ABA调控种子发育的研究进展
2023-11-01宋松泉唐翠芳雷华平费思恬陈海波
宋松泉 唐翠芳 雷华平 费思恬 陈海波



摘 要:种子发育是一个复杂的生物学过程,受各种遗传和外界因素的调节,显著影响农作物特别是禾谷类作物的种子活力和产量与质量。脱落酸(ABA)是调控种子发育和萌发最重要的植物激素之一,其活性水平、信号转导及其LAFL网络在种子发育包括胚胎发生和成熟过程的调控中起关键作用。该文主要综述了近年来ABA调控种子发育的研究取得的重要进展,包括ABA代谢和信号转导对种子发育的调控,ABA与种子成熟转录因子(AFL-B3、FUS3、ABI3、LEC2等)的作用,以及ABA在种子发育中的作用机制,并提出了需要进一步研究的科学问题, 为深入理解种子发育的分子机制提供参考,从而提高种子的活力、产量和质量。
关键词: ABA代谢, 脱落酸, 转录因子网络, 种子发育, 信号转导
中图分类号:Q944
文献标识码:A
文章编号:1000-3142(2023)09-1553-15
收稿日期:2023-05-20
基金项目:国家科技支撑计划项目(2012BAC01B05); 郴州国家可持续发展议程创新示范区建设省级专项(2022sfq06)。
第一作者: 宋松泉(1957-), 博士, 研究员, 研究方向为种子生理与生物技术, (E-mail) sqsong2019@163.com
*通信作者
Research progress on seed development regulated by ABA
SONG Songquan 1,2*, TANG Cuifang 1,3, LEI Huaping 1, FEI Sitian 1, CHEN Haibo 1
( 1. Nanling Research Institute for Modern Seed Industry, Xiangnan University, Chenzhou 423099, Hunan, China; 2. Institute of Botany, Chinese
Academy of Sciences, Beijing 100093, China; 3. College of Life Sciences, Hunan Normal University, Changsha 410081, China )
Abstract:Seed development is a complex biological process that is controlled by various genetic and external factors, and significantly affects the seed vigor, yield and quality of crops, especially cereal plant crops. Abscisic acid (ABA) is one of the most important phytohormones that regulate seed development and germination, and plays a key role in regulation of seed development through its activity level, signaling, and LAFL network, including embryogenesis and maturation process. In recent years, important progresses have been acquired in the research of seed development regulated by ABA. In the present paper, we have mainly reviewed the research achievements in this field, including the regulation of ABA metabolism and signaling on seed development, the action between ABA and transcription factors of seed maturation (AFL-B3, FUS3, ABI3, LEC2, etc.), and the action mechanism of ABA in seed development. In addition, we also propose some scientific questions that need to be further investigated in this field to provide some information for deeplyunderstanding the molecular mechanism of seed development, so as to improve seed vigor and increasing yield and quality.
Key words: abscisic acid (ABA) metabolism, abscisic acid, network of transcription factor, seed development, signaling
在大多數被子植物中,种子是双受精(double fertilization)过程的产物,其中一个精核与卵细胞融合产生二倍体的合子,另一个精核与双核中央细胞融合形成三倍体的初生胚乳核(Baroux & Grossniklaus, 2019)。随后,单细胞合子经过细胞分裂和分化发育成为胚(Verma et al., 2021);而初生胚乳核通过一系列有丝分裂发育成为多核细胞后,细胞化成为胚乳(Li & Berger, 2012; Batista et al., 2019)。种皮由胚珠的珠被发育而成,外珠被形成外种皮,内珠被形成内种皮。种子发育(seed development)过程可分为胚胎发生(embryogenesis)和成熟(maturation)两个主要阶段 (Ali et al., 2022; Kozaki & Aoyanagi, 2022)。胚胎发生包括胚、胚乳和种皮(母体来源)的形成与结构发育,其特征是高度协调的细胞分裂与分化(Kozaki & Aoyanagi, 2022)。种子成熟从胚胎发生结束时开始,当种子在生理上独立于亲本植物时结束(Ali et al., 2022)。种子成熟显著地影响农作物特别是禾谷类作物种子的活力和产量与质量。
种子发育是一个复杂的生物学过程,包括储藏物(如碳水化合物、蛋白和脂类)的积累、耐脱水性(desiccation tolerance)的获得、生长停滞和进入休眠(dormancy) (Bewley et al., 2013; Jo et al., 2019)。研究表明,种子发育受各种遗传和外界因素的调节,其中植物激素在种子发育调控中起关键作用(Shu et al., 2016; Kozaki & Aoyanagi, 2022)。在胚胎发生早期,生长素(auxin)通过影响顶端-基底端极性(apical-basal polarity)的形成和维管发育在拟胚体(embryonic body)建立中起重要作用。细胞分裂素(cytokinin)与生长素一起通过细胞分裂、发育和分化促进生长。油菜素内酯(brassinosteroid)调节胚珠的数量、种子的大小和形状,拮抗ABA的抑制作用也参与种子的萌发(Kozaki & Aoyanagi, 2022)。脱落酸(abscisic acid, ABA)和赤霉素(gibberellin, GA)被认为是拮抗调节种子发育的主要激素(Shu et al., 2016; Sano & Marion-Poll, 2021)。研究表明,GA在种子的正常发育中起重要作用。豌豆(Pisum sativum) GA缺陷突变体不能产生正常的种子(Swain et al., 1997)。豌豆GA 2-氧化酶(GA 2-oxidase, GA2ox)基因在拟南芥(Arabidopsis thaliana)种子中的过表达引起种子败育(seed abortion) (Singh et al., 2010)。番茄(Solanum lycopersicu)果实中GA2ox的过表达导致果实重量、种子数量和萌发率降低(Chen et al., 2016)。核心GA信号转导途径主要由GA受体GID1 (GA INSENSITIVE DWARF1)、DELLA (Asp-Glu-Leu-Leu-Ala)蛋白、F-box蛋白和DELLA调控的靶因子组成(Nelson & Steber, 2016)。当GA缺乏时,DELLA蛋白比较稳定,可抑制GA的反应;当GA存在时,GID1与GA的结合促进GID1-GA-DELLA复合物的形成, 从而促进其与SLY1 (SLEEPY 1)/GID2 F-box蛋白结合和多泛素化DELLA, 并通过26S蛋白酶体靶向降解DELLA。这样就解除了GA反应的DELLA抑制(Nelson & Steber, 2016; 宋松泉等, 2020; Sohn et al., 2021)。在拟南芥DELLA因子中,RGL2 (REPRESSOR OF GA-LIKE2)在抑制种子萌发中起主要作用(Sohn et al., 2021)。
近年来,植物激素ABA在调控种子发育中的研究取得了重要进展(Sano & Marion-Poll, 2021; Smolikova et al., 2021; Ali et al., 2022; Verma et al., 2022)。本文主要综述了本领域的研究成果,包括ABA代谢和信号转导对种子发育的调控,ABA与种子成熟转录因子的作用,以及ABA在种子发育中的作用机制,并提出了在本领域需要进一步研究的科学问题, 为深入理解种子发育的分子机制提供参考,从而提高种子活力和增加产量与质量。
1 ABA代谢与信号转导对种子发育的调控
1.1 种子发育过程中ABA水平的变化
在拟南芥种子发育过程中,整个果实(长角果)和种子中的ABA水平在发育中期(约开花后9 d)达到峰值,随后下降;但果实中的ABA水平在开花后12 d又开始增加直到发育后期(约开花后21 d) (Kanno et al., 2010; Kozaki & Aoyanagi, 2022)。然而,当合子组织缺乏ABA时,母体组织中合成的ABA会被转移到合子组织的胚中(Kanno et al., 2010)。合子组织中合成的ABA的主要作用是诱导和/或维持种子休眠;母体来源的ABA影响拟南芥成熟种子吸胀时释放的黏液层厚度(Kanno et al., 2010)。
在小麦(Triticum aestivum)种子发育过程中,ABA的水平有2个峰值,其中发育后期(授粉后35~40 d)合成的ABA与种子的休眠水平相关(Tuan et al., 2018)。水稻(Oryza sativa)和小黑麦(triticale)种子发育过程中的ABA水平只有一个峰值。在水稻种子中,与休眠诱导有关的ABA积累发生在种子发育的早期和中期(授粉后10~20 d),比小麦种子早(Gu et al., 2011; Liu et al., 2014)。在小黑麦种子中,ABA积累的峰值约为授粉后35 d,在种子水分大量丧失之前(Fidler et al., 2016)。
1.2 ABA代谢对种子发育的调控
活性ABA通过一条间接的途径从叶黄素(xanthophyll) [例如玉米黄质(zeaxanthin)、紫黄质(violaxanthin)和新黄质(neoxanthin)]合成(Marion-Poll & Leung, 2006)。3个关键酶负责ABA生物合成的连续步骤,如玉米黄质环氧化酶(zeaxanthin epoxidase, ZEP)、9-顺式-环氧类胡萝卜素双加氧酶(9-cis-epoxycarotenoid dioxygenase, NCED)和脱落醛氧化酶(abscisic aldehyde oxidase, ABAO) (Dejonghe et al., 2018)。
ZEP基因最初在拟南芥和皱叶烟草(Nicotiana plumbaginifolia)中被鉴定出来。其ABA缺陷突变体(aba1/aba2)在玉米黃质氧化为环氧玉米黄质(antheraxanthin)和紫黄质中受损,这被认为是ABA生物合成的初始步骤(Sano & Marion-Poll, 2021)。在水稻中,在ABA合成过程中玉米黄质的氧化存在缺陷,发现了一个具有胎萌的突变体Tos17(Ali et al., 2022)。通过遗传筛选在玉米(Zea mays)中鉴定的其他ABA营养缺陷型突变体(vp2、vp5、vp7和vp9)存在ZEP活性缺陷,阻碍了类胡萝卜素生物合成的早期步骤(Ali et al., 2022)。综上表明,玉米黄质氧化是植物中ABA合成的一个重要且保守的阶段。目前,从全反式紫黄质(all-trans-violaxanthin)和全反式新黄质(all-trans-neoxanthin)到9-顺式紫黄质(9-cis- violaxanthin)和9-顺式新黄质(9-cis-neoxanthin)的转化还不清楚。然而,North等(2007)发现ABA4负责从全反式紫黄质转化为全反式新黄质,为这些转化的研究提供了一些线索。
ABA生物合成的第二个关键基因NCED最初在玉米胎生突变体vp14 (viviparous 14)中被克隆。vp14突变体在ABA生物合成的步骤中存在9-顺式-环氧类胡萝卜素的氧化缺陷,并在干种子中表现出ABA含量降低(Tan et al., 1997)。在拟南芥中,NCED2、NCED3、NCED5、NCED6和NCED9被认为是VP14的同源基因,参与ABA生物合成的限速步骤(Nambara & Marion-Poll, 2005)。此外,分别从大豆(Glycine max)、番茄和二穗短柄草(Brachypodium distachyon)中鑒定出的PvNCED1、LeNCED1和BdNCED1也在ABA生物合成和种子发育过程中具有重要作用(Barrero et al., 2012)。综上表明,叶黄素的氧化裂解是ABA生物合成的主要步骤,可调节种子的发育。
脱落醛(abscisic aldehyde)的氧化是ABA生物合成的最后步骤,其中脱落醛被氧化成为ABA (Dejonghe et al., 2018)。在番茄中鉴定的脱落醛氧化为ABA的缺陷突变体是flacca和sitiens (Taylor et al., 1988)。在拟南芥中鉴定的脱落醛氧化酶3 (abscisic aldehyde oxidase 3, AAO3),在种子中的ABA生物合成的最后两个步骤中起作用,其表达也在种子成熟中后期的胚维管组织中被观察到(Seo et al., 2004)。
1.3 ABA信号转导对种子发育的调控
核心ABA信号转导组分包括ABA受体PYR/PYL/RCAR (pyrabactin resistance 1/pyrabactin resistance 1-like/regulatory components of ABA receptor)家族、A组2C型蛋白磷酸酶(Group A Type 2C protein phosphatase, PP2C)和蔗糖非发酵-1-相关的蛋白激酶2 (sucrose non-fermenting-1-related protein kinase 2, SnRK2) (Nonogaki, 2019a, b; Lim et al., 2022) (图1)。
在拟南芥中,PYR/PYL/RCAR蛋白家族的14个成员被证明在种子中具有重要作用,例如pyr1/prl1/prl2/prl4四重突变体和pyl十二重突变体表现出种子休眠变弱,对ABA不敏感(Ma et al., 2009; Zhao et al., 2018)。此外,水稻中ospyl七重突变体在种子萌发过程中对ABA不敏感(Miao et al., 2018)。
在ABA缺乏时,PYL蛋白释放PP2C,并激活其磷酸酶功能(Ma et al., 2009)。PP2C蛋白包括ABA不敏感1/2 (ABA-INSENSITIVE 1/2, ABI1/2)和ABA过敏感萌发1/3 (ABA-HYPERSENSITIVE GERMINATION 1/3, AHG1/3),通过蛋白磷酸化抑制下游ABA信号转导蛋白的活性,从而阻断下游ABA信号转导网络的功能(Park et al., 2009)。因此,PP2C在ABA信号转导系统中起负调控因子的作用,而在敲除突变体时则表现出对ABA过敏感和种子休眠减弱(Yoshida et al., 2006)。研究表明,EAR1 (ENHANCER OF ABA CO-RECEPTOR 1)能与PP2C蛋白(即ABI1/2、HAB1/2 (Hypersensitive to ABA 1/2) 和AHG1/3)一起作用来增加PP2C的活性(Wang et al., 2018)。与EAR1一样,PR5K2 (PR5 receptor-like kinase 2)通过增加ABI1/2的磷酸化来抑制ABA信号转导(Baek et al., 2019)。此外,DOG1 (DELAY OF GERMINATION 1)与血红素结合,并与AHG1相互作用以阻止其磷酸酶功能,并增加种子休眠程度(Nishimura et al., 2018)。综上表明,PP2C能够被PYL受体或被其他蛋白调节,但在种子发育过程中PP2C、PYL与其他调控因子(DOG1、PR5K2和EAR1)之间的相互关系尚不清楚。
在ABA存在时,PYR/PYL/RCAR蛋白与ABA和PP2C蛋白结合,以抑制PP2C的磷酸酶活性,从而释放SnRK2并使其发挥功能。研究表明,拟南芥PYL蛋白家族的所有成员都能与PP2C家族成员相互作用,并在ABA介导的反应中起作用(Zhao et al., 2013)。在拟南芥中,总共3种SnRK2 (SnRK2.2、SnRK2.3和SnRK2.6)被发现作为ABA信号转导网络的正调控因子参与种子发育的许多过程,如脱绿(de-greening)、种子储藏产物的积累、耐脱水性的获得和萌发(Finkelstein et al., 2008)。ABA信号转导终止子(ABA signaling terminator, ABT)是一种WD40蛋白,能够有效地阻断ABA信号转导,在种子萌发和幼苗建立中起重要作用。ABT以PYR1/PYL/RCAR-PP2C依赖的方式被ABA诱导,并与PYR1/PYPL/RCAR和PP2C蛋白相互作用,干扰PYR1/4和ABI1/2之间的相互作用,从而阻断ABA信号转导(Wang et al., 2020)。
此外,SnRK2的主要靶点是ABF [ABRE (ABA RESPONSIVE ELEMENT) binding factor]。ABF家族由9个成员组成,包括ABF1、ABF2/AREB1 (ABRE BINDING PROTEIN 1)、ABF3、ABF4/AREB2、AREB3、ABI5、bZIP15、bZIP67和bZIP亚家族EEL,主要参与ABA介导的转录调控(Nakashima et al., 2009)。ABI5的转录能够被SnRK2通过与ABI5启动子中的ABRE顺式元件专一地结合来激活,进而在拟南芥种子成熟后期和吸胀的种子中激活ABA介导的转录活性。此外,另一个关键因子ABI3与ABI5转录因子相互作用,并与ABI5共同作用以促进下游ABA反应基因的转录,这两个基因均能被RAV1 (RELATED TO ABI3/VP1)通过与其启动子结合进行调控(Ali et al., 2022)。有趣的是,ABI5也通过与PYL11和PYL12的启动子结合来调节ABA的反应,从而直接调控萌发过程中的转录。当ABI5突变时,由PYL11和PYL12过表达所引起的ABA过敏感反应被完全或部分受损(Zhao et al., 2020)。
2 ABA与种子成熟转录因子
通过遗传筛选发现,LAFL基因在ABA介导的种子发育中起重要作用。LAFL基因包括AFL-B3 (AFL clade of B3 domain plant-specific transcription factor)、FUS3 (FUSCA3)、ABI3、LEC2 (LEAFY COTYLEDON 2),以及CBF (CCAAT-binding transcription factor)或NF-Y (nuclear factor Y)的HAP3亚基、LEC1和L1L (LEC1-LIKE) (Smolikova et al., 2021; Kozaki & Aoyanagi, 2022) (图2)。LAFL基因的突变影响种子发育的许多方面,如种子成熟时储藏物含量下降,耐脱水性和ABA水平降低以及休眠变弱(Holdsworth et al., 2008; Jia et al., 2014)。除种子发育外,LAFL网络还调控一些与植物发育有关的基因,如锌指因子(zinc finger factor) PEI1、AP2 (APETALA2)家族因子BBM (BABY BOOM)、NAC因子CUC1 (CUP-SHAPED COTYLEDON 1)和MADS box因子FLC (FLOWERING LOCUS C)的基因(Jia et al., 2014)。
AFL因子通过RY顺式元件(RY cis-element)激活靶基因,RY顺式元件被B3-DNA结合结构域识别(Braybrook et al., 2006)。LEC1和L1L作为NF-Y复合物的一个亚基,与CCAAT DNA基序结合(Miller, 2016)。对拟南芥和大豆靶基因上游区域LEC1结合位点的全基因组分析表明,LEC1除了调控CCAAT基序外,还在种子成熟过程中调控基因的启动子中富含G-box、ABRE-like、RY和BPC1顺式元件,表明LEC1通过与一些其他种类的转录因子相互作用来调节靶基因(Jo et al., 2019)。
遗传分析表明,在LAFL基因之间的相互作用比较复杂(图2)。LEC1能够激活ABI3、FUS3和LEC2的表达,而LEC2的异位表达能够上调LEC1、ABI3和FUS3 (To et al., 2006; Stone et al., 2008)。ABI3和FUS3相互正向调控,并调控自身的表达(To et al., 2006; Mnke et al., 2012)。此外,L1L被FUS3调控(Yamamoto et al., 2010)。ChIP (chromatin immunoprecipitation)分析表明,LEC1调控L1L (Junker et al., 2012),而FUS3调控LEC1、FUS3和ABI3 (Wang & Perry, 2013)。
除了LAFL基因外,ABI5及其相关的bZIP转录因子也与ABRE结合,参与种子成熟的调控。ABI5是ABA信号转导的关键参与者(Collin et al., 2021)。种子成熟过程中一组LAFL调控的重要基因包括胚胎发生晚期丰富(LATE EMBRYOGENESIS ABUNDANT, LEA)基因,其启动子中具有RY和ABRE基序,并被ABI3和ABI5相关的bZIP转录因子的组合调控(Alonso et al., 2009)。因此,ABA信号转导被ABI5及其相关的bZIP因子通过与ABI3的N-端COAR (co-activator/co-represso)结构域物理相互作用整合到LAFL网络中(Alonso et al., 2009)。在其他LAFL因子的靶基因启动子中也发现了ABRE,表明LAFL的其他组分可能被ABA共同调控(Junker et al., 2012; Wang & Perry, 2013)。
在拟南芥中,外源ABA增加FUS3的表达(Kagaya et al., 2005),以及FUS3诱导ABA的增加(Gazzarrini et al., 2004)。因此,FUS3和ABA是相互的正调控因子(Braybrook & Harada, 2008)。此外,FUS3的表达也能够被生长素正向调控(Gazzarrini et al., 2004)。
3 ABA在种子发育中的功能
3.1 儲藏物的积累
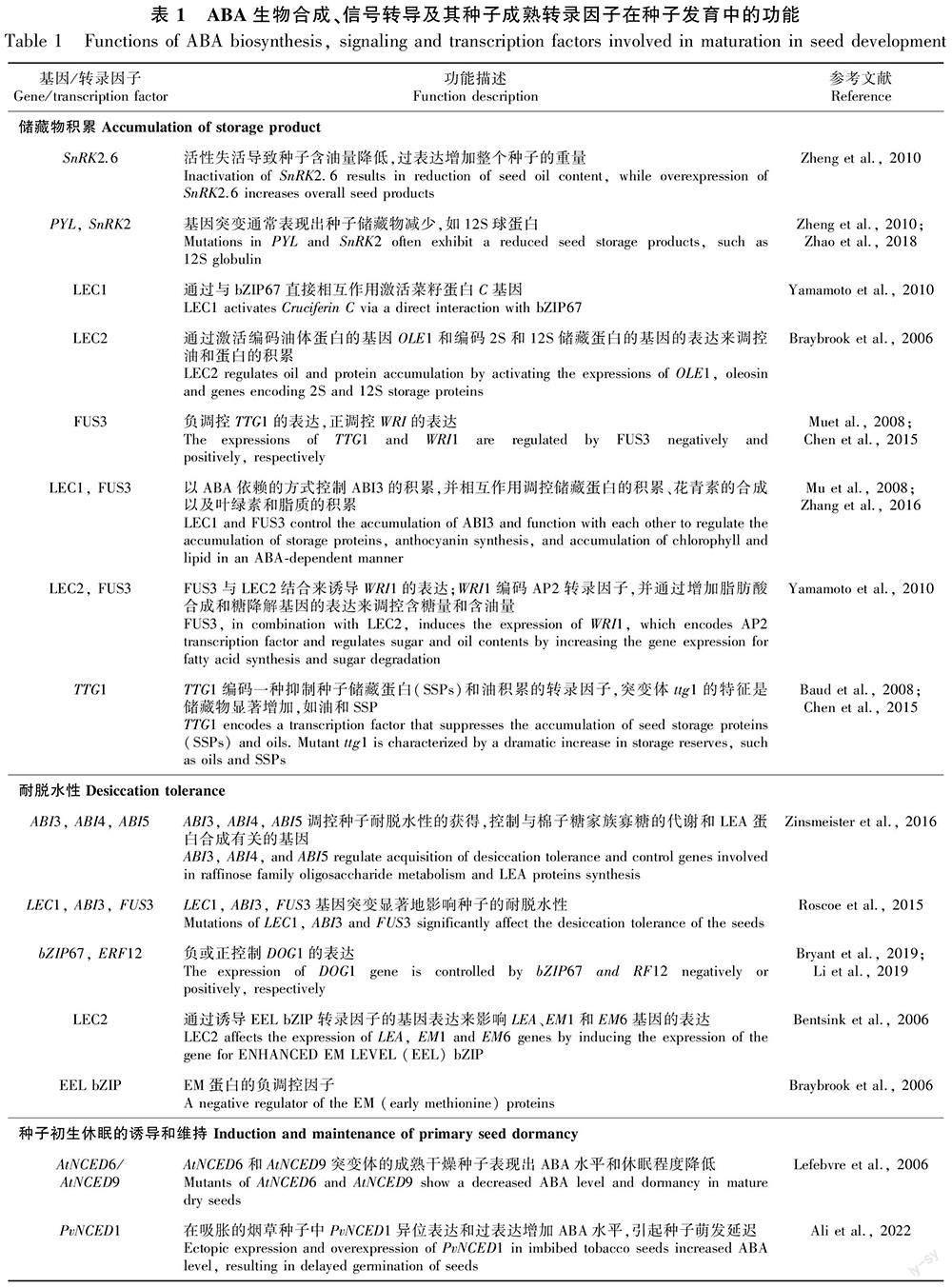
在种子成熟过程中,储藏化合物如种子储藏蛋白(seed storage protein, SSP)、脂质和碳水化合物的积累与ABA的水平和信号转导密切相关(Finkelstein, 2013) (表1)。ABA信号转导组分如PYL和SnRK2的突变通常表现为种子储藏物减少(Nakashima et al., 2009; Zheng et al., 2010; Zhao et al., 2018)。SnRK2.6失活导致种子的含油量降低,而SnRK2.6过表达则增加整个种子的重量(Zheng et al., 2010)。SnRK2三重突变体(snrk2.2/3/6)和pyl十二重突变体通常表现出种子储藏物减少,如12S球蛋白(Nakashima et al., 2009; Zhao et al., 2018)。玉米和水稻中的淀粉生物合成受蔗糖和ABA的协同调控(Huang et al., 2016; Chen et al., 2019)。
LAFL基因参与储藏物积累的调控。LEC1和FUS3在成熟过程中以ABA依赖的方式控制ABI3的积累,并相互作用调控储藏蛋白[包括拟南芥储藏蛋白3 (At2S3)和菜籽蛋白C (cruciferin C, CRC)]的积累、花青素的合成以及叶绿素和脂质的积累(Mu et al., 2008; Zhang et al., 2016)。LEC1通过与bZIP67的直接相互作用激活CRC (Yamamoto et al., 2010)。
FUS3负调控TTG1 (TRANSPARENT TESTA GLABRA1)的表达,TTG1编码一种抑制拟南芥中SSP和油积累的转录因子(Chen et al., 2015)。ttg1突变体的特征是储藏物显著增加,如SSP和油 (Baud et al., 2008)。FUS3可能通过抑制TTG1导致储藏物的积累(Chen et al., 2015)。FUS3与LEC2结合也诱导WRI1 (WRINKLED 1)的表达;WRI1编码AP2转录因子,并通过增加脂肪酸合成和糖降解基因的表达来调控种子中的含糖量和含油量(Yamamoto et al., 2010)。FUS3与抑制TTG1表达和增加WRI1表达一起促进储藏油的积累。这种储藏油的积累通过激活WRI1被LEC1和AFL基因调控(Mu et al., 2008)。此外,LEC2通过激活编码油体蛋白(oleosin)的基因OLE1和编码2S和12S储藏蛋白的基因表达来调控油和蛋白的积累(Braybrook et al., 2006)。
在种子成熟过程中,除了LAFL基因外,其他因子也参与储藏物的积累。bZIP67与L1L和NF-YC2 (NUCLEAR FACTOR-YC2)一起调控FAD3 (FATTY ACID DESATURASE 3),该酶在种子成熟期间对ω-3脂肪酸的储藏具有一定作用(Mendes et al., 2013)。DOGL4 (DOG1-LIKE4)基因的表达被ABA诱导,在种子成熟过程中调控一些种子储藏蛋白的表达,包括CRC、白蛋白和油体蛋白(Sall et al., 2019)。
3.2 耐脱水性的获得
种子的耐脱水性是植物在长期进化过程中保证物种生存和繁衍的适应性机制,在农作物种子保存和植物种质资源长期保存中起关键作用(Smolikova et al., 2021; 宋松泉等, 2022)。种子的耐脱水性机制在种子成熟后期被激活,以及与LEA蛋白、小分子量热休克蛋白(small heat shock protein, sHSP)、非还原性寡糖和不同化学性质的抗氧化物的积累有关(Smolikova et al., 2021; 宋松泉等, 2022)。成熟和耐脱水性的主要调控因子是ABA和DOG1蛋白,它们控制转录因子网络,包括LEC1、LEC2、FUS3、ABI3、ABI5、AGL67、PLATZ1、PLATZ2 (Smolikova et al., 2021) (图2)。
LEA基因的表达被ABI3和ABI5调控(Bies-Ethève et al., 2008)。ABI3也调控种子专一的热休克因子(heat shock factor) HSFA9的表达(Kotak et al., 2007)。LEA和HSP基因的表达被DOG1通过ABI5/ABI3增加;以及它们的表达增加种子中含N化合物的储藏,从而促进种子休眠和提高种子生活力(Dekkers et al., 2016)。研究表明,在种子成熟过程中DOG1的表达分别被bZIP67和ERF12 (ETHYLENE RESPONSE FACTOR 12)负调控或正调控(Bryant et al., 2019; Li et al., 2019)。在蒺藜苜蓿(Medicago truncatula)和豌豆中,ABI3、ABI4和ABI5被认为是调控种子耐脱水性获得的主要中枢,以调控与棉子糖家族寡糖(raffinose family oligosaccharide, RFO)代谢和LEA蛋白合成有关的基因(Zinsmeister et al., 2016) (表1)。
LEC1、ABI3或FUS3的突变显著地影响种子的耐脱水性,表明激活种子的耐脱水性都需要这3种转录因子的(Roscoe et al., 2015)。LEC2通过诱导EEL (ENHANCED EM LEVEL) bZIP转录因子的基因表达来影响LEA、EM1 (THE EARLY METHIONINE 1)和EM6基因的表达,从而参与耐脱水性的建立(Bentsink et al., 2006)。EEL bZIP转录因子是拟南芥中EM蛋白的负调控因子(Braybrook et al., 2006)。
3.3 种子初生休眠的诱导与维持
休眠是一种暂时的静止状态,是野生植物种子在不利环境条件下避免萌发和确保下一代繁衍的重要特征;而对于栽培作物,具有迅速和整齐萌发的种子被选择以获得作物高产与优质。此外,种子休眠特别是收获休眠(harvest dormancy)的缺乏是不理想的农艺性状,因为它可能导致收获前萌发(preharvest sprouting, PHS),这是禾谷类作物栽培中所面临的严重问题,以及非休眠突变体可能降低种子的寿命 (Finkelstein et al., 2008; Tuanet al., 2018)。种子在储藏物合成后和成熟结束时开始脱水,并储存新合成的ABA,进入休眠。一些证据表明,ABA是這些过程的关键调控因子(Finkelstein et al., 2008; Nambara et al., 2010)。ABA生物合成、感知和信号转导的突变影响种子休眠(Nakashima et al., 2009; Zhao et al., 2018) (表1)。
拟南芥AtNCED6和AtNCED9突变体的成熟干燥种子表现出ABA水平和休眠程度降低(Lefebvre et al., 2006),其他的ABA缺陷突变体,如aba1和aba2/3,也显示出休眠水平降低(Kozaki & Aoyanagi, 2022)。拟南芥ODR1 [suppressor of RDO5 (REDUCED DORMANCY 5)]与bHLH57一起作用,并在NCED6和NCED9的上游起作用,控制ABA合成与种子休眠(Liu et al., 2020)。大豆PvNCED1基因在吸胀的烟草(Nicotiana tabacum)种子中异位表达和过表达提高了ABA水平,并引起种子萌发延迟。在番茄中,LeNCED1的过表达也通过提高种子中的ABA水平来增加休眠(Ali et al., 2022)。在小麦中,2个TaABA8′OH1同源基因(TaABA8′OH1A和TaABA8′-OH1D;AtCYP707的同源基因)的突变导致ABA含量和休眠程度的增加(Chono et al., 2013)。TsNCED1也与较高的ABA含量和PHS抗性增加有关(Fidler et al., 2016)。ABA信号转导组分的突变,如水稻ospyl七重突变体和snrk2.2/3/6三重突变体,也导致水稻和拟南芥种子的成熟前萌发(Nakashima et al., 2009; Miao et al., 2018)。
在拟南芥中,AtMYB96直接激活ABA合成基因(NCED2、NCED5、NCED6和NCED9)和失活GA生物合成基因(AtGA3ox1和AtGA20ox1)来诱导种子的初生休眠(Lee et al., 2015)。AtABI4通过直接与AtNECD6的启动子区域相互作用增加ABA的生物合成,与GA失活基因AtGA2ox7的启动区域相互作用抑制GA的积累来增加种子休眠(Shu et al., 2013, 2016)。
LAFL基因的成员也参与休眠的获得。成熟种子中胚的生长停滞由FUS3、LEC1和LEC2控制,它们的突变体都不能完全使胚的生长停止,并表现出成熟前萌发(Gubler et al., 2005)。玉米VP1基因是拟南芥ABI3的同源基因,是最早鉴定和表征的一种ABA信号转导的关键组分。VP1突变导致玉米收获前萌发和胚的成熟中断。小麦、水稻和高粱(Sorghum bicolor)的VP1基因也与休眠的水平以及对ABA和收获前萌发的敏感性有关(Kozaki & Aoyanagi, 2022)。在玉米中,LAFL基因的成员被VP8 (编码一种假定的肽酶)调控(Suzuki et al., 2008)。水稻中VP8同源基因PLA3 (PLATOCHRON 3/GO (COLIATH))和拟南芥中AMP1 (ALTERDMERISTEM PROGRAM 1)的突变表现出休眠变弱(Griffifiths et al., 2011)。ABI5在小麦和豌豆种子成熟过程中也具有重要的休眠诱导作用(Zinsmeister et al., 2016; Yamasaki et al., 2017; Utsugi et al., 2020)。在高粱中,SbABI4和SbABI1通过直接与SbGA2ox3的启动子结合增强其转录,从而延长种子休眠(Cantoro et al., 2013)。
DOG1和RDO5已经被鉴定是两个主要的休眠基因,似乎独立于植物激素包括ABA起作用 (Bentsink et al., 2006; Xiang et al., 2014; Carrillo-Barral et al., 2020)。RDO5是PP2C蛋白磷酸酶家族的一个成员,但不表现出磷酸酶活性(Xiang et al., 2014),而DOG1是一个功能未知的蛋白(Carrillo-Barral et al., 2020)。DOG1和RDO5的突变分别完全解除或减少种子休眠(Bentsink et al., 2006; Xiang et al., 2014)。遗传分析表明,DOG1和ABA对于正常的种子休眠都是必需的(Bentsink et al., 2006; Nakabayashi et al., 2012)。
DOG1与4种磷酸酶相互作用,其中2种属于A分支2C型蛋白磷酸酶,即AHG1和AHG3(图1)。ABA途径和DOG1途径在PP2C磷酸酶水平上汇合:DOG1抑制AHG1和AHG3,而ABA抑制其他的PP2C磷酸酶和AHG3。通过抑制PP2C磷酸酶,ABA和DOG1促进和维持种子休眠(Antoni et al., 2012; Née et al., 2017)。DOG1也是种子成熟的许多过程所必需的,部分是通过干扰ABA信号转导组分(Dekkers et al., 2016)。
OsSDR4 (SEED DORMANCY 4)被认为是一种与种子休眠有关的调控因子,在水稻中具有未知的功能(Sugimoto et al., 2010)。在拟南芥中,AtSDR4L (SDR4-LIKE)通过调节DOG1和GA途径中的RGA-LIKE2 (编码DELLA蛋白RGL2)来调控休眠释放和萌发(Cao et al., 2019)。Liu等(2020)推测,AtODR1 (用于逆转rdo5)是OsSDR4的一个直系同源基因,与bHLH57一起在AtNCED6和AtNCED9的上游起作用,以控制拟南芥中的ABA合成和种子休眠。
3.4 种子脱绿
在种子成熟过程中,SnRK2和ABI3基因被鉴定为脱绿过程的重要组分(Delmas et al., 2013)。snrk2.2/snrk2.3/snrk2.6三重突变体在种子发育过程中表现为对ABA不敏感,并产生绿色种子(Nakashima et al., 2009; Zhao et al., 2018)。研究发现,拟南芥abi3-6突变体表现出缺乏脱绿,ABI3通过调控SGR (STAY GREEN, AtSGR1和AtSGR)基因的表达来控制胚的脱绿,这些基因是由Mendel I位点编码的SGR基因的同源基因(Armstead et al., 2007; Delmas et al., 2013)。ABI5也调控豆科植物种子的脱绿和种子寿命(Verdier et al., 2013; Zinsmeister et al., 2016)。
4 结束语
种子发育是一个复杂的过程,包括胚胎发生和成熟阶段,主要特征是储藏物的积累、耐脱水性的获得、生长停滞和获得休眠,并显著地影响种子活力和产量与质量(Kozaki & Aoyanagi, 2022)。植物激素ABA对种子发育的调控主要是通过ABA代谢、信号转导及其LAFL网络实现的(Sano & Marion-Poll, 2021; Ali et al., 2022)。尽管近年来ABA调控种子发育的研究已取得了重要进展,但是仍然有一些重要的科学问题尚不清楚。例如,內源ABA水平的调节通过类胡萝卜素途径合成,通过8′-羟基化作用(8′-hydroxylation)失活;ABA葡糖基转移酶(ABA glucosyltransferase)能将ABA转化成为ABA-葡糖酯(ABA-glucose ester, ABA-GE),作为ABA的储存池;ABA-GE又能被β-葡糖苷酶(β-glucosidase)水解成为ABA和葡萄糖(Sano & Marion-Poll, 2021)。这些酶及其基因如何响应发育或者环境变化以维持种子发育所需的正常ABA水平尚不清楚。
種子成熟和耐脱水性的主要调控因子是ABA和DOG1蛋白,它们控制转录因子网络,如LEC1、LEC2、FUS3、ABI3、ABI5、AGL67、PLATZ1、PLATZ2 (Smolikova et al., 2021)。核心ABA途径和DOG1途径在PP2C汇合。值得注意的是,在整合发育条件或者环境信号时PP2C优先响应哪一条途径,以及这两条途径怎样协调也还不够清楚。虽然DOG1是种子休眠的主要调控因子之一,但其分子功能仍然没有被确定(Nonogaki, 2019a; Sano & Marion-Poll, 2021)。
在种子成熟过程中,GA的水平被FUS3和LEC2下调,从而抑制与生物活性GA合成有关的酶(Kozaki & Aoyanagi, 2022)。在拟南芥中,GA信号转导通过激活LEC1以增加胚胎发生晚期生长素的积累来促进胚的发育。GA信号转导抑制因子DELLA与LEC1相互作用,从而促进YUC (YUCCA)基因的表达,并通过增加生长素的积累来促进胚胎发生。GA触发DELLA的降解,以解除其对LEC1的抑制,导致激活胚胎发生所必需的基因(Hu et al., 2018)。然而,GA在胚胎发生中的详细功能尚不清楚(Kozaki & Aoyanagi, 2022)。生长素促进ABI3的表达,ABI3通过激活ARF (AUXIN RESPONSE FACTOR)基因诱导胚胎同一性基因(Kozak & Aoyanagi, 2022)。同样,生长素通过诱导ABI3表达来刺激ABA信号转导,从而控制种子休眠(Liu et al., 2013)。因此,其他植物激素及其与ABA的相互作用对种子发育的调控值得进一步研究。
目前,虽然许多参与种子成熟的转录因子已经在分子和遗传水平上被鉴定和表征,但对早期胚胎发生的转录调控研究仍然很少。此外,转录因子的活性被一些遗传和表观遗传因素的严格控制,然而对这些因素的了解也不完整(Verma et al., 2022)。这些问题的深入研究将有助于理解种子发育的分子机制,从而为提高种子活力和增加产量与质量提供新知识和新技术。
参考文献:
ALI F, QANMBER G, LI F, et al., 2022. Updated role of ABA in seed maturation, dormancy, and germination[J]. J Adv Res, 35: 199-214.
ALONSO R, OATR-SNCHEZ L, WELTMEIER F, et al., 2009. A pivotal role of the basic leucine zipper transcription factor bZIP53 in the regulation of Arabidopsis seed maturation gene expression based on heterodimerization and protein complex formation[J]. Plant Cell, 21(6): 1747-1761.
ANTONI R, GONZALEZ-GUZMAN M, RODRIGUEZ L, et al., 2012. Selective inhibition of clade A phosphatases type 2C by PYR/PYL/RCAR abscisic acid receptors[J]. Plant Physiol, 158(2): 970-980.
ARMSTEAD I, DONNISON I, AUBRY S, et al., 2007. Cross-species identification of Mendel’s I locus[J]. Science, 315(5808): 73.
BAEK D, KIM MC, KUMAR D, et al., 2019. AtPR5K2, a PR5-like receptor kinase, modulates plant responses to drought stress by phosphorylating protein phosphatase 2Cs[J]. Front Plant Sci, 10: 1146.
BAROUX C, GROSSNIKLAUS U, 2019. Seeds — An evolutionary innovation underlying reproductive success in flowering plants[M]. Curr Top Dev Biol, 131: 605-642.
BARRERO JM, JACOBSEN JV, TALBOT MJ, et al., 2012. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon[J]. New Phytol, 193(2): 376-86.
BATISTA RA, MORENO-ROMERO J, QIU Y, et al., 2019. The MADS-box transcription factor PHERES1 controls imprinting in the endosperm by binding to domesticated transposons[J]. eLife, 8: e50541.
BAUD S, DUBREUCQ B, MIQUEL M, et al., 2008. Storage reserve accumulation in Arabidopsis: Metabolic and developmental control of seed filling[J]. Arab Book Am Soc Plant Biol, 6: e0113.
BENTSINK L, JOWETT J, HANHART CJ, et al., 2006. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis[J]. Proc Natl Acad Sci USA, 103(45): 17042-17047.
BEWLEY JD, BRADFORD KJ, HILHORST HWM, et al., 2013. Seed: physiology of development, germination and dormancy[M]. 3rd ed. New York: Springer.
BIES-ETHVE N, GAUBIER-COMELLA P, DEBURES A, et al., 2008. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana[J]. Plant Mol Biol, 67(1/2): 107-124.
BRAYBROOK SA, HARADA JJ, 2008. LECs go crazy in embryo development[J]. Trends Plant Sci, 13(12): 624-630.
BRAYBROOK SA, STONE SL, PARK S, et al., 2006. Genes directly regulated by Leafy Cotyledon 2 provide insight into the control of embryo maturation and somatic embryogenesis[J]. Proc Natl Acad Sci USA, 103(9): 3468-3473.
BRYANT FM, HUGHES D, HASSANI-PAK K, et al., 2019. Basic LEUCINE ZIPPER TRANSCRIPTION FACTOR 67 transactivates DELAY OF GERMINATION1 to establish primary seed dormancy in Arabidopsis[J]. Plant Cell, 31(6): 1276-1288.
CANTORO R, CROCCO CD, BENECH-ARNOLD RL, et al., 2013. In vitro binding of Sorghum bicolor transcription factors ABI4 and ABI5 to a conserved region of a GA 2-OXIDASE promoter: Possible role of this interaction in the expression of seed dormancy[J]. J Exp Bot, 64(18): 5721-5735.
CAO H, HAN Y, LI J, et al., 2019. Arabidopsis thaliana SEED DORMANCY 4-LIKE regulates dormancy and germination by mediating the gibberellin pathway[J]. J Exp Bot, 71(3): 919-933.
CARRILLO-BARRAL N, DEL CARMEN RODRGUEZ-GACIO M, MATILLA AJ, 2020. Delay of germination 1 (DOG1): A key to understanding seed dormancy[J]. Plants, 9(4): 480.
CHEN T, LI G, ISLAM MR, et al., 2019. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship[J]. BMC Plant Biol, 19(1): 525.
CHEN S, WANG X, ZHANG L, et al., 2016. Identification and characterization of tomato gibberellin 2-oxidases (GA2oxs) and effects of fruit-specific SlGA2ox1 overexpression on fruit and seed growth and development[J]. Hortic Res, 3: 16059.
CHEN M, ZHANG B, LI C, et al., 2015. TRANSPARENT TESTA GLABRA 1 regulates the accumulation of seed storage reserves in Arabidopsis[J]. Plant Physiol, 169(1): 391-402.
CHONO M, MATSUNAKA H, SEKI M, et al., 2013. Isolation of a wheat (Triticum aestivum L.) mutant in ABA 8′-hydroxylase gene: Effect of reduced ABA catabolism on germination inhibition under field condition[J]. Breed Sci, 63(1): 104-115.
COLLIN A, DASZKOWSKA-GOLEC A, SZAREJKO I, 2021. Updates on the role of ABSCISIC ACID INSENSITIVE 5 (ABI5) and ABSCISIC ACID-RESPONSIVE ELEMENT BINDING FACTORs (ABFs) in ABA signaling in different developmental stages in plants[J]. Cells, 10(8): 1996.
DEJONGHE W, OKAMOTO M, CUTLER SR, 2018. Small molecule probes of ABA biosynthesis and signaling[J]. Plant Cell Physiol, 59(8): 1490-1499.
DEKKERS BJ, HE H, HANSON J, et al., 2016. The Arabidopsis DELAY OF GERMINATION 1 gene affects ABSCISIC ACID INSENSITIVE 5 (ABI5) expression and genetically interacts with ABI3 during Arabidopsis seed development[J]. Plant J, 85(4): 451-465.
DELMAS F, SANKARANARAYANAN S, DEB S, et al., 2013. ABI3 controls embryo degreening through Mendel’s I locus[J]. Proc Natl Acad Sci USA, 110(40): E3888-E3894.
FIDLER J, ZDUNEK-ZASTOCKA E, PRABUCKA B, et al., 2016. Abscisic acid content and the expression of genes related to its metabolism during maturation of triticale grains of cultivars differing in pre-harvest sprouting susceptibility[J]. J Plant Physiol, 207: 1-9.
FINKELSTEIN R, 2013. Abscisic acid synthesis and response[J]. Arab Book, 11: e0166.
FINKELSTEIN R, REEVES W, ARIIZUMI T, et al., 2008. Molecular aspects of seed dormancy[J]. Ann Rev Plant Biol, 59: 387-415.
GAZZARRINI S, TSUCHIYA Y, LUMBA S, et al., 2004. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid[J]. Dev Cell, 7(3): 373-385.
GRIFFIFITHS J, BARRERO JM, TAYLOR J, et al., 2011. ALTERED MERISTEM PROGRAM 1 is involved in development of seed dormancy in Arabidopsis[J]. PLoS ONE, 6: e20408.
GU XY, FOLEY ME, HORVATH DP, et al., 2011. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice[J]. Genetics, 189(4): 1515-1524.
GUBLER F, MILLAR AA, JACOBSEN JV, 2005. Dormancy release, ABA and pre-harvest sprouting[J]. Curr Opin Plant Biol, 8(2): 183-187.
HOLDSWORTH MJ, BENTSINK L, SOPPE WJ, 2008. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination[J]. New Phytol, 179(1): 33-54.
HU Y, ZHOU L, HUANG M, et al., 2018. Gibberellins play an essential role in late embryogenesis of Arabidopsis[J]. Nat Plants, 4(5): 289-298.
HUANG H, XIE S, XIAO Q, et al., 2016. Sucrose and ABA regulate starch biosynthesis in maize through a novel transcription factor, ZmEREB156[J]. Sci Rep, 6: 27590.
JIA H, SUZUKI M, MCCARTY DR, 2014. Regulation of the seed to seedling developmental phase transition by the LAFL and VAL transcription factor networks[J]. Wiley Interdiscipl Rev Dev Biol, 3(1): 135-145.
JO L, PELLETIER JM, HARADA JJ, 2019. Central role of the LEAFY COTYLEDON 1 transcription factor in seed development[J]. J Integr Plant Biol, 61(5): 564-580.
JUNKER A, MNKE G, RUTTEN T, et al., 2012. Elongation-related functions of LEAFY COTYLEDON 1 during the development of Arabidopsis thaliana[J]. Plant J, 71(3): 427-442.
KAGAYA Y, OKUDA R, BAN A, et al., 2005. Indirect ABA-dependent regulation of seed storage protein genes by FUSCA3 transcription factor in Arabidopsis[J]. Plant Cell Physiol, 46(2): 300-311.
KANNO Y, JIKUMARU Y, HANADA A, et al., 2010. Comprehensive hormone profiling in developing Arabidopsis seeds: Examination of the site of ABA biosynthesis, ABA transport and hormone interactions[J]. Plant Cell Physiol, 51(12): 1988-2001.
KOTAK S, VIERLING E, BAUMLEIN H, et al., 2007. A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis[J]. Plant Cell, 19(1): 182-195.
KOZAKI A, AOYANAGI T, 2022. Molecular aspects of seed development controlled by gibberellins and abscisic acids[J]. Int J Mol Sci, 23(3): 1876.
LEE HG, LEE K, SEO PJ, 2015. The Arabidopsis MYB96 transcription factor plays a role in seed dormancy[J]. Plant Mol Biol, 87(4/5): 371-381.
LEFEBVRE V, NORTH H, FREY A, et al., 2006. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy[J]. Plant J, 45(3): 309-319.
LI J, BERGER F, 2012. Endosperm: Food for humankind and fodder for scientific discoveries[J]. New Phytol, 195(2): 290-305.
LI XY, CHEN TT, LI Y, et al., 2019. ETR1/RDO3 regulates seed dormancy by relieving the inhibitory effect of the ERF12-TPL complex on DELAY OF GERMINATION 1 expression[J]. Plant Cell, 31(4): 832-847.
LIM J, LIM CW, LEE SC, 2022. Core components of abscisic acid signaling and their post-translation modification[J]. Front Plant Sci, 13: 895698.
LIU Y, FANG J, XU F, et al., 2014. Expression patterns of ABA and GA metabolism genes and hormone levels during rice seed development and imbibition: A comparison of dormant and non-dormant rice cultivars[J]. J Genet Genom, 41(6): 327-338.
LIU F, ZHANG H, DING L, et al., 2020. REVERSAL OF RDO5 1, a homolog of rice seed dormancy 4, interacts with bHLH57 and controls ABA biosynthesis and seed dormancy in Arabidopsis[J]. Plant Cell, 32(6): 1933-1948.
LIU X, ZHANG H, ZHAO Y et al., 2013. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis[J]. Proc Natl Acad Sci USA, 110(38): 15485-15490.
MA Y, SZOSTKIEWICZ I, KORTE A, et al., 2009. Regulators of PP2C phosphatase activity function as abscisic acid sensors[J]. Science, 324(5930): 1064-1068.
MARION-POLL A, LEUNG J, 2006. Abscisic acid synthesis, metabolism and signal transduction[M]//HEDDEN P, THOMAS SG. Plant hormone signalling. Ann Plant Rev, 24: 1-35.
MENDES A, KELLY AA, VAN ERP H, et al., 2013. bZIP67 regulates the omega-3 fatty acid content of Arabidopsis seed oil by activating fatty acid desaturase 3[J]. Plant Cell, 25(8): 3104-3116.
MIAO C, XIAO L, HUA K, et al., 2018. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity[J]. Proc Natl Acad Sci USA, 115(23): 6058-6063.
MILLER M, 2016. Interactions of CCAAT/enhancer-binding protein β with transcriptional co-regulators[J]. Postepy Biochem, 62: 343-348.
MNKE G, SEIFERT M, KEILWAGEN J, et al., 2012. Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon[J]. Nucl Acids Res, 40(17): 8240-8254.
MU J, TAN H, ZHENG Q, et al., 2008. LEAFY COTYLEDON 1 is a key regulator of fatty acid biosynthesis in Arabidopsis[J]. Plant Physiol, 148(2): 1042-1054.
NAKABAYASHI K, BARTSCH M, XIANG Y, et al., 2012. The time required for dormancy release in Arabidopsis is determined by DELAY OF GERMINATION 1 protein levels in freshly harvested seeds[J]. Plant Cell, 24(7): 2826-2838.
NAKASHIMA K, FUJITA Y, KANAMORI N, et al., 2009. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy[J]. Plant Cell Physiol, 50(7): 1345-1363.
NAMBARA E, MARION-POLL A, 2005. Abscisic acid biosynthesis and catabolism[J]. Ann Rev Plant Biol, 56: 165-185.
NAMBARA E, OKAMOTO M, TATEMATSU K, et al., 2010. Abscisic acid and the control of seed dormancy and germination[J]. Seed Sci Res, 20(2): 55-67.
NE G, KRAMER K, NAKABAYASHI K, et al., 2017. DELAY OF GERMINATION 1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy[J]. Nat Commun, 8: 72.
NELSON SK, STEBER CM, 2016. Gibberellin hormone signal perception: Down-regulating DELLA repressors of plant growth and development[M]//HEDDEN P, THOMAS SG. The gibberellins. Ann Plant Rev, 49: 153-187.
NISHIMURA N, TSUCHIYA W, MORESCO JJ, et al., 2018. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme[J]. Nat Commun, 9: 2132.
NONOGAKI H, 2019a. Seed germination and dormancy: The classic story, new puzzles, and evolution[J]. J Integr Plant Biol, 61(5): 541-563.
NONOGAKI H, 2019b. ABA responses during seed development and germination[J]. Adv Bot Res, 92: 171-217.
NORTH HM, DE ALMEIDA A, BOUTIN JP, et al., 2007. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers[J]. Plant J Cell Mol Biol, 50(5): 810-824.
PARK SY, FUNG P, NISHIMURA N, et al., 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins[J]. Science, 324(5930): 1068-1071.
ROSCOE TT, GUILLEMIINOT J, BESSOULE JJ, et al., 2015. Complementation of seed maturation phenotypes by ectopic expression of ABSCISIC ACID INSENSITIVE 3, FUSCA3 and LEAFY COTYLEDON 2 in Arabidopsis[J]. Plant Cell Physiol, 56(6): 1215-1228.
SALL K, DEKKERS BJW, NONOGAKI M, et al., 2019. DELAY OF GERMINATION 1-LIKE 4 acts as an inducer of seed reserve accumulation[J]. Plant J, 100(1): 7-19.
SANO N, MARION-POLL A, 2021. ABA metabolism and homeostasis in seed dormancy and germination[J]. Int J Mol Sci, 22(10): 5069.
SEO M, AOKI H, KOIWAI H, et al., 2004. Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds[J]. Plant Cell Physiol, 45(11): 1694-1703.
SHU K, CHEN Q, WU Y, et al., 2016. ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels[J]. Plant J, 85(3): 348-361.
SHU K, ZHANG H, WANG S, et al., 2013. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis[J]. PLoS Genet, 9(6): e1003577.
SINGH DP, FILARDO FF, STOREY R, et al., 2010. Overexpression of a gibberellin inactivation gene alters seed development, KNOX gene expression, and plant development in Arabidopsis[J]. Physiol Plant, 138(1): 74-90.
SMOLIKOVA G, LEONOVA T, VASHURINA N, et al., 2021. Desiccation tolerance as the basis of long-term seed viability[J]. Int J Mol Sci, 22(1): 101.
SOHN SI, PANDIAN S, KUMAR TS, et al., 2021. Seed dormancy and pre-harvest sprouting in rice — An updated overview[J]. Int J Mol Sci, 22(21): 11804.
SONG SQ, LIU J, HUANG H, et al., 2020. Gibberellin metabolism and signaling and its molecular mechanism in regulating seed germination and dormancy[J]. Sci Sin Vitae, 50(6): 599-615.[宋松泉, 劉军, 黄荟, 等, 2020. 赤霉素代谢与信号转导及其调控种子萌发与休眠的分子机制[J]. 中国科学: 生命科学, 50(6): 599-615.]
SONG SQ, LIU J, TANG CF, et al., 2022. Research progress on the physiology and its molecular mechanism of seed desiccation tolerance[J]. Sci Agric Sin, 55(6): 1047-1063.[宋松泉, 刘军, 唐翠芳, 等, 2022. 种子耐脱水性的生理及分子机制研究进展[J]. 中国农业科学, 55(6): 1047-1063.]
STONE SL, BRAYBROOK SA, PAULA SL, et al., 2008. Arabidopsis LEAFY COTYLEDON 2 induces maturation traits and auxin activity: Implications for somatic embryogenesis[J]. Proc Natl Acad Sci USA, 105(8): 3151-3156.
SUGIMOTO K, TAKEUCHI Y, EBANA K, et al., 2010. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice[J]. Proc Natl Acad Sci USA, 107(13): 5792-5797.
SUZUKI M, LATSHAW S, SATO Y, et al., 2008. The maize Viviparous 8 locus, encoding a putative ALTERED MERISTEM PROGRAM1-like peptidase, regulates abscisic acid accumulation and coordinates embryo and endosperm development[J]. Plant Physiol, 146(3): 1193-1206.
SWAIN SM, REID JB, KAMIYA Y, 1997. Gibberellins are required for embryo growth and seed development in pea[J]. Plant J, 12(6): 1329-1338.
TAN BC, SCHWARTZ SH, ZEEVAART JA, et al., 1997. Genetic control of abscisic acid biosynthesis in maize[J]. Proc Natl Acad Sci USA, 94(22): 12235-12240.
TAYLOR IB, LINFORTH RST, AL-NAIEB RJ, et al., 2010. The wilty tomato mutants flacca and sitiens are impaired in the oxidation of ABA aldehyde to ABA[J]. Plant Cell Environ, 11(8): 739-745.
TO A, VALON C, SAVION G, et al., 2006. A network of local and redundant gene regulation governs Arabidopsis seed maturation[J]. Plant Cell, 18(7): 1642-1651.
TUAN PA, KUMAR R, REHAL PK, et al., 2018. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals[J]. Front Plant Sci, 9: 668.
UTSUGI S, ASHIKAWA I, NAKAMURA S, et al., 2020. TaABI5, a wheat homolog of Arabidopsis thaliana ABA insensitive 5, controls seed germination[J]. J Plant Res, 133(2): 245-256.
VERDIER J, LALANNE D, PELLETIER S, et al., 2013. A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of Medicago truncatula seeds[J]. Plant Physiol, 163(2): 757-774.
VERMA S, ATTULURI VPS, ROBERT HS, 2021. An essential function for auxin in embryo development[J]. Cold Spring Harbor Perspect Biol, 13(4): a039966.
VERMA S, ATTULURI VPS, ROBERT HS, 2022. Transcriptional control of Arabidopsis seed development[J]. Planta, 255(4): 90.
WANG K, HE J, ZHAO Y, et al., 2018. EAR1 negatively regulates ABA signaling by enhancing 2C protein phosphatase activity[J]. Plant Cell, 30(4): 815-834.
WANG F, PERRY SE, 2013. Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development[J]. Plant Physiol, 161(3): 1251-1264.
WANG Z, REN Z, CHENG C, et al., 2020. Counteraction of ABA mediated inhibition of seed germination and seedling establishment by ABA signaling terminator in Arabidopsis[J]. Mol Plant, 13(9): 1284-1297.
XIANG Y, NAKABAYASHI K, DING J, et al., 2014. REDUCED DORMANCY 5 encodes a protein phosphatase 2C that is required for seed dormancy in Arabidopsis[J]. Plant Cell, 26(11): 4362-4375.
YAMAMOTO A, KAGAYA Y, USUI H, et al., 2010. Diverse roles and mechanisms of gene regulation by the Arabidopsis seed maturation master regulator FUS3 revealed by microarray analysis[J]. Plant Cell Physiol, 51(12): 2031-2046.
YAMASAKI Y, GAO F, JORDAN MC, et al., 2017. Seed maturation associated transcriptional programs and regulatory networks underlying genotypic difference in seed dormancy and size/weight in wheat (Triticum aestivum L.)[J]. BMC Plant Biol, 17: 154.
YOSHIDA T, NISHIMURA N, KITAHATA N, et al., 2006. ABA hypersensitive germination 3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs[J]. Plant Physiol, 140(1): 115-126.
ZHANG YQ, LU X, ZHAO FY, et al., 2016. Soybean GmDREBL increases lipid content in seeds of transgenic Arabidopsis[J]. Sci Rep, 6: 34307.
ZHAO Y, CHAN Z, XING L, et al., 2013. The unique mode of action of a divergent member of the ABA-receptor protein family in ABA and stress signaling[J]. Cell Res, 23(12): 1380-1395.
ZHAO H, NIE K, ZHOU H, et al., 2020. ABI5 modulates seed germination via feedback regulation of the expression of the PYR/PYL/RCAR ABA receptor genes[J]. New Phytol, 228(2): 596-608.
ZHAO Y, ZHANG Z, GAO J, et al., 2018. Arabidopsis duodecuple mutant of PYL ABA receptors reveals PYL repression of ABA-independent SnRK2 activity[J]. Cell Rep, 23(11): 3340-3351.
ZHENG Z, XU X, CROSLEY RA, et al., 2010. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis[J]. Plant Physiol, 153(1): 99-113.
ZINSMEISTER J, LALANNE D, TERRASSON E, et al., 2016. ABI5 is a regulator of seed maturation and longevity in legumes[J]. Plant Cell, 28(11): 2735-2754.
(責任编辑 李 莉 王登惠)
