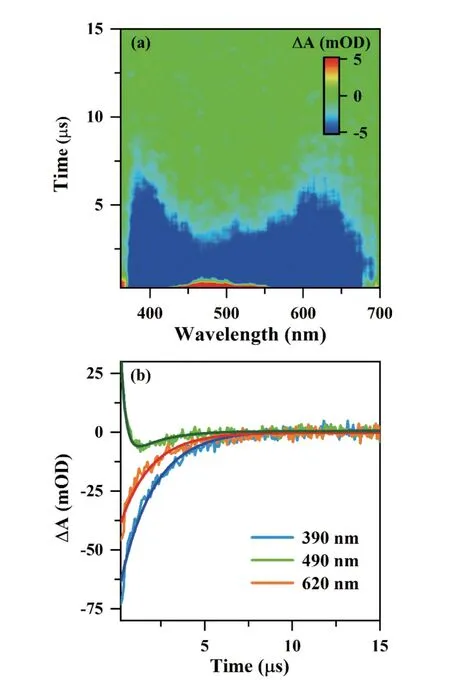
FIG.1 TA spectra of 0.1 g/L CQDs in ethanol: (a) pseudo color TA plot,and (b) TA kinetic traces at different probe wavelengths.
wherep1>0,p2>0,τPIA and τSE correspond to their lifetimes of stimulated emission and photoinduced absorption features.Here τPIA=0.29 µs and τSE-490=1.76 µs for CQDs.The long-lifetime SE and short-lifetime PIA caused the weaker TA spectrum at 490 nm.In the TA spectra at 390 nm and 620 nm,only the SE feature appeared,which obeys a single exponential (p1=0)decayed with τSE-390=1.93 µs and τSE-620=1.76 µs.Comparing the three SE lifetimes at the three probe wavelengths,the τSE-490 was the same as the τSE-620 but shorter than the τSE-390.This indicated the PL at 490 nm and 620 nm originated from the same excited state,and the PL at 390 nm came from another higher energy state.
Increasing the MoS2concentration gradually quenched the PL of CQDs in ethanol as shown inFIG.2.The Stern-Volmer plot in the inset revealed the relationship between the intensity ratioI0/Iand MoS2concentration.In the plot,I0andIare the PL intensities in the absence and presence of MoS2in CQD solutions.The rising curve was fitted using the following formula[34,35]:
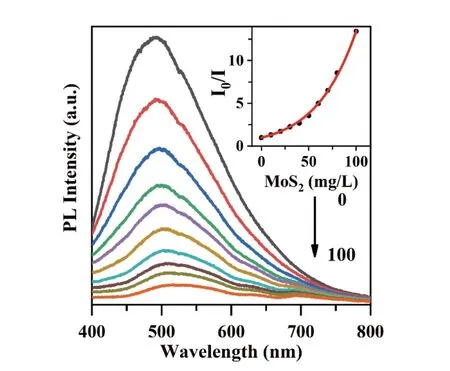
FIG.2 PL quenching of CQDs induced by MoS2 in ethanol.The concentrations of MoS2 were 0,10,20,30,40,50,60,70,80,and 100 mg/L,respectively.Inset: Stern-Volmer plot.
where [MoS2] is the concentration of MoS2;KSV andKqare the Stern-Volmer constant and exponential factor.The fitting parameters areKSV=5.79×10-14L/g,Kq=26.2 L/g.
To study PL quenching dynamics,CQD-MoS2composites and excited-state interaction between them were measured by TA.FIG.3shows the TA spectra of CQD-MoS2composites changed with increasing MoS2concentrations.InFIG.3(a),the positive PIA CQD features at 430-560 nm inFIG.1(a) turned weaker even and eventually disappeared in the CQD-MoS2composite at 10 mg/L of MoS2.With MoS2added,SE features were distributed symmetrically around the 490 nm PL peak and disappeared above 630 nm,as shown inFIG.3(b).There is no spectral feature in the TA plot for MoS2with the concentration below 50 mg/L (FIG.S2(a) in SM),and it would not influence the TA spectrum of the CQD-MoS2composite.When the concentration of MoS2increased to 50 mg/L,the SE feature of CQDMoS2inFIG.3(c) dramatically weakened.The disappearing SE blue-shifted to 580 nm.In addition,two new positive PIA signals in the CQD-MoS2composite appeared at 460 nm and 600 nm,consistent with the TA spectrum of the 50 mg/L MoS2(FIG.S2(b) in SM),and indicated that PIA came from MoS2.Increasing MoS2to 100 mg/L,SE of CQDs turned much faster at 470-660 nm inFIG.3(d).The positive PIA of MoS2(FIG.S2(c) in SM) disturbed the TA spectrum of the CQD-MoS2composite.
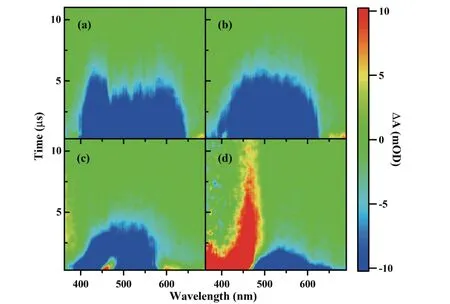
FIG.3 Pseudo color TA plot of the CQD-MoS2 composite with differnt MoS2 concentrations of (a) 10 mg/L,(b) 20 mg/L,(c) 50 mg/L,and (d) 100 mg/L in ethanol.
Further investigation of the TA evolution of CQDMoS2composite was carried out by measuring the TA kinetic trajectory at the PL peak of CQD-MoS2as shown inFIG.4.The fitting lifetime of CQD-MoS2composite can be obtained (Table S1 in SM).PIA of CQDs gradually decreased with increasing MoS2and disappeared at 20 mg/L (FIG.4(a)-(c)).SE strengthened as the concentration of MoS2increased from 0 to 40 mg/L.InFIG.4(e),TA trace of CQD-MoS2obeyed a single-exponent (p1=0) decay with τSE=1.73 µs(FIG.S3 in SM),which was nearly equal to the τSE-490 and τSE-620 of CQDs inFIG.1.Therefore,the lifetimes of PIA and SE were constant and their weightsp1,p2were influenced by the MoS2concertation.TA trajectories were well fitted by Eq.(1) with τPIA=0.29 µs and τSE=1.78 µs (FIG.4(a)-(e)).With additional MoS2

FIG.4 TA kinetic trajectories and corresponding fitting curve of CQD-MoS2 composites in ethanol with different MoS2 concentrations at probe wavelengths of (a-e) 490 nm,(f) 500 nm and 530 nm.Red lines in (a-e) are their global fitted curves at 0-40 mg/L MoS2.
adding to the CQD solutions,SE weakened and decayed faster.TA traces inFIG.4(f) were single-exponent decay with τSEbeing 1.30 µs and 0.77 µs at 50 mg/L and 100 mg/L MoS2,respectively.
TA spectra of CQDs-MoS2composites revealed their excited-state interactions.As shown inFIG.5,electrons in the highest occupied molecular orbital (HOMO) of CQDs were excited to the lowest unoccupied molecular orbital (LUMO) by absorbing a photon.The electron in LUMO relaxed [36] and produced the intrinsic PL emission (PL1),which extended the SE1 with τSE-390=1.93 µs in the TA plot of CQDs.Defect states could also capture the electron [37],and then downward emited PL2 (defect emission) or upward absorbed another photon,which resulted in SE2τSE=1.78 µs and PIA with τPIA=0.29 µs.

FIG.5 Schematic energy levels of CQDs-MoS2 composites.HOMO: highest occupied molecular orbital,LUMO:lowest unoccupied molecular orbital,Abs: absorption,PL1:intrinsic emission,PL2: defect emission.Path 1: LUMO of CQDs transfers energy to MoS2,Path 2: Defect state of CQDs transfers energy to MoS2.
With adding MoS2to CQD solutions,a spectral overlap occurred between the PL emission of CQDs and the absorption of MoS2(FIG.S4 in SM),which indicated that PL quenching of CQDs might originate from the energy transfer to MoS2[38,39].Two energy transfer pathways existed in the CQDs-MoS2composite.One path was the energy transfer from the LUMO of CQDs to MoS2,which quenched the intrinsic emission PL1 and SE1features in TA spectra [40].The other energy transfer pathway occurred from defect states of CQDs to MoS2,upward absorption and downward defect emission from the defect state were sequentially suppressed [41].The suppressed upward absorption lowered PIA in TA measurements.SE2also decreased with adding MoS2along with quenched PL2.In the CQDs-MoS2composite,LUMO electron populations and CQD defect states were suppressed by energy transfer to MoS2.
IV.CONCLUSION
The excited state interactions in CQD-MoS2composites were investigated by TA spectroscopy.SE1(1.93 µs@390 nm),PIA (0.29 µs),and SE2features(1.76 µs@490 nm) were observed in the TA spectra of CQDs.The PL of CQD solutions quenched with the gradual addition of MoS2.Intrinsic emission was quenched by the energy transfer from the LUMO of CQDs to MoS2.Upward absorptions of defect states were suppressed by another energy transfer from the defect state of CQDs to MoS2,which decreased PIA with a smallerp1.SE2in the CQD-MoS2composite predominated above 20 mg/L MoS2.The second energy transfer process quenched downward defect emission.The exploration of energy transfer dynamics not only offered useful guidance for interactions in complex systems but also applied to optical devices and sensing.
Supplementary materials:The absorption and emission spectra of CQDs and MoS2,pseudo color TA plots of MoS2,TA kinetic trajectory of CQDs-MoS2,fitting curves and parameters are provided.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No.61805134 and No.1197 4229);Applied Basic Research Program in Shanxi Province,China (No.201801D221016 and No.202103021 223254);Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi(No.2020L0235 and No.2021L257);Linfen Key Research and Development Program (No.2028);Graduate Innovation Project in Shanxi Province (No.2022Y498).
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
- Controllable Modulation of Morphology and Property of CsPbCl3 Perovskite Microcrystals by Vapor Deposition Method
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
